- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Upon infection with SARS-CoV-2, the virus that causes COVID-19, most people will develop no or mild symptoms. However, a small percentage of the population will become severely ill,
and some will succumb to death. The clinical severity of COVID-19 has a close connection to the dysregulation of the patient’s immune functions. We previously developed a simple,
nanoparticle-enabled blood test that can determine the humoral immune status in animals. In this study, we applied this new test to analyze the immune function in relation to disease
severity in COVID-19 patients. From the testing of 153 COVID-19 patient samples and 142 negative controls, we detected a drastic decrease of humoral immunity in COVID-19 patients who
developed moderate to severe symptoms, but not in patients with no or mild symptoms. The new test may be potentially used to monitor the immunity change and predict the clinical risk of
patients with COVID-19. SIMILAR CONTENT BEING VIEWED BY OTHERS CELLULAR IMMUNITY REFLECTS THE PERSISTENT SYMPTOMS AMONG COVID-19 RECOVERED PATIENTS IN JAPAN Article Open access 08 July 2023
A MULTIPLEX CHEMILUMINESCENT IMMUNOASSAY FOR SEROLOGICAL PROFILING OF COVID-19-POSITIVE SYMPTOMATIC AND ASYMPTOMATIC PATIENTS Article Open access 02 February 2021 IMMUNE RESPONSE IN
COVID-19: WHAT IS NEXT? Article Open access 17 May 2022 INTRODUCTION Coronavirus disease 2019 (COVID-19), a viral infectious disease that is caused by Severe Acute Respiratory Distress
Syndrome Virus-2 (SARS-CoV-2) virus infection, has become a global pandemic following an initial outbreak in the People’s Republic of China at the end of 2019 and beginning of 2020. Until
October 2021, 242 million people around the world have been infected with the virus and 4.9 million people have died of COVID-191,2. Upon infection, while most people will show no or mild
symptoms, a significant portion of the population will develop moderate to severe symptoms that require hospitalization, aggressive treatments such as mechanical ventilation, intensive care,
and some eventually succumb to death3,4,5. The mortality rate based on the number of confirmed positive cases varies from on average 2–3% to as high as ~ 10% in some countries1. Extensive
studies have found that the immune response in COVID-19 patients is rather complicated, varies significantly from person to person, and patients’ immune response dynamics have a direct
association with their clinical outcome6,7,8,9,10,11,12,13,14. Of particular interest, patients who developed severe symptoms or died of COVID-19 have reduced number of T cells and their
immune responses are skewed towards type 2, antibody-mediated immunity15,16,17,18,19,20,21. These patients are more likely to experience cytokine storm that can lead to uncontrolled
inflammation of the body, tissue damage, and eventually organ failure. In contrast, asymptomatic patients or patients with mild symptoms appear to have stronger type 1, cell-mediated
immunity than patients with more severe symptoms22. In the fight against infectious pathogens, especially intracellular pathogens such as viruses, type 1 immune response is the frontline
defense against the pathogen from the immune system. If type 1 immune response fails to stop the infection, type 2 immune response is initiated, clearing the virus by producing
virus-specific antibodies23. These antibodies include binding antibodies and neutralizing antibodies. While binding antibodies can only bind to virus or virus antigens, neutralizing
antibodies bind with the virus in a specific way that render the virus particle no longer infectious or pathogenic24. The level of binding antibodies is determined by using immunoassays such
as ELISA and quantitated as antibody titer. The level of neutralizing antibodies is analyzed by evaluating the viability of live virus using techniques such as plaque reduction assay.
Higher anti-virus antibody titers were found in patients with more severe symptoms compared to those with only mild symptoms17,18. On the other hand, neutralizing antibodies with strong
potency in the COVID-19 patients is a predictor of survival25,26. While type 1 immunity is dominated with cell-mediated immune responses and type 2 immunity leads primarily to
antibody-mediated immune responses, both types are inter-connected through the humoral immune system, particularly, the immunoglobin G (IgG) subclasses. In humans, IgG1 and IgG3 are
associated with type 1 immune response, and IgG4 is linked to type 2 immune response27,28. In bovine, IgG2 is associated with type 1 and IgG1 is associated with type 2 immunity29. In murine
models, IgG2a is a specific indicator of type 1 immunity and mouse IgG1 is a marker of type 2 immunity30. Under typical, healthy conditions, there is a balance between type 1 and type 2
immunity-associated IgG subclasses. Upon infection or other changes of physiological conditions such as pregnancy or parturition, such balance may be disturbed29,31. The ratio of IgG
subclasses in blood serum or plasma is often used as a marker to evaluate the relative balance of type 1 and type 2 immune responses in humans and animals32,33,34. We previously developed a
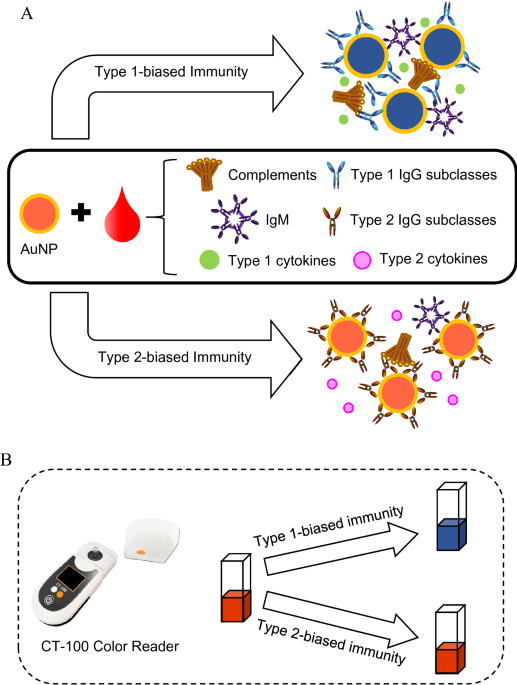
rapid blood test, D2Dx immunity test, that can detect and monitor the humoral immune status and responses in laboratory and farm animals35,36,37,38,39. The test uses a gold nanoparticle
(AuNP) as a pseudo virus pathogen to probe the humoral immunity in a blood plasma or serum sample. The AuNP is designed to react with type 1 and type 2 immunity-associated IgG subclasses
non-specifically, but differently. Upon interaction with type 1 immunity-associated IgG subclasses, such as human IgG1, IgG3, bovine IgG2 and murine IgG2a, the AuNPs will form large
aggregates, leading to a strong color change of the AuNP solution (Fig. 1). This color change is detected using a handheld colorimeter as shown in Fig. 1. The AuNP probe can also interact
with type 2-associated IgG subclass, i.e., IgG4 for human, IgG1 for bovine, and IgG1 for murine. However, these IgG subclasses will not lead to AuNP aggregate formation, hence, no color
change of the assay solution. To illustrate the principle of this test, we present here a kinetic study of the AuNP interaction with different IgG subclasses. Figure 2A is the reaction
kinetics between the AuNP probe with IgG subclasses indicative of type 1 immunity, i.e., human IgG1 and IgG3, bovine IgG2, and mouse IgG2a, while Fig. 2B is the reaction kinetics between the
AuNP probe and type 2 indicating subclasses, namely, bovine IgG1, human IgG4, mouse IgG1. The association of human IgG2, mouse IgG2b and mouse IgG3 in type 1 versus type 2 immunity is not
as clearly understood as other IgG subclasses, but the results are presented here as well. As clearly demonstrated from the study, all type 1-related IgG subclasses, regardless if it is from
human, bovine or murine sources, caused dramatic color change of the assay solution; while all IgG subclasses associated with type 2 immunity, led to almost no color change of the assay
solution. In a blood plasma or serum sample, different IgG subclasses will compete to interact with the AuNP probe, and the measured color change of the assay solution is a quantitative
indication of the relative balance of type 1 and type 2 immunity. A high D2Dx test score is indicative of a stronger type 1 immunity and weaker type 2 immunity; while a low test score
correlates to a weaker type 1 immunity and stronger type 2 immunity. It should be noted here that although the AuNP probe is designed to primarily react with the type 1 and type 2
immunity-associated IgG subclasses differently, other molecules and biochemicals, especially complement proteins are also involved in the interaction with the AuNP probe, as specifically
proven in our published study35. Previously, we have used this new test primarily to study and monitor the humoral immune status and type 1/type 2 immunity balance change in laboratory
animal models and agricultural farm animals35,36,37,38. Our studies show that the test can detect active immune response in animals upon virus infections, immune function development in
neonatal animals, immune status change in dairy cows associated with pregnancy and parturition. Based on these animal studies, we hypothesize that the D2Dx immunity test may be able to
detect the immune status change in COVID-19 patients. Furthermore, if the clinical severity of the COVID-19 patients is associated with the relative balance between the type 1 versus type 2
immunity, the test may be able to detect this difference and the result may be used to predict the clinical risk of the patients. RESULTS AND DISCUSSION In this study, we tested 142 negative
control samples and 153 COVID-19 positive samples (Table 1). Figure 3 is the D2Dx immunity test scores of the six study cohorts. P values calculated from student t-test were listed in the
plot for different cohort pairs. Our first statistical analysis was focused on the comparison of the negative control cohort (normal-OH) vs the three COVID-19 patient cohorts, since these
samples were collected at the same period from April to August 2020 from the same clinical site, Orlando Health. The average test score of the normal-OH, asymptomatic/mild, moderate and
severe cohort is 0.069, 0.056, 0.029 and 0.032, respectively. Statistically significant difference was observed in the following cohort pairs: normal-OH vs asymptomatic/mild (P value
0.0048); normal-OH vs moderate (P value 1.1E−14); normal-OH vs severe (P value 7E−16), asymptomatic/mild vs moderate (P value 5.25E−06), and asymptomatic/mild vs severe (P value 2.47E−05).
The difference between moderate and severe cohort is not significant (P value > 0.05). The combined test scores of the moderate and severe cohort are significantly lower than the
asymptomatic/mild cohort (P value less than 0.0001). We also compared the differences between the three negative control cohorts, normal-OH, normal-USA, and normal-DR. There is no difference
between the normal-USA and normal-DR cohort (P value 0.296). Although we observed a slight difference between the normal-OH cohort and the normal-USA or normal-DR cohort (P value 0.01), the
average test scores of the three cohorts are very close, in between 0.063 and 0.069. Compared to the difference we observed from the negative control versus the COVID-19 positive samples,
this difference is rather insignificant. Furthermore, the samples in the normal-USA and normal-DR cohort have been stored for almost two years before testing. It is most likely that the
biological activity of these two cohort samples has changed slightly, leading to a very slightly lower average score in these two cohorts (average score 0.062 and 0.064 for normal-USA and
normal-DR cohort) than the more recently collected normal-OH cohort samples (average score 0.069). The comparison of the three negative control cohorts confirms that the D2DX test is a
highly robust test, and the difference we observed from the normal control samples and COVID-19 positive samples are indeed caused by the patients’ active disease status and different immune
responses in the patients. As initially hypothesized, we believe the difference observed from COVID-19 patients with different degrees of clinical severity can be attributed to their
difference in type 1 versus type 2 immunity. Patients who developed no or mild symptoms upon infection must have had stronger type 1 immunity and had the capability to maintain this strong
type 1 immune response. In D2Dx immunity test, these patients presented relatively high scores. Patients who develop moderate to severe symptoms are those who did not or could not mount a
sufficient type 1 immune response, leading to an invoked type 2 response to help control the infection. In this study, we analyzed the anti-SARS-CoV-2 IgG antibody level in 9 randomly
selected patient samples. We found a strong negative correlation between the D2Dx immunity test score and the anti-virus IgG titer (Fig. 4), with a Spearman correlation coefficient of −
0.77. This finding, in agreement with many published studies by others15,16,17,18,19,20,21,22, provided strong evidence to support our hypothesis that patients that progressed into more
severe clinical symptoms developed a strong type 2, antibody-mediated immunity, hence, showed lower scores in the D2Dx immunity test. We also analyzed the anti-SARS-CoV-2 IgM antibody level
in 8 out of the 9 samples selected for IgG antibody titer analysis (one sample was not available by the time IgM titer analysis was conducted). We found a moderate positive correlation
between the D2Dx immunity test scores and the anti-virus IgM antibody titer, with a Spearman correlation coefficient of 0.53 (data not shown). This observation suggests that the production
of IgM antibody at early stage of infection is protective and patients who can produce stronger IgM antibody response may have lower clinical risk40,41. Immune response to viral infection is
a highly dynamic process. We analyzed the correlation of the immunity test scores of COVID-19 patients in the moderate and severe cohort with their days from symptom onset and blood
collection. This analysis is presented in three graphs: Fig. 5A is the correlation analysis of the moderate cohort; Fig. 5B is the correlation of the severe cohort by including 21 samples
covering symptom onset days from 0 to 27 days; and Fig. 5C is the correlation of the severe cohort including only 16 samples covering symptom onset days from 0 to 14 days. With the moderate
cohort, we found no correlation between the symptom onset days and the immunity test scores (correlation coefficient 0.09). With the severe cohort, when all 21 samples are considered, we
found a moderate positive correlation (correlation coefficient 0.54) between the immunity test scores and the symptom onset days. When we limit the days from symptom onset to blood
collection to 14 days (2 weeks) in the severe cohort, a strong positive correlation (correlation coefficient 0.73) was found between the immunity test scores and the symptom onset days. Due
to limited data, we have in this analysis, the correlation results should be treated with caution. However, the preliminary data indicates that there appears to be a significant dynamic
change in the immune response in COVID-19 patients who develop more severe symptoms, while this change is not seen in patients with moderate symptoms. In summary, we reported here an
extremely simple and rapid test that can detect the immune status change in COVID-19 patients and the differences between COVID-19 patients with milder symptoms versus those with more severe
symptoms and poor clinical outcome. Although this is a preliminary study, many interesting results were observed and the potential of the test as a point-of-care clinical test for COVID-19
patient management was demonstrated. The test involves a simple one-step process, and the result is obtained in less than 1 min. Although we used blood plasma samples collected through full
blood draw in the current study, the test can use blood samples obtained from finger prick, since only a few µL of plasma sample is needed to perform the test. More extensive clinical
studies should be conducted to further validate the test. In this study, we did not evaluate the potential influences of clinical interventions, comorbidities, or other demographic factors
including age, sex, race or ethnicities of the patients on the test results. Our main objective was to evaluate the applicability of D2Dx test to differentiate three groups of subjects with
mild/asymptomatic, moderate, severe disease symptoms after SARS-CoV-2 infection. A small percentage of the patients, especially patients in the severe clinical cohorts received COVID-19
related treatments such as Tocilizumab and/or hydroxychloroquine prior to or at the time of the blood sample collection. These confounding factors may or may not impact the test results and
warrant further investigations. In addition to predicting and monitoring the clinical outcome of COVID-19 patients, the novel D2Dx technology we report here may provide a valuable new tool
to accelerate the design and development of new vaccines for COVID-19 and other viral infectious diseases. The balance of Th1/Th2 immune response to a vaccination is an important factor to
be considered in the design of the vaccine. A vaccine that primarily elicit a Th2 immune response may prevent the initiation of a Th1 immune response, and sometime may exacerbate the disease
following infection42. A recently completed study by Swanson et al. shows that the Oxford-AstraZeneca COVID-19 vaccine, AZD1222, induced strong spike-specific CD4 + Th1 and CD8 + T-cell
responses in vaccinated individuals43. A study by Sahin et al. on Pfizer BNT162b2 vaccine to COVID-19 also revealed a strong type 1 immune response44. These two studies suggest an effective
COVID-19 vaccine should primarily elicit Th1-biased immune response. With its simplicity, low cost, and rapid results, D2Dx immunity test may be used to quickly assess the Th1 and Th2
response profile generated by vaccination and monitor such responses kinetically over time. Such data can provide further guidance in the design strategies to improve the efficacy of future
new vaccines. METHODS HUMAN BLOOD SAMPLE SOURCES AND COLLECTIONS All methods were carried out in accordance with relevant guidelines and regulations. The blood samples collected in BD
Vacutainer Plus Blood Collection Tubes containing K2EDTA were centrifuged at 2000_g_ for separation into cells and plasma as supernatant. The blood plasma samples were obtained from two
different sources. Plasma samples of deidentified healthy controls (n = 80) were previously obtained and archived from Boca Biolistics (Boca Raton, Florida) in December 2018 for a different
study. Among the 80 samples, 42 were collected in the United States and 38 samples were collected in Dominican Republic. All donors were healthy donors without any reported or known
infectious diseases when the samples were collected. The samples were aliquoted and stored at − 80 °C upon receipt until used for this study. Prior to the use for D2Dx assay, the samples
were thawed for overnight at 4 °C, and then left to equilibrate at room temperature for 2 h before testing. The samples were tested without any dilution or other treatment. We collected
blood samples from COVID-19 patients and healthy volunteer donors at Orlando Health. The separated plasma supernatants were aliquoted and stored in freezer (− 30 °C). Of which, 62 were from
healthy donors and 153 were from COVID-19 positive cases. The study (OH IRB # 20.095.06) was approved by Orlando Health IRB#2. Informed consent was obtained from each patient. Informed
consent statement from healthy individual donors was obtained as well. COVID-19 patients were treated at various hospitals within the Orlando Health hospital system as in-patients or
out-patients. The IRB also approved to use remnant blood plasma or serum samples from patients and volunteers from a previous serology validation study. The clinical statuses of the study
patients were obtained from the patient’s medical record, or by self-reporting by volunteer donors. PATIENT COHORTS AND DEMOGRAPHIC INFORMATION In this study, we tested 142 negative control
samples and 153 COVID-19 positive samples. Table 1 summarizes the clinical status and sample size of each study cohorts. We included three negative control groups. Previously, we collected
80 blood plasma samples from healthy donors in December 2018, about 1 year before the COVID-19 outbreak. The samples were collected at two geographic locations. A total of 42 samples were
from the United States (Normal-USA cohort) and 38 samples were from Dominican Republic (Normal-DR cohort). A third cohort of 62 samples were collected at Orlando Health from healthy
volunteer donors (Normal-OH cohort) from April to August 2020, in the same time period when we collected COVID-19 positive samples. These volunteer donors were tested negative in
anti-SARS-CoV-2 IgG and IgM serology test, and never reported any clinical symptoms associated with COVID-19. The 153 COVID-19 positive samples were collected between April to August 2020 in
Orlando Health (Orlando, Florida). In this study, we used the World Health Organization (WHO) Eight Category Ordinal Scale for Clinical Improvement45, as used in the seminal Remdesivir
trial46, to rank the clinical severity of the patients and group the patients into different cohorts. The uninfected controls are assigned with a 0 score. Patients who were tested positive
but exhibited no or only mild symptoms were assigned with a score of 1 (ambulatory with no limitation of activities) or 2 (ambulatory with limitation of activities). These patients were
either not hospitalized or were hospitalized for unrelated conditions and found to be positive. From score 3 and above, all patients were hospitalized and treated in various hospitals at
Orlando Health. Blood samples were collected during patients’ stay in the hospital. The symptoms exhibited by the patient at the time of blood draw were documented. According to the clinical
symptom severity, the patient was assigned with the Ordinal Scale from 3 to 7: 3—patients were hospitalized, but no oxygen therapy; 4—patients require oxygen by mask or nasal prongs;
5—patients require non-invasive ventilation or high flow mask; 6—patients require intubation and mechanical ventilation; 7—patients require ventilation and additional organ support such as
vasopressors, RRT, and ECMO. We did not include samples from patients who died of COVID-19 in our study, which would be scale 8. In keeping with the Eight Category Ordinal Scale, patients
with scale 1–2 were further grouped as asymptomatic/mild cohort (38 samples); patients with scale 3–4 are grouped as moderate cohort (54 samples); and patients with scale of 5 and above are
group as severe cohort (61 samples). D2DX IMMUNITY TEST OF BLOOD PLASMA SAMPLES D2Dx immunity test kits (catalog D2Dx-hu-500, lot number hu08012020) were received from Nano Discovery Inc.
(Orlando, Florida). Each kit contains the AuNP reagent and cuvettes for 500 tests. A handheld colorimeter reader device, CT-100 from Nano Discovery Inc. was used to read the test result. The
specific composition and chemical structure of the AuNP reagent is proprietary information of Nano Discovery Inc. The AuNP reagent was manufactured, formulated, and calibrated using the
CT-100 reader according to an internal quality control standard established by Nano Discovery Inc. A 50 μL of the AuNP reagent solution was first placed into a cuvette using a micropipette.
Then 10 μL of an undiluted blood plasma sample was added. After mixing the assay solution for 5 s using a mini-vortex mixer, the cuvette was placed in the cuvette holder in CT-100, and the
result was read automatically in 30 s. The response of the test was reported directly as the absorbance change of the assay solution over a 30-s of reaction time. KINETIC INTERACTION STUDY
OF AUNP WITH IGG SUBCLASSES The study of the interaction between the AuNP reagents and IgG subclasses from bovine, human and murine was conducted using the following materials: Bovine IgG1
(pep003, Bio-Rad, 1 mg/mL); bovine IgG2 (pep004, Bio-Rad, 1 mg/mL); human IgG1 (ab90283, Abcam, 3 mg/mL); human IgG2 (ab90284, Abcam, 2.2 mg/mL); human IgG3 (ab118462, Abcam, 2.2 mg/mL);
human IgG4 (ab183266, Abcam, 1.5 mg/mL); mouse IgG1 (02-6100, Thermofisher, 1 mg/mL); mouse IgG2a (02-6200, Thermofisher, 1 mg/mL); mouse IgG2b (02-6300, Thermofisher, 1 mg/mL); mouse IgG3
(IMG5119A, Novus Biological, 0.5 mg/mL). The kinetic study was conducted using a LaMotte model 3250 colorimeter. To an optical cuvette, 100 µL AuNP reagent from the D2Dx immunity test kit
(D2Dx-hu-500) was added. Then 10 µL of the IgG subclass protein solution was added. Following mixing for 5 s using a mini vortex mixer, the cuvette was placed in the colorimeter, and the
absorbance change of the assay solution was recorded every 30 s for a total reaction time of 3 min. SARS-COV-2 SPECIFIC ANTIBODY MEASUREMENTS An enzyme-linked immunosorbent assay (ELISA) was
used to detect SARS-CoV-2 specific IgG and IgM antibodies in plasma samples from patients (positive by PCR test with nasopharyngeal samples) and from donors with or without symptoms, who
were tested positive or negative by PCR. Qualitative serology ELISA kits from Creative Diagnostics (Shirley, NY, USA) were used to measure anti-SARS-CoV-2 IgG (product # DEIASL019) and IgM
(product # DEIASL020) levels in plasma samples. Briefly, 100 μL of negative and positive control were added to the positive and negative control wells without dilution. Ten microliters (10
μL) of plasma samples were added to the wells containing 100 µL of dilution buffer, mixed thoroughly, sealed with cover film, and incubated at 37 °C for 30 min. The plate was washed five
times using 300 µL of 1 × diluted wash buffer. After completing all the assay steps according to the product instructions, the absorbance of the assay solutions at 450 nm was measured using
a plate reader (Synergy H1 Hybrid Reader, BioTek, USA). An OD value of ≥ 0.50 for the positive control and ≤ 0.10 for the negative control sample were obtained, confirming the validity of
the assay, per product instruction. The following cutoff values, as provided in the product manual, were used for results interpretation: < 0.3, negative; ≥ 0.3 to < 0.5, intermediate;
≥ 0.50, positive. STATISTICAL ANALYSIS Statistical differences of test results between different cohorts were analyzed using student t test, two-sample assuming unequal variances. P values
< 0.05 were considered as significant difference. The numbers of asterisks indicate significance levels of P values, for example, the symbols of *, **, ***, and **** represent P values of
≤ 0.05, ≤ 0.01, ≤ 0.001, and ≤ 0.0001, respectively. If there is no significant difference (P > 0.05) between the groups, the results are presented as “ns”, namely, not significant.
Spearman’s rank-order correlation was used to analyze the correlation between the D2Dx immunity test scores of COVID-19 patients in the severe symptom cohort and the days from symptom onset
to blood draw. The strength of the correlation was interpreted according to the scale suggest by Akoglu47: correlation coefficient 1—perfect; 0.7–0.9—strong positive correlation;
0.4–0.6—moderate positive correlation; 0.1–0.3—weak positive correlation; and 0—zero correlation. Both student t test and Spearman’s rank-order correlation was conducted using the data
analysis function in Microsoft Office 2010 Excel software. CHANGE HISTORY * _ 21 DECEMBER 2021 A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-04067-0 _
REFERENCES * Johns Hopkins Coronavirus Resource Center, COVID-19 Global Cases. Johns Hopkins University. Accessed 21 Oct 2021. https://coronavirus.jhu.edu/ (2021). * CDC Coronavirus Disease
2019 (COVID-19). CDC. Accessed 21 Oct 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/index.html (2021). * Guan, W. _et al._ Clinical characteristics of coronavirus disease
2019 in China. _N. Engl. J Med._ 382, 1708–1720 (2020). Article CAS PubMed Google Scholar * Zhou, F. _et al._ Clinical course and risk factors for mortality of adult inpatients with
COVID-19 in Wuhan, China: A retrospective cohort study. _Lancet_ 395, 1054–1062 (2020). Article CAS PubMed PubMed Central Google Scholar * Huang, C. _et al._ Clinical features of
patients infected with 2019 novel coronavirus in Wuhan, China. _Lancet_ 395, 497–506 (2020). Article CAS PubMed PubMed Central Google Scholar * Tay, M. Z., Poh, C. M., Rénia, L.,
MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: Immunity, inflammation and intervention. _Nat. Rev. Immunol._ 20, 363–374 (2020). Article CAS PubMed Google Scholar * Merad, M.
& Martin, J. C. Pathological inflammation in patients with USA COVID-19: A key role for monocytes and macrophages. _Nat. Rev. Immunol._ 20, 355–362 (2020). Article CAS PubMed PubMed
Central Google Scholar * Zhong, J., Tang, J., Ye, C. & Dong, L. The immunology of COVID-19: Is immune modulation an option for treatment?. _Lancet Rheumatol._ 2, 428–436 (2020).
Article Google Scholar * Prompetchara, E., Ketloy, C. & Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. _Asian Pac. J.
Allergy Immunol._ 38, 1–9 (2020). CAS PubMed Google Scholar * Chowdhury, M. A., Hossain, N., Kashem, M. A., Shahid, Md. A. & Alam, A. Immune response in COVID-19: A review. _J.
Infect. Public Health_ 13, 1619–1629 (2020). Article PubMed PubMed Central Google Scholar * Yazdanpanah, F., Hamblin, M. R. & Rezaei, N. The immune system and COVID-19: Friend or
foe?. _Life Sci._ 256, 117900 (2020). Article CAS PubMed PubMed Central Google Scholar * Vabret, N. _et al._ Immunology of COVID-19: Current state of the science. _Immunity_ 52, 910–941
(2020). Article CAS PubMed PubMed Central Google Scholar * Garcia, L. F. Immune response, inflammation, and the clinical spectrum of COVID-19. _Front. Immunol._ 11, 1441 (2020).
Article CAS PubMed PubMed Central Google Scholar * Pierce, C. A. _et al._ Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. _Sci. Transl. Med._ 12,
eabd5487 (2020). Article CAS PubMed PubMed Central Google Scholar * Diao, B. _et al._ Reduction and functional exhaustion of T cells in patients with coronavirus disease 19 (COVID-19).
_Front. Immunol._ 11, 827 (2020). Article CAS PubMed PubMed Central Google Scholar * Odak, I. _et al._ Reappearance of effector T cells is associated with recovery from COVID-19.
_EBioMed._ 57, 102885 (2020). Article Google Scholar * Long, Q. _et al._ Antibody response to SARS-CoV-2 in patients with COVID-19. _Nat. Med._ 26, 845–848 (2020). Article CAS PubMed
Google Scholar * Zhao, J. _et al._ Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. _Clin. Infect. Dis._ 71, 2027–2034 (2020). Article CAS PubMed Google
Scholar * Wang, Z. _et al._ Elevated serum IgM level indicate poor outcome in patients with coronavirus disease pneumonia: A retrospective case-control study. _medRxiv_ 20, 1108 (2020).
Google Scholar * Roncati, L., Nasillo, V., Lusenti, B. & Riva, G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. _Ann. Hematol._ 99, 1419–1420 (2020).
Article CAS PubMed PubMed Central Google Scholar * Roncati, L. & Lusenti, B. The moonlighting protein able to explain the Th1 immune lockdown in severe COVID-19. _Med. Hypotheses_
143, 110087 (2020). Article CAS PubMed PubMed Central Google Scholar * Sekine, T. _et al._ Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. _Cell_
183, 158–168 (2020). Article CAS PubMed PubMed Central Google Scholar * Spellberg, B. & Edwards, J. E. Type 1/type 2 immunity in infectious diseases. _Clin. Infect. Dis._ 32, 76–102
(2001). Article CAS PubMed Google Scholar * Ali, M. G. _et al._ Recent advances in therapeutic applications of neutralizing antibodies for virus infections: An overview. _Immunol. Res._
68, 325–339 (2020). Article CAS PubMed PubMed Central Google Scholar * Garcia-Beltran, W. F. _et al._ COVID-19 neutralizing antibodies predict disease severity and survival. _medRxiv_
584, 353 (2020). Google Scholar * Khoury, D. S. _et al._ Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. _Nat. Med._ 27,
1205–1211 (2021). Article CAS PubMed Google Scholar * Hjelholt, A., Christiansen, G., Sørensen, U. S. & Birkelund, S. IgG subclass profiles in normal human sera of antibodies
specific to five kinds of microbial antigens. _Pathog. Dis._ 67, 206–213 (2013). Article CAS PubMed Google Scholar * Aalberse, R. C., Stapel, S. O., Schuurman, J. & Rispens, T.
Immunoglobulin G4: An odd antibody. _Clin. Exp. Allergy_ 39, 469–477 (2009). Article CAS PubMed Google Scholar * Sasaki, M., Davis, C. L. & Larson, B. L. Production and turnover of
IgG1 and IgG2 immunoglobulins in the bovine around parturition. _J. Dairy Sci._ 59, 2046–2055 (1976). Article CAS PubMed Google Scholar * Rostamian, M., Sohrabi, S., Kavosifard, H. &
Niknam, H. M. Lower levels of IgG1 in comparison with IgG2a are associated with protective immunity against _Leishmania tropica_ infection in BALB/c mice. _J. Microbiol. Immunol. Infect._
50, 160–166 (2017). Article CAS PubMed Google Scholar * Bradford, B. J., Yuan, K., Farney, J. K., Mamedova, L. K. & Carpenter, A. J. Invited review: Inflammation during the
transition to lactation: New adventures with an old flame. _J. Dairy Sci._ 98, 6631–6650 (2015). Article CAS PubMed Google Scholar * Coffman, R. L., Lebman, D. A. & Rothman, P.
Mechanism and regulation of immunoglobulin isotype switching. _Adv. Immunol._ 54, 229–270 (1993). Article CAS PubMed Google Scholar * Kawasaki, Y. _et al._ Evaluation of T helper-1/-2
balance on the basis of IgG subclasses and serum cytokines in children with glomerulonephritis. _Am. J. Kidney Dis._ 44, 42–49 (2004). Article CAS PubMed Google Scholar * Holdsworth, S.
R., Kitching, A. R. & Tipping, P. G. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. _Kidney Int._ 55, 1198–1216 (1999). Article CAS
PubMed Google Scholar * Zheng, T. _et al._ A single-step gold nanoparticle–blood serum interaction assay reveals humoral immunity development and immune status of animals from neonates to
adults. _ACS Infect. Dis._ 5, 228–238 (2019). Article CAS PubMed Google Scholar * Tsai, C. _et al._ A rapid blood test to monitor the immune status change of dairy cows and to evaluate
their disease risk during the periparturient period. _Sens. Int._ 2, 100078 (2021). Article Google Scholar * Zheng, T. _et al._ A rapid blood Test to monitor immunity shift during
pregnancy and potential application for animal health management. _Sens. Int._ 1, 100009 (2020). Article Google Scholar * Zheng, T. _et al._ A rapid blood test to determine the active
status and duration of acute viral infection. _ACS Infect. Dis._ 3, 866–873 (2017). Article CAS PubMed Google Scholar * Zheng, T. & Huo, Q. A nanoparticle pseudo pathogen for rapid
detection and diagnosis of virus Infection. _Sens. Int._ 1, 100010 (2020). Article PubMed Google Scholar * Shen, L. _et al._ Delayed specific IgM antibody responses observed among
COVID-19 patients with severe progression. _Emerg. Microbes Infect._ 9, 1096–1101 (2020). Article CAS PubMed PubMed Central Google Scholar * Liu, X. _et al._ Patterns of IgG and IgM
antibody response in COVID-19 patients. _Emerg. Microbes Infect._ 9, 1269–1274 (2020). Article CAS PubMed PubMed Central Google Scholar * Rosenthal, K. S. & Zimmerman, D. H.
Vaccines: All things considered. _Clin. Vaccine Immunol._ 13, 821–829 (2006). Article CAS PubMed PubMed Central Google Scholar * Swanson, P. A. II. _et al._ T-cell mediated immunity
after AZD1222 vaccination: A polyfunctional spike-specific Th1 response with a diverse TCR repertoire. _medRxiv_ 27, 113 (2021). Google Scholar * Sahin, U. _et al._ BNT162b2 vaccine induces
neutralizing antibodies and poly-specific T cells in humans. _Nature_ 595, 572–577 (2021). Article ADS CAS PubMed Google Scholar * WHO R&D Blueprint novel Coronavirus COVID-19
Therapeutic Trial Synopsis. WHO https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (2020). * Beigel, J. H. _et al._ Remdesivir for the treatment of Covid-19
—Preliminary report. _N. Engl. J. Med._ 383, 992–994 (2020). Article Google Scholar * Akoglu, H. User’s guide to correlation coefficients. _Turk. J. Emerg. Med._ 18, 91–93 (2018). Article
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study is supported by intramural funding from Nano Discovery Inc. and Orlando Health. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Translational Research and Specialty Diagnostic Laboratory, Arnold Palmer Hospital for Children, Orlando Health, 110 Bonnie Loch Court, Orlando, FL, 32806, USA
Chirajyoti Deb, Allan N. Salinas, Bassam Abomoelak & Devendra I. Mehta * Nano Discovery Inc., 1060 Woodcock Road Suite 131, Orlando, FL, 32803, USA Tianyu Zheng * Center for Nursing
Research, Orlando Health, 1414 Kuhl Ave, Orlando, FL, 32806, USA Aurea Middleton & Daleen Penoyer * Center for Digestive Health and Nutrition, Arnold Palmer Hospital for Children,
Orlando Health, 60 Gore St., Orlando, FL, 32806, USA Katelyn Kern, Vijay Mehta, Laura Irastorza & Devendra I. Mehta * Internal Medicine Group, Orlando Health, 1414 Kuhl Ave, Orlando, FL,
32806, USA Rahul Borsadia * Critical Care Medicine, Orlando Health, 1414 Kuhl Ave, Orlando, FL, 32806, USA Charles Hunley * Department of Chemistry and NanoScience Technology Center,
University of Central Florida, 12424 Research Parkway Suite 400, Orlando, FL, 32826, USA Qun Huo Authors * Chirajyoti Deb View author publications You can also search for this author
inPubMed Google Scholar * Allan N. Salinas View author publications You can also search for this author inPubMed Google Scholar * Tianyu Zheng View author publications You can also search
for this author inPubMed Google Scholar * Aurea Middleton View author publications You can also search for this author inPubMed Google Scholar * Katelyn Kern View author publications You can
also search for this author inPubMed Google Scholar * Daleen Penoyer View author publications You can also search for this author inPubMed Google Scholar * Rahul Borsadia View author
publications You can also search for this author inPubMed Google Scholar * Charles Hunley View author publications You can also search for this author inPubMed Google Scholar * Bassam
Abomoelak View author publications You can also search for this author inPubMed Google Scholar * Vijay Mehta View author publications You can also search for this author inPubMed Google
Scholar * Laura Irastorza View author publications You can also search for this author inPubMed Google Scholar * Devendra I. Mehta View author publications You can also search for this
author inPubMed Google Scholar * Qun Huo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.D. conceived and designed the study, contributed
on the design and development of IRB protocol provided supervision and guidance on the sample processing and tests (serology and D2Dx immunity test), conducted tests, data analysis, and
prepared and edited manuscript. D.M. conceived and designed the study, contributed on the design and development of IRB protocol provided supervision and guidance on the sample processing
and tests (serology) and D2Dx immunity test), conducted data analysis, and edited manuscript. A.S. conducted and coordinated blood sample acquisitions, clinical data mining, data entry, data
analysis, experiments on serology and D2Dx immunity tests; managed other experimental processes, and edited manuscript. A.M. helped with patient enrollment, coordinated blood sample
collections, clinical data collections, clinical data mining, etc., reviewed manuscript. K.K. helped with developing the IRB protocol, helped with patient enrollment, blood sample
collection, and clinical data collection, reviewed manuscript. D.P. contributed on the design and development of IRB protocol, supervised patient enrolment, clinical sample, and data
collections, coordinated the clinical samples and data collection, reviewed and edited manuscript. R.B. treated COVID-19 patients, guided patient enrollment, ordered blood sample collection,
heled with clinical data generation, etc. C.H. treated covid-19 patients, guided patient enrollment, ordered blood sample collection, helped with clinical data generation, etc. B.A., helped
with experiments on serology test, managed other experimental processes, and reviewed manuscript. V.M. helped with clinical data collection, clinical data entry, reviewed and edited
manuscript. L.I. helped with clinical data collection, clinical data entry, reviewed and edited manuscript. T.Z. designed and developed the reader device for D2Dx immunity test, prepared the
reagent kits for the test, contributed on the design and development of IRB protocol, conducted data analysis and prepared manuscript. Q.H. conceived and designed the study, provided
supervision and guidance on the D2Dx immunity test, conducted data analysis and prepared manuscript. CORRESPONDING AUTHORS Correspondence to Chirajyoti Deb, Devendra I. Mehta or Qun Huo.
ETHICS DECLARATIONS COMPETING INTERESTS QH is a shareholder and officer in Nano Discovery Inc. No conflict of interest claim from all other authors. ADDITIONAL INFORMATION PUBLISHER'S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. The original online version of this Article was revised: The
original version of this Article contained an error in the key for Figure 3, where the light green colour indicating “Severe” was incorrectly labelled as “D2Dx test score.” RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Deb, C.,
Salinas, A.N., Zheng, T. _et al._ A 1-minute blood test detects decreased immune function and increased clinical risk in COVID-19 patients. _Sci Rep_ 11, 23491 (2021).
https://doi.org/10.1038/s41598-021-02863-2 Download citation * Received: 25 June 2021 * Accepted: 11 November 2021 * Published: 06 December 2021 * DOI:
https://doi.org/10.1038/s41598-021-02863-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative