- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Triplet excitons play an important role in the physics of organic emitters used in organic light-emitting diodes, bio-imaging, and security inks. Triplet exciton dynamics is
influenced by the emitters and the environment surrounding them, but there is no effective way to alter triplet dynamics using external triggers. Here we demonstrate rapid and reversible
control of the triplet dynamics of the emitter coronene via an external heavy-atom effect induced by external gases. Strong interaction between the emitter molecule and gases is achieved by
encapsulating the emitter in a metal organic framework. Exposure to xenon, which has a large spin-orbit coupling, accelerates the radiative decay of triplets, leading to a stronger
phosphorescence that decays more quickly than under vacuum. By contrast, excitons can be non-radiatively quenched through exposure to oxygen. This fast and reversible regulation of triplet
dynamics may provide a new platform for responsive photo-switches, optical storage, and molecular computers. SIMILAR CONTENT BEING VIEWED BY OTHERS HIDDEN TRIPLET STATES AT HYBRID
ORGANIC–INORGANIC INTERFACES Article 26 July 2024 RECENT PROGRESS IN TRIPLET ENERGY TRANSFER SYSTEMS TOWARD ORGANIC AFTERGLOW MATERIALS Article Open access 21 March 2025 LANTHANIDE-DOPED
INORGANIC NANOPARTICLES TURN MOLECULAR TRIPLET EXCITONS BRIGHT Article 25 November 2020 INTRODUCTION Triplet excitons of organic molecules used as phosphors1,2 and photo-absorbers3,4 play an
important role in the physics of optoelectronic devices, such as organic light-emitting diodes5,6,7 and photovoltaics8. Devising new ways to control triplet excitons will help advance the
development of a wide variety of emitters7, photo reactions9,10, and organic semiconductor devices11,12,13. In a simple system undergoing photoluminescence, the key processes affecting
triplet excitons are the generation process of intersystem crossing (ISC) from a singlet excited state to a triplet excited state and the deactivation processes of radiative
(phosphorescence) and non-radiative transition from a triplet excited state to the singlet ground state. The rate constants of ISC (_k_isc) and of radiative emission from triplets (_k_p) are
strongly influenced by the chemical structure of the emitting molecules and can be tuned from 10−2 to 106 s−1 by exploiting spin-orbit coupling through the inclusion of heavy atoms like
bromine14, iridium15,16,17, and platinum18,19. The rate constant of non-radiative decay from triplet excited state (_k_nr(_T_)) is affected by factors related to the environment surrounding
the emitters, such as the host matrix20,21, emitter concentration22, and temperature (_T_) through processes such as energy transfer to other molecules23 and thermal deactivation24.
Minimization of _k_nr(_T_) by using rigid host matrices like steroids21, polymers25, clathrate compounds20,26, mixed crystals14, and metal organic frameworks (MOFs)27,28,29 has been
reported. Because of the competing radiative and non-radiative recombination processes, precisely designed molecules and optimized molecular environments are required to obtain efficient
room-temperature phosphorescence (RTP) from organic molecules. RTP can easily be reduced by increasing temperature24, introducing triplet quenchers23, or enhancing molecular motion to
increasing _k_nr(_T_). However, no effective way exists to enhance RTP by increasing _k_p via simple external triggers. Although several approaches, such as the use of photochromic
emitters30 and pH-sensitive molecules31 and the introduction of potassium iodine32 or xenon (Xe)33 to induce an external heavy-atom effect, have been reported, these systems exhibit very
slow responsivity and poor reversibility. In general, the external heavy-atom effect is used to describe the changes in the optical properties of an organic emitter caused by the presence of
heavy atoms that are not incorporated in the emitter molecules. Although this effect increases all spin-flipping processes _k_isc, _k_p, and _k_nr(_T_)34, the degree of enhancement depends
on various factors such as the spin-orbit coupling constants35,36, the distance between a heavy atom and an organic emitter37,38, the concentration of the heavy atoms32,35,39, the presence
of ionic interactions40, and the formation of charge transfer complexes between a heavy atom and emitter41. In some cases, the external heavy atom increases _k_nr(_T_) more than _k_p41.
However, in most cases, the heavy-atom effect enhances phosphorescence more than non-radiative processes40. For example, sodium halide more effectively enhances the _k_p than the _k_nr(_T_)
of 2-naphthalenesulfonate35. Thus, _k_p of an emitter can be reversibly controlled by introduction and removal of external heavy atoms. Recently, we demonstrated long-lived RTP with an
observed phosphorescence lifetime (_τ_phos) of 22 s under vacuum by embedding deuterated coronene (coronene-_d_12) into the zeolitic imidazolate framework ZIF-827. The large surface area of
ZIF-8 and low-doping concentration of coronene-_d_12 in ZIF-8 allow for gas adsorption and desorption. Here we report a rapid and reversible enhancement of _k_p in emitters encapsulated in
MOFs by introducing heavy-atom gases as an external trigger (Fig. 1a). We expose coronene-_d_12 encapsulated in ZIF-8 (coronene-_d_12@ZIF-8) to the gas argon (Ar) mixed with various
concentrations of Xe, which has a large spin-orbit coupling constant, at room temperature, and investigate the dependence of the emission spectra, phosphorescence quantum yield (_Φ_phos),
and _τ_phos on the concentration of Xe and the timing of the gas exposure. Both _k_p and _k_nr(_T_) can be tuned by introducing Xe and air, allowing for the full control of long-lived
triplet excitons. RESULTS OPTICAL PROPERTIES OF CORONENE-_D_ 12@ZIF-8 UNDER VARIOUS CONDITIONS A thin film of coronene-_d_12 encapsulated in ZIF-8 (coronene-_d_12@ZIF-8) was fabricated
according to the literature27. The concentration of coronene-_d_12 in ZIF-8 is 1.03 wt%, which means that coronene-_d_12@ZIF-8 still has enough space to accommodate extra molecules27. The
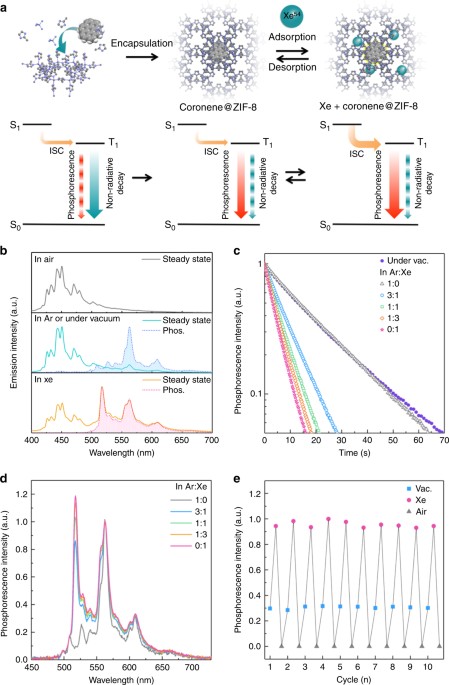
emission spectra under steady-state excitation and the emission decay profiles of the film were obtained in ambient air, Ar, and Xe and under vacuum at room temperature (Fig. 1). Because
phosphorescence is quenched by the oxygen in air23, only fluorescence was observed in air under steady-state excitation. On the other hand, dual steady-state emission from fluorescence and
phosphorescence was observed under vacuum and in Ar and Xe (Fig. 1b), and a slowly decaying phosphorescence was observed after stopping the excitation. The large surface area originating
from the porous structure of ZIF-8 leads to a good affinity for the gases42,43, so the external gas molecules can easily interact with the solid-state coronene-_d_12 in ZIF-827. While air
easily quenched the phosphorescence, the inert gas Ar does not affect the emission spectra or the observed phosphorescence lifetime (_τ_phos), which were nearly identical to those under
vacuum (Fig. 1c). On the other hand, both the phosphorescence spectra and _τ_phos were drastically different in Xe even though it is also an inert gas (Fig. 1b, c). Because, the ZIF-8 pores
can adsorb Xe molecules44, this result can be attributed to an external heavy-atom effect induced by Xe. The yellow phosphorescence with peaks of 487, 509, 527, 539, 563, and 608 nm in Ar
changes into green phosphorescence with peaks of 517, 525, 529, 563, 594, and 609 nm in Xe. Interestingly, the phosphorescence peak maximum under Xe is at 517 nm, which corresponds to the
0-0 transition of coronene-_d_1232. Thus, the symmetry forbidden 0-0 transition of coronene-_d_12 becomes partially allowed because of spin-orbit coupling with Xe. A similar phenomenon was
reported by combining aromatic hydrocarbons with heavy atoms, such as coronene with potassium iodide in ethanol32 or benzene with Xe33,45. Moreover, the delayed fluorescence observed in Ar
at 300 K disappeared in Xe because of the faster _k_p27. In addition to accelerating _k_p, the heavy-atom effect also increases _k_isc. Thus, the phosphorescence component in the
steady-state emission increases compared to that in Ar, and the ratio of phosphorescence to the total emission (_I_phos/_I_total) for 100-ms-long photo-excitation (see the Methods section
for measurement details) increases from 0.26 in Ar to 0.70 in Xe (Table 1). The _τ_phos of coronene-_d_12@ZIF-8 in Xe was 4.7 s, which is one-fifth of that in Ar (20.2 s). Although an
acceleration of _k_nr(_T_) is often the origin of a reduction in _τ_phos, the phosphorescent quantum yield (_Φ_phos) calculated from the total photoluminescence quantum yield (_Φ_total) and
_I_phos/_I_total was found to be 6.2%, which is much larger than that in Ar (3.7%) (Table 1 and Supplementary Figure 1). Therefore, the shorter _τ_phos originates from an enhanced _k_p.
Altering the concentration of Xe provides a simple method to control _τ_phos. We measured the film in mixtures of Ar and Xe with Xe concentrations of 25, 50, and 75% (Fig. 1c, d).
Phosphorescence intensity shows a linear relationship with the concentration of Xe (Supplementary Figure 2). Increasing the concentration of Xe increased _Φ_phos because of the accelerated
_k_p, but _Φ_total decreased since _k_isc also accelerated, leading to a reduced fluorescence contribution (Table 1). These results clearly indicate that the strength of the external
heavy-atom effect can be tuned with the concentration of the external gas. Even though the atomic size (4.4 Å) of Xe is slightly larger than the window size of ZIF-8 (3.4 Å), Xe atoms can
pass through the windows of ZIF-8 and interact with the coronene-_d_12 because the window size can be expanded by the rotation of the imidazole linkers44. Since there is no strong
encapsulation effect between the Xe atoms and the pores of ZIF-8, Xe atoms can be removed by exposing the film to a different gas or placing the film under vacuum. Therefore, the effects of
the external gases on coronene-_d_12@ZIF-8 are highly reversible. To demonstrate this reversibility, the steady-state phosphorescence intensity of the coronene-_d_12@ZIF-8 film was observed
over multiple cycles of sequential exposure to vacuum, Xe, and air (Fig. 1e). Each environment had a distinct phosphorescence intensity that was nearly constant over the multiple cycles.
Moreover, the phosphorescence intensity did not change even after we stored the film in air for several months. These results indicate that the modification of the triplet dynamics is fully
reversible. RESPONSIVITY OF TRIPLET EXCITONS TO EXTERNAL GASES The response of the emission to the external gases is rapid and reversible, enabling the direct control of accumulated triplet
excitons via the external heavy-atom effect. The emission of the film under vacuum exhibited a clear response within 1 s of introducing Xe after stopping the photo-excitation, and the
response time was largely determined by the time it takes to manually open and close the gas valves of the measurement system and not the migration speed of the gas (Supplementary Figures 3
and 4). The response of the emission decay of coronene-_d_12@ZIF-8 film to the introduction of Xe after stopping the excitation along with the decay in Xe and under vacuum are shown in Fig.
2 and Supplementary Movies 1-3. Long-lived phosphorescence from coronene-_d_12@ZIF-8 under vacuum is clearly visible more than 1 min after stopping the excitation light (Fig. 2a and
Supplementary Movie 1). Because the Xe gas accelerates both _k_isc and _k_p, an initially more intense phosphorescence with a faster decay was observed in Xe (Fig. 2b and Supplementary Movie
2). When we introduced Xe after stopping the excitation under vacuum, the phosphorescence intensity rapidly increased and the emission color became greener. (Figs. 2c, 3a and 3d and
Supplementary Movie 3). Introduction of Xe accelerates _k_p, so the accumulated triplet excitons are more rapidly converted into phosphorescence, leading to a phosphorescence intensity that
quickly increases before beginning to decay at a faster rate than under vacuum (Fig. 3d). Because introducing Xe increases _k_p, the integrated phosphorescence decay when exposed to Xe after
being under vacuum was slightly larger than that in a constant Ar atmosphere by 1.1 times (Supplementary Figure 5), which also indicates that _k_p is accelerated more than the _k_nr(_T_) in
the presence of Xe. By contrast, the long-lived phosphorescence was completely quenched when we introduced air (Fig. 3d). In general, triplet exciton quenching by oxygen in the solid matrix
is much slower than that in solution because of the slow migration speed of gases23. However, MOFs adsorb oxygen into their cavities in a moment because of its large surface area, leading
to fast-response time. The enhanced emission intensity and shorter _τ_phos indicate that we can regulate the phosphorescence by the introduction of Xe at arbitrary times (Fig. 3e and
Supplementary Figure 6). The _τ_phos can also be easily controlled by varying the concentration of Xe gas (Fig. 3f and Supplementary Figure 6). All of these results indicate that both the
phosphorescence intensity and _τ_phos can be easily and rapidly regulated through the introduction of a controlled amount of heavy atoms. DISCUSSION In summary, we demonstrated the use of
the external heavy-atom effect to achieve fast, rapid, and reversible control of triplet excitons in organic semiconductors. A host matrix of ZIF-8 provides not only a rigid environment for
the guest emitter but also a high affinity for external gases. According to the combination of these functions, we can easily regulate the conversion of the accumulated triplet excitons into
emission. The timing and speed of extraction of accumulated excitons as emission can be controlled by introducing gas mixtures with various concentrations of Xe. Alternatively, the
accumulated excitons can be completely extinguished through non-radiative processes by introducing air. This fast and reversible control of triplet excitons in a MOF matrix could open a new
platform for fast-response photo-switches, reversible optical storage, and molecular computers. METHODS PREPARATION OF CORONENE-_D_ 12@ZIF-8 FILM A coronene-_d_12@ZIF-8 film was prepared by
using ship-in-a-bottle synthesis following the procedure from literature27. A dimethylformamide (DMF) solution of coronene-_d_12, Zn(NO3)2·6H2O, and 2-methylimidazole (H-MeIm) was heated at
140 °C for 24 h in a Teflon-lined autoclave reactor. After cooling to room temperature, the activation steps were performed under low pressure (<10−3 Pa) using a turbo molecular pump. The
doping concentration of coronene-_d_12 into ZIF-8 was calculated according to the method in a previous report. OPTICAL MEASUREMENTS The long-lived photoluminescence spectra and decay
profiles under various conditions were obtained using a measurement system with the configuration shown in Supplementary Figure 4. A coronene-_d_12@ZIF-8 film was first activated by the
turbo molecular pump (HiPace80, Pfeiffer vacuum), then a gas mixture, the concentration of which was controlled by a gas mixer (Kofloc, PMG-1A), was introduced into the sample chamber.
Notably, this system contains several manual gas valves used for the introduction of the external gases. The coronene-_d_12@ZIF-8 film was placed in a sample chamber with a quartz window and
excited by a UV light source (MORITEX MUV-202U) with a bandpass filter (340 ± 5 nm). The emission spectra and emission decay profiles were recorded using a multichannel spectrometer
(PMA-12, Hamamatsu Photonics) with a longpass filter (370 nm). The _Φ_total of coronene-_d_12@ZIF-8 in various environments were measured with an absolute photoluminescence quantum yield
spectrometer (Quantaurus-QY, Hamamatsu Photonics) at room temperature under a vacuum (10−3 Pa). Here, _Φ_flu (photoluminescence quantum yield of the fluorescence) and _Φ_phos were calculated
from the emission intensity at all wavelengths obtained using the PMA-12. The PMA-12 was used to measure the emission integrated over 100-ms intervals. The total emission intensity
(_I_total) for 100-ms-long photo-excitation was measured by integrating all of the measurements from the PMA-12 with the first measurement starting at the same time as the photo-excitation.
The fluorescence intensity (_I_flu) was estimated by subtracting the intensity from the second 100-ms measurement interval, which roughly corresponds to the phosphorescence during the first
100-ms interval because of the slow phosphorescence decay, from the intensity during the first 100-ms measurement interval, which includes the photo-excitation period. The phosphorescence
intensity (_I_phos) was measured from the integrated intensity for all of the measurements from the second 100-ms measurement interval on with the intensity of the second 100-ms measurement
interval counted twice to roughly account for the phosphorescence during excitation. DATA AVAILABILITY The authors declare that the data support the findings of this study are available from
the authors upon reasonable request. REFERENCES * Hirata, S. Recent advances in materials with room temperature phosphorescence: photophysics for triplet exciton stabilization. _Adv. Opt.
Mater_. 5, 1700116 (2017). * Mukherjee, S. & Thilagar, P. Recent advances in purely organic phosphorescent materials. _Chem. Commun._ 51, 10988–11003 (2015). Article CAS Google Scholar
* Hirata, S., Totani, K., Yamashita, T., Adachi, C. & Vacha, M. Large reverse saturable absorption under weak continuous incoherent light. _Nat. Mater._ 13, 938–946 (2014). Article
CAS Google Scholar * Su, W., Cooper, T. M. & Brant, M. C. Investigation of reverse-saturable absorption in brominated porphyrins. _Chem. Mater._ 4756, 1212–1213 (1998). Article Google
Scholar * Kabe, R., Notsuka, N., Yoshida, K. & Adachi, C. Afterglow organic light-emitting diode. _Adv. Mater._ 28, 655–660 (2016). Article CAS Google Scholar * Adachi, C., Baldo,
M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. _J. Appl. Phys._ 90, 5048–5051 (2001). Article CAS Google
Scholar * Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. _Nature_ 492, 234–238 (2012). Article CAS
Google Scholar * Righini, M. et al. External quantum efficiency above 100% in a singlet-exciton-fission–based organic photovoltaic cell. _Science_ 340, 334–338 (2013). Article Google
Scholar * Leveque, C., Chenneberg, L., Corce, V., Ollivier, C. & Fensterbank, L. Organic photoredox catalysis for the oxidation of silicates: applications in radical synthesis and dual
catalysis. _Chem. Commun._ 52, 9877–9880 (2016). Article CAS Google Scholar * Horie, K., Ando, H. & Mita, I. Photochemistry in polymer solids. 8. Mechanism of photoreaction of
benzophenone in poly(vinyl alcohol). _Macromolecules_ 20, 54–58 (1987). Article CAS Google Scholar * Hoshino, S. & Suzuki, H. Electroluminescence from triplet excited states of
benzophenone. _Appl. Phys. Lett._ 69, 224–226 (1996). Article CAS Google Scholar * Nakanotani, H. et al. High-efficiency organic light-emitting diodes with fluorescent emitters. _Nat.
Commun._ 5, 4016 (2014). Article CAS Google Scholar * Kido, J. & Iizumi, Y. Fabrication of highly efficient organic electroluminescent devices. _Appl. Phys. Lett._ 73, 2721–2723
(1998). Article CAS Google Scholar * Bolton, O., Lee, K., Kim, H.-J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. _Nat.
Chem._ 3, 205–210 (2011). Article CAS Google Scholar * You, Y. & Park, S. Y. Phosphorescent iridium(III) complexes: toward high phosphorescence quantum efficiency through ligand
control. _Dalton Trans_. 0, 1267–1282 (2009). * Xie, Z., Ma, L., Kathryn, E., Jin, A. & Lin, W. Porous phosphorescent coordination polymers for oxygen sensing. _J. Am. Chem. Soc._ 132,
922–923 (2010). Article CAS Google Scholar * Li, L. et al. Dynamic entangled framework based on an iridium-organic unit showing reversible luminescence turn-on sensing. _Inorg. Chem._ 54,
8872–8874 (2015). Article CAS Google Scholar * Li, K. et al. Highly phosphorescent platinum(II) emitters: photophysics, materials and biological applications. _Chem. Sci._ 7, 1653–1673
(2016). Article CAS Google Scholar * Zhou, B. G., Wong, W., Poon, S., Ye, C. & Lin, Z. Symmetric versus unsymmetric platinum (II) bis(aryleneethynylene)s with distinct electronic
structures for pptical power limiting / optical transparency trade-off optimization. _Adv. Funct. Mater._ 19, 531–544 (2009). Article CAS Google Scholar * Scypinski, S. & Love, L. J.
C. Room-temperature phosphorescence of polynuclear aromatic hydrocarbons in cyclodextrins. _Anal. Chem._ 56, 322–327 (1984). Article CAS Google Scholar * Hirata, S. et al. Efficient
persistent room temperature phosphorescence in organic amorphous materials under ambient conditions. _Adv. Opt. Mater._ 23, 3386–3397 (2013). CAS Google Scholar * Kawamura, Y., Brooks, J.,
Brown, J. J., Sasabe, H. & Adachi, C. Intermolecular interaction and a concentration-quenching mechanism of phosphorescent Ir(III) complexes in a solid film. _Phys. Rev. Lett._ 96,
17404 (2006). Article Google Scholar * Ji, S. et al. Real-time monitoring of luminescent lifetime changes of PtOEP oxygen sensing film with LED/photodiode-based time-domain lifetime
device. _Analyst_ 134, 958–965 (2009). Article CAS Google Scholar * Hirata, S. et al. Reversible thermal recording media using time-dependent persistent room temperature phosphorescence.
_Adv. Opt. Mater._ 1, 438–442 (2013). Article Google Scholar * Reineke, S. et al. Highly efficient, dual state emission from an organic semiconductor. _Appl. Phys. Lett._ 103, 93302
(2013). Article Google Scholar * Montes-Navajas, P. & Garcia, H. Cucurbituril complexation enhances intersystem crossing and triplet lifetime of 2,4,6-triphenylpyrylium ion. _J. Phys.
Chem. C_ 114, 2034–2038 (2010). Article CAS Google Scholar * Mieno, H., Kabe, R., Notsuka, N., Allendorf, M. D. & Adachi, C. Long-lived room-temperature phosphorescence of coronene in
zeolitic imidazolate framework ZIF-8. _Adv. Opt. Mater._ 4, 1015–1021 (2016). Article CAS Google Scholar * Yang, X. & Yan, D. Long-afterglow metal-organic frameworks: reversible
guest-induced phosphorescence tunability. _Chem. Sci._ 7, 4519–4526 (2016). Article CAS Google Scholar * Yang, X. & Yan, D. Strongly enhanced long-lived persistent room temperature
phosphorescence based on the formation of metal-organic hybrids. _Adv. Opt. Mater._ 4, 897–905 (2016). Article CAS Google Scholar * Katsurada, Y., Hirata, S., Totani, K., Watanabe, T.
& Vacha, M. Photoreversible on-off recording of persistent room-temperature phosphorescence. _Adv. Opt. Mater._ 3, 1726–1737 (2015). Article CAS Google Scholar * Yang, Y., Wang, K. Z.
& Yan, D. Ultralong persistent room temperature phosphorescence of metal coordination polymers exhibiting reversible pH-responsive emission. _ACS Appl. Mater. Interfaces_ 8, 15489–15496
(2016). Article CAS Google Scholar * Najbar, J., Rodakiewicz-nowak, J. & Chodkowska, A. External heavy atom effect on the triplet state of aromatic hydrocarbons. _J. Lumin._ 17,
449–465 (1978). Article CAS Google Scholar * Hsu, Y. & Johnson, P. M. External heavy atom effects on benzene in rare gas hosts. _J. Chem. Phys._ 59, 136–142 (1973). Article CAS
Google Scholar * Siegel, S. & Judeikis, H. S. Effect of external heavy atoms on decay processes from the triplet state. _J. Chem. Phys._ 42, 3060–3068 (1965). Article CAS Google
Scholar * White, W. & Seybold, P. G. External heavy-atom effect on the room-temperature luminescence of adsorbed dyes. _J. Phys. Chem._ 81, 2035–2040 (1977). Article CAS Google
Scholar * Giachino, G. G. & Kearns, D. R. Nature of the external heavy‐atom effect on radiative and nonradiative singlet–triplet transitions. _J. Chem. Phys._ 52, 2964–2974 (1970).
Article CAS Google Scholar * Minaev, B. Theoretical study of the external heavy atom effect on phosphorescence of free-base porphin molecule. _Spectrochim. Acta Part A Mol. Biomol.
Spectrosc._ 60, 3213–3224 (2004). Article Google Scholar * Najbar, J. & Barzyk, W. The influence of iodide ions on radiative (T1→S0 and radiationless (S1→T1, and T1→S0) transitions in
the fluorene molecule. _J. Lumin._ 8, 242–251 (1974). Article Google Scholar * Najbar, J. & Munro, I. H. External heavy atom effects on the decay of the triplet state of aromatic
hydrocarbons III. The decay functions of fluorescence and phosphorescence of carbazole in the presence of KI. _J. Lumin._ 17, 135–148 (1978). Article CAS Google Scholar * Kim, J. P., Xie,
Z., Creer, M., Liu, Z. & Yang, J. Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of cystic fibrosis. _Chem. Sci._ 8, 550–558 (2017). Article CAS
Google Scholar * Hamai, S. & Kudou, T. External heavy atom effects of 6-deoxy-6-iodo-α-cyclodextrin on the room-temperature phosphorescence of 6-bromo-2-naphthol and 3-bromoquinoline in
aqueous solutions. _J. Photochem. Photobiol. A Chem._ 113, 135–140 (1998). Article CAS Google Scholar * McCarthy, M. C., Varela-Guerrero, V., Barnett, G. V. & Jeong, H.-K. Synthesis
of zeolitic imidazolate framework films and membranes with controlled microstructures. _Langmuir_ 26, 14636–14641 (2010). Article CAS Google Scholar * Jiang, J. Q., Yang, C. X. & Yan,
X. P. Zeolitic imidazolate framework-8 for fast adsorption and removal of benzotriazoles from aqueous solution. _ACS Appl. Mater. Interfaces_ 5, 9837–9842 (2013). Article CAS Google
Scholar * Gallaba, D. H., Albesa, A. G. & Migone, A. D. Evidence of gate-opening on xenon adsorption on ZIF-8: An adsorption and computer simulation study. _J. Phys. Chem. C_ 120,
16649–16657 (2016). Article CAS Google Scholar * Cundall, R. B. & Pereira, L. C. Solvent effects on the photoluminescence of benzene_-_ h6 and benzen_e_ -d6 at 77 K. _Rev. Port Quim._
20, 13–20 (1978). CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Japan Science and Technology Agency (JST), ERATO, Adachi Molecular Exciton
Engineering Project under JST ERATO Grant Number JPMJER1305, Japan, the International Institute for Carbon Neutral Energy Research (WPI-I2CNER) sponsored by the Ministry of Education,
Culture, Sports, Science and Technology (MEXT), and MEXT/JSPS KAKENHI Grant Number JP 15K21220. We thank Dr. W. J. Potscavage Jr. for his assistance with the preparation of this manuscript.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Organic Photonics and Electronics Research (OPERA), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan Hiroyuki
Mieno, Ryota Kabe & Chihaya Adachi * JST ERATO Adachi Molecular Exciton Engineering Project, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan Hiroyuki Mieno, Ryota Kabe
& Chihaya Adachi * International Institute for Carbon Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan Chihaya Adachi Authors *
Hiroyuki Mieno View author publications You can also search for this author inPubMed Google Scholar * Ryota Kabe View author publications You can also search for this author inPubMed Google
Scholar * Chihaya Adachi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.K. conceived the project. H.M. and R.K. conducted the
experiments. H.M, R.K., and C.A discussed the results and co-wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Ryota Kabe or Chihaya Adachi. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY MOVIE 1 SUPPLEMENTARY MOVIE 2
SUPPLEMENTARY MOVIE 3 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Mieno, H., Kabe, R. & Adachi, C. Reversible control of triplet dynamics in metal-organic framework-entrapped organic emitters via external gases. _Commun Chem_ 1, 27
(2018). https://doi.org/10.1038/s42004-018-0027-x Download citation * Received: 11 January 2018 * Accepted: 20 March 2018 * Published: 03 May 2018 * DOI:
https://doi.org/10.1038/s42004-018-0027-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative