- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The capacity of pluripotent embryonic stem cells (ES cells) to proliferate and differentiate makes them promising tools in the field of cell therapy. In spite of the controversy
surrounding the numerous ethical questions raised by this technology, it has been shown to have therapeutic potential for heart, lung, liver, bone and connective tissue regeneration. In
addition, a very attractive aspect of this technology is its potential for the treatment of cerebral pathology. A number of studies using ES cell transplants report the differentiation of ES
cells in the brain or spinal cord of rodents, and the improvement of locomotor and/or cognitive deficits caused by brain injury. This review offers a synthesis of recent advances in the
field of both human and rodent stem cell manipulation to select populations of neurons, astrocytes and oligodendrocytes. In parallel, this review emphasizes the striking similarities that
exist between genetically programmed embryonic development of the nervous system and the differentiation of ES cells _in vitro_. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICIENT GENERATION
OF FUNCTIONAL NEURONS FROM MOUSE EMBRYONIC STEM CELLS VIA NEUROGENIN-2 EXPRESSION Article 18 August 2023 ENHANCED PRODUCTION OF MESENCEPHALIC DOPAMINERGIC NEURONS FROM LINEAGE-RESTRICTED
HUMAN UNDIFFERENTIATED STEM CELLS Article Open access 05 December 2023 MULTIPOTENT NEURAL STEM CELLS ORIGINATING FROM NEUROEPITHELIUM EXIST OUTSIDE THE MOUSE CENTRAL NERVOUS SYSTEM Article
Open access 10 April 2025 MAIN Embryonic stem (ES) cells are isolated directly in culture from the inner cell mass (ICM) of pre-implanted embryos (blastocyst) (1,2). They are self-renewing,
pluripotent and capable of contributing to all the tissues of the embryo _in vivo_, and into the majority of cell types _in vitro_, with the exception of extra-embryonic tissue types, at
least in the case of mouse ES cells (3). Interestingly, this differentiation process closely follows a genetic program similar to that turned on during embryonic development. Two principal
methods are used for the differentiation of ES cells into neural cells. The first involves the formation of embryoid bodies, within which cells differentiate into the three germ layers and
provide morphogenetic signals that are present in the embryo (4). The second method consists of culturing ES cells in various media conditions to direct neural differentiation and thereby to
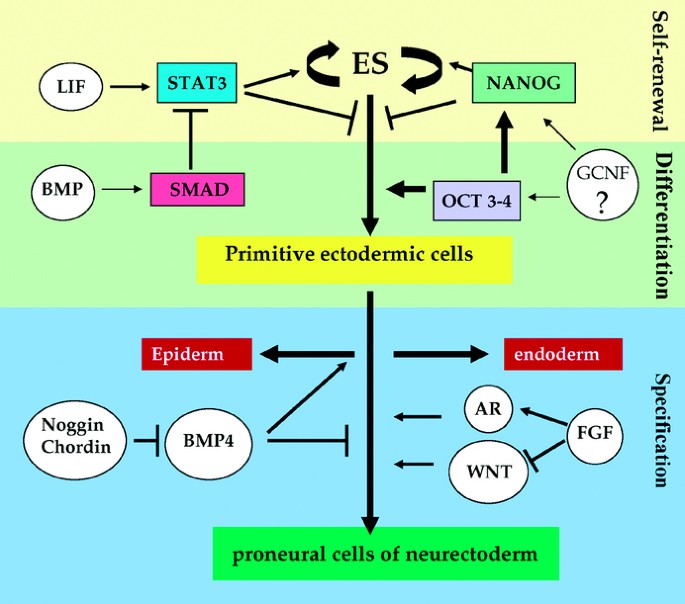
generate quasi-homogenous populations of neurons or glia. SELF-RENEWAL OF ES CELLS ES cells, which represent the very first stem cells of an embryo, are capable of self-renewal, _i.e._ they
can theoretically undergo an infinite number of cell divisions without loss of potential. This process requires the activation of the STAT pathway by either IL-6 or LIF for promoting cell
cycle progression and for the maintenance of the undifferentiated state (Fig. 1) (5,6). _In vitro_, the activation of STAT3 by LIF is sufficient for the self-renewal of mouse ES cells.
Conversely, in the absence of STAT3 activation by LIF, mouse ES cells differentiate spontaneously (5,7,8). However, LIF exhibits no obvious effects on human ES cell proliferation (9),
suggesting that the role of Stat3 signaling in the self-renewal mechanism may differ between mouse and human ES cells. BMP signaling, involving the interaction of BMP (bone morphogenic
proteins) with BMPR1 receptors and the subsequent activation of Smad effectors, also plays a critical role in ES cell self-renewal. BMPR1 is highly expressed in mouse ES cells but
down-regulated when cells differentiate. However BMP action is directly dependent on the presence of LIF in mouse ES cells. In its absence, BMP acts as a strong inducer of mesodermal
differentiation while also inhibiting neuronal specification. These interactions between BMP and LIF pathways may occur downstream at the level of CBP/p300 proteins to regulate gene
expression (10). In addition, two homeodomain transcription factors, NANOG and OCT4, that are highly expressed in the ICM and epiblastic cells of pre-implanted embryos also seem to exert
strict control over the self-renewal _versus_ the initiation of the differentiation process of stem cells (11–13). Permanent inactivation of the oct4 gene prevents the establishment of
pluripotent cell populations, and leads to developmental arrest (13), whereas stable transfection of OCT4 maintains self-renewal and pluripotency of human ES cells (14). However, in culture
conditions that induce neurogenesis, the loss of expression of oct4 in ES cells encourages the formation of the endoderm, whereas its overexpression allows neuronal differentiation even in
the absence of appropriate culture conditions (15). Similarly, the constitutive expression of NANOG in ES cells sustains cell potentials, while Nanog deprivation initiates differentiation
into endodermal cells (16). Recent results indicate that OCT4 and NANOG act in concert on the proliferation and differentiation of ES cells, by means of molecular mechanisms that are most
likely different but whose pathways cross talk (17). DIFFERENTIATION OF ES CELLS INTO PRECURSOR CELLS OF THE NEURECTODERM (SPECIFICATION) Neurectoderm is specified on the dorsal side of the
embryonic ectoderm in part due to the inhibition of signaling pathways induced by proteins of the TGFb-family, such as BMP and nodal, by molecules secreted by the dorsal lip of the
blastopore, also known as the organizer (18). Inhibition of BMP signaling along with the level of WNT and FGF signaling allows the neurectoderm to establish a rostro-caudal identity. As
demonstrated over the last six decades in _Xenopus_, BMP4 inhibition by the factors Noggin, Follistatin and Chordin secreted from the organizer, is sufficient to induce neuralization,
leading to the concept of the “default model.” For instance, neural fate specification can be induced directly from mouse ES cells cultured at low density in the absence of any inducing
supplements, suggesting that a default mechanism may prevail in the mouse to direct ES toward a neural stem cell stage (19). In contrast, expression patterns of BMP and their antagonists do
not fit the default model in chick but also in mouse embryos, supporting the idea that other factors and signaling pathways, including FGFs and WNT may also be required for neural induction
(for review, 18). Subsequently BMPs are also involved in the establishment of dorso-ventral identity of the neural tube (see section III). Due to the absence of a three-dimensional
structure, ES cells in culture depend upon the addition of factors for efficient neural induction. Experiments show that the neural induction pathway in ES cells is similar to that
identified during embryonic development. Undifferentiated human ES cells show high expression levels of nodal and lefty (its own antagonist) and also of SMAD proteins, the downstream
effectors of BMP and Nodal signaling pathways (20). High levels of active SMAD2/3 are correlated with maintenance of human ES cells in a pluripotent state (21). When differentiation of ES
cells is initiated, a decrease in SMAD 2/3 activation and the expression of nodal and lefty is observed (20). In addition, the WNT signaling pathway interacts with the BMP signaling pathway
to regulate the levels of SMAD 2/3 (20). Interestingly in this context, there is a difference between mouse ES cells and human ES cells. While mouse ES cells maintain pluripotency in the
presence of WNT signaling as assessed by the expression of Oct3/4, this effect is not correlated with levels of active SMAD2/3. In contrast, in human ES cells, maintenance of Oct3/4
expression is correlated with active SMAD 2/3 (21). The results discussed in the preceding paragraph thus suggest that the balance between the induction of cell fates and the maintenance of
pluripotency in human ES cells may be due to a conserved reciprocal interaction involving similar signaling pathways. The FGF signaling pathway also intersects with the BMP signaling
pathway, and for human ES cells, the addition of FGF-2 helps to maintain the pluripotency of ES cells (22,23). However, it is the balance between BMP, WNT and FGF signaling pathways that
determines whether addition of exogenous FGF2 leads to maintenance of human ES cells in an undifferentiated state or whether FGF2 helps in expanding a population of FGF-dependent neural
precursor cells. The organizer secretes antagonists of BMP signaling such as Noggin, Chordin and Follistatin, whose loss reduces specification of ectoderm to neurectoderm. The contextual
role of these signaling pathways is highlighted by the fact that ES cells simultaneously produce BMP4 as well as the inhibitors of its own signaling pathway, Noggin and Chordin, at very low
concentrations. Therefore either the absence of exogenous BMP4 (24) or overexpression of Noggin or Chordin leads to an increase in the number of differentiated colonies generated from ES
cells. A selective inhibition of the WNT pathway in ES cells prevents neural differentiation and enhances the regulated expression of the specific inhibitors of neural differentiation
(25–27). In parallel, FGF4, by inducing the expression of neuronal markers, appears sufficient but not absolutely necessary for the differentiation of ES cells in culture. While its presence
assures the proliferation of differentiating cells, its absence induces neural differentiation (6,28,29). Finally, endogenous retinoic acid (RA) is also required for the establishment of
neural specification in embryos, as well as the induction of neural differentiation of embryoid bodies in culture (30). To summarize, these results indicate that ES cells undergo
specification to generate neural progenitors cells according to mechanisms similar to those that occur during embryonic development _in vivo_. In particular, inhibitors of BMP4 induce
neuralization in concert with supporting factors such as WNT, FGF and RA. The action of BMP is context-dependent as suppression of BMP signaling in floating aggregates of human ES cells
leads to induction of neural tissue (31) while in a different context the interaction of these signals gives rise to differentiation of cardiac muscle (32). The signaling pathways
responsible for neural specification are also involved in the acquisition of dorso-ventral identity at a later stage (see next section). PRONEURAL CELLS DERIVED FROM ES CELLS ACQUIRE BOTH
ROSTROCAUDAL AND DORSOVENTRAL IDENTITIES OF STEM CELLS IN THE EMBRYO Retinoic acid has been identified as a caudalizing factor that, along with FGF and WNT, is important for the neuronal
fate of ES cells during the specification of the rostrocaudal axis of the embryonic brain (33,34). Further, in ES cells, the addition of RA along with aggregation of cells leads to neural
differentiation in a dose-dependent manner (35). When mouse ES cells are specified to become neural progenitor cells, the phenotype obtained is consistent with RA acting as a caudalizing
molecule. Thus the absence of RA results in the appearance of neuronal progenitor cells which express the anterior forebrain markers emx1/2 and nkx2.1, as well as Bf1, a telecenphalic marker
(36), while low doses of RA result in the generation of cells that express markers for midbrain neurons (37). High RA concentrations result in a caudal phenotype with expression of
posterior markers such as Hoxc5/6 instead of anterior markers (_i.e._ Otx2 or En1), and in concert with sonic hedgehog (SHH) signaling, lead to the differentiation of motor neurons (38). At
the level of the anterior neural plate, Wnt signaling exclusion is required for the acquisition of telencephalic characteristics, whereas its activation is required for caudal specification
(39). The inhibition of the FGF signaling pathway suppresses the expression of the RA receptor, and the overexpression of RARa, the RA receptor, restores the effects of FGF, demonstrating
the interaction between the RA and FGF pathways in specifying the rostrocaudal neural axis, and suggesting that RARa is a direct target for the FGF signaling pathway (40). BMP4
DIFFERENTIATES ES-DERIVED PRONEURAL CELLS INTO NEURONAL PRECURSORS AND NEURAL CREST CELLS (DORSALIZATION) In the embryo, BMP4 is secreted by the roof plate of the neural tube and forms a
dorsal gradient. Depending upon the position of neural precursors along the rostrocaudal axis, the local concentration of BMP-4 specifies the neural phenotype outcomes. Thus in culture
conditions (low RA concentrations) that anteriorize the neural precursors, the absence of SHH as a ventralizing factor induces the generation of pallial telencephalic (Pax6+) neurons (36).
Furthermore, in serum-free culture conditions compatible with telecenphalic specification (no RA addition and repression of WNT and nodal), Pax6-+ cells can be further differentiated into
neural retinal precursors (41). Neural progenitor cells derived from ES cells grown in defined media when exposed to BMP4 will also progressively acquire the characteristics of dorsal
neurons and neural crest cells (35). Thus the presence of BMP4 increases the number of cells expressing markers of the neural crest (including snail, slug and Msx1) while reducing the
expression of ventral markers (_i.e._ nkx2.2 and HNF3β). In addition, depending upon the dose used, BMP4 induces the differentiation of neural crest precursors into sensory neurons
(Brn3a/Peri), or autonomic neurons (TH/Peri). The above results suggest that the role of BMP4 as a dorsalizing factor in the embryo is also observed in ES cells that have been specified to
undergo a neural fate. (Fig. 2). SHH INDUCES VENTRAL DIFFERENTIATION IN PRONEURAL CELLS DERIVED FROM ES CELLS TO GENERATE MOTONEURONS. In the mouse embryo, SHH is initially produced by the
notochord, and then by the floor plate cells of the neural tube. This peptide diffuses along a concentration gradient in the ventral portion of the neural tube to allow the local
differentiation of ventral precursors (18); the loss of SHH or the interruption of its signaling pathway results in the dorsalization of the embryo by the expansion of regions under the
control of BMP4, leading to a pathology known in humans as holoprosencephaly (42), whereas constitutive expression of SHH triggers a lethal overall outgrowth of embryonic neural tube and
suppresses the differentiation of dorsal regions (43). Gene mutations leading to partial gain of hedgehog functions result in the formation of multiple cancers including medulloblastomas and
skin nevoid basal cell carcinomas, also known as Gorlin's syndrome (44). Several studies have shown that embryoid bodies derived from murine ES cells express two of the three members
of the hedgehog family, Indian hedgehog (ihh) as well as Sonic (shh; unpublished data), at the level of the outer visceral endoderm, and direct effectors of its signaling cascade (_i.e._
patched and gli1) in the inner endoderm (45). A recent study has shown that along with its role in dorsoventral patterning, SHH is also required for the specification of neurectoderm as well
as the responsiveness of ES cells to other neural inducers such as RA (46). Thus, in the presence of increasing concentrations of SHH, differentiating mouse ES cells expressing markers for
the neural crest and for dorsal neurons (Pax7 and Math1) are reduced or disappear, while cells expressing ventral markers (nkx2.2 and HNF3β) are augmented (35) (Fig. 2). Mouse embryoid
bodies cultured in serum-free medium supplemented with SHH and FGF-8, develop high yields of TuJ1-positive neuroblasts that express either dopaminergic or serotoninergic markers (46) and may
represent _in vitro_ models of ventral midbrain-hindbrain neurons. In mouse ES cells that have been have been cultured on stromal cells to induce neural differentiation, the presence of
higher concentrations of SHH results in an increase in the differentiation of neuronal cells expressing ventral markers (nkx2.2 and HNF3β) along with a decrease in the markers for neural
crest and for dorsal neurons (Pax7 and Math1). At a later time during differentiation other markers that identify more specialized populations of cells, such as basal telencephalic
motoneurons or brachiomotor and visceral neurons, appear (35,36) (Fig. 2). Similar motor neuron phenotypes were also obtained with human ES cells that were “caudalized” by exposure to RA and
“ventralized” in the presence of SHH (46). Finally, SHH signaling, along with FGF8 signaling, leads to the specification and differentiation of dopaminergic neurons. Differentiation of
neural progenitors in both mouse and human ES cells into dopaminergic neurons is correlated with the expression of genes such as Nurr1, Lmx1b and Ptx3 that are involved in the patterning and
differentiation of dopaminergic neurons during embryonic development (47). Overexpression of the nuclear receptor Nurr1 has been shown to potentiate the effects of SHH and FGF8 on neuronal
differentiation of mouse ES cells (48). Mouse ES cell lines expressing Nurr1 develop functional characteristics of dopaminergic (DA) neurons after transplantation into the brain of rat
models of Parkinson's disease (49). Similar dopaminergic differentiation has been achieved using human ES cells (50); unfortunately, however, these DA-derived human ES cells do not seem
to survive and /or retain their dopaminergic phenotype when grafted into rat brain (51). DIFFERENTIATION OF GLIA (ASTROCYTES AND OLIGODENDROCYTES In contrast to the strong interest in
neuron generation, _in vitro_ differentiation of ES cells into glia has received very limited attention. During development, gliogenesis occurs at a later time than neurogenesis in the
ventral and dorsal zones of the neural tube. This timing is partially controlled by SHH and involves the expression of the transcription factors olig2 and nkx2.2 (52). Olig2 is alternatively
expressed in ventral oligodendrocytic (OL) progenitors or suppressed in neurons and astrocytes. OL fate is reduced or delayed in nkx2.2 or olig2−/−mice, whereas progenitor cells expressing
olig2 successfully myelinize axons in culture and _in vivo_ in the injured spinal cord (29,53). ES cells can differentially generate glial cell populations including astrocytes,
oligodendrocytes and microglia, according to a sequential process whose dynamics resemble that of the mechanisms involved _in utero_ (54,55). Thus, olig2-overexpressing ES cells selectively
generate oligodendrocytes and motor neurons (56) and FGF2 and SHH act synergistically to induce OL progenitor formation from embryoid bodies (46) (Fig. 2). The generation of highly-purified
olidendrocyte progenitors from mouse ES cells has been recently reported using culture conditions that combined FGF-2, PDGF and T3 supplements (57). Neural differentiation of mouse and human
ES cells always give rise to a fraction of “contaminating” GFAP-positive cells among the desired populations of neuron or oligodendrocyte progenitors, suggesting that these ES-derived
neural stem cell progenitors share the potential to generate both neuronal and glial lineages. However, the differentiation of astrocytes also appears to be under the influence of BMP4 at
the level of the dorsal neural tube, suggesting that at least a fraction of astrocytic populations may have an origin distinct from that of ventral OL progenitors. Despite the lack of
interest in cell therapy involving ES-derived astrocytes, replenishment may soon become useful as a therapeutic approach in the dramatic case of Alexander's disease, a fatal neurologic
illness characterized by white-matter degeneration and the formation of astrocytic cytoplasmic inclusions called Rosenthal fibers, due to mutations in the gene encoding GFAP proteins (58).
Finally, it is also worth noting here that neural stem cells found in both mouse and human adult brains are characterized by the expression of GFAP, suggesting that they may share common
properties with astrocytes (59,60). CONCLUSION The results discussed above, taken as a whole, demonstrate that the neural differentiation of ES cells in culture progresses according to a
genetic program similar to that observed in the embryo. In the absence of a three-dimensional structure, ES cells are _a priori_ capable of giving rise not only to all populations of neurons
(motor, sensory and associative) produced _in vivo_, but also to cells in the oligo-astroglial lineage. The culture of ES cells in the presence of extracellular morphogenetic signals
originally identified in embryos as inducers of the positional patterning of specialized cell populations can generate similar cell subtypes, according to a program comparable in certain
respects to that occurring in the embryo. In this context, the use of naïve or pre-differentiated ES cells appears to be a definite advance in the treatment of animal models of human
neurodegenerative disorders. Using this approach a number of recent studies involving the transplantation of stem cells into the brain of mice exhibiting neuronal pathologies similar to
human disorders have met with success (49). However, in the case of leukoencephalopathies, the goal is to generate oligodendrocytes or Schwann cells from ES cells, to myelinate or
remyelinate CNS axons on transplantation. While mouse ES cells exhibit this capacity, oligodendrocyte differentiation from human ES cells remains to be optimized before it can be potentially
tested in human pathologies (such as Pelizaeus-Merzbacher disease) (61). One technical limitation that is being rapidly overcome is that most of the human ES cell lines currently used in
_in vitro_ studies are contaminated with bovine or murine determinants that are the result of culture conditions, precluding their use in cell therapy. Therefore, new culture protocols based
on the use of human feeder cells or artificial three dimensional substrates have recently shown potential to pave the way for human ES cells in cellular therapy (62). ABBREVIATIONS * BMP:
bone morphogenic proteins * ES: embryonic stem cells REFERENCES * Evans MJ, Kaufman MH 1981 Establishment in culture of pluripotential cells from mouse embryos. _Nature_ 292: 154–156 Article
CAS PubMed Google Scholar * Martin GR 1981 Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. _Proc Natl Acad
Sci USA_ 78: 7634–7638 Article CAS PubMed PubMed Central Google Scholar * Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N 2000
Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. _Mol Med_ 6: 88–95 Article CAS PubMed PubMed Central Google Scholar *
Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S 1997 Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. _Proc Natl Acad Sci
USA_ 94: 3801–3804 Article CAS PubMed PubMed Central Google Scholar * Li M, Sendtner M, Smith A 1995 Essential function of LIF receptor in motor neurons. _Nature_ 378: 724–727 Article
CAS PubMed Google Scholar * O'Shea KS 2004 Self-renewal vs. differentiation of mouse embryonic stem cells. _Biol Reprod_ 71: 1735–1765 Google Scholar * Nichols J 2001 Introducing
embryonic stem cells. _Curr Biol_ 11: R503–R505 Article CAS PubMed Google Scholar * Humphrey RK, Beattie GM, Lopez AD, Bucay N, King CC, Firpo MT, Rose-John S, Hayek A 2004 Maintenance
of pluripotency in human embryonic stem cells is STAT3 independent. 2522–2530 * Nakashima H, Otsuka T, Ohba Y, Akahoshi M, Nagano S, Ogami E, Arinobu Y, Miyake K, Inoue Y, Niiro H, Kaji Y,
Niho Y 1999 Two polymorphisms within interleukin-3 (hIL3) gene detected by mismatch PCR/RFLP. _Genes Immun_ 1: 156–168 Article CAS PubMed Google Scholar * Rao M 2004 Conserved and
divergent paths that regulate self-renewal in mouse and human embryonic stem cells. _Dev Biol_ 275: 269–286 Article CAS PubMed Google Scholar * Niwa H 2001 Molecular mechanism to
maintain stem cell renewal of ES cells. _Cell Struct Funct_ 26: 137–148 Article CAS PubMed Google Scholar * Nichols J, Smith A, Buehr M 1998 Rat and mouse epiblasts differ in their
capacity to generate extraembryonic endoderm. _Reprod Fertil Dev_ 10: 517–525 Article CAS PubMed Google Scholar * Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers
I, Schöler H, Smith A 1998 Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. _Cell_ 95: 379–391 Article CAS PubMed Google Scholar
* Gerrard L, Zhao D, Clark AJ, Cui W 2005 Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency.
_Stem Cells_ 23: 124–133 Article CAS PubMed Google Scholar * Shimozaki K, Nakashima K, Niwa H, Taga T 2003 Involvement of Oct3/4 in the enhancement of neuronal differentiation of ES
cells in neurogenesis-inducing cultures. _Development_ 130: 2505–2512 Article CAS PubMed Google Scholar * Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M,
Maeda M, Yamanaka S 2003 The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. _Cell_ 113: 631–642 Article CAS PubMed Google Scholar * Kuroda
T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T 2005 Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. _Mol Cell
Biol_ 25: 2475–2485 Article CAS PubMed PubMed Central Google Scholar * Stern CD 2005 Neural induction: old problem, new findings, yet more questions. _Development_ 132: 2007–2021
Article CAS PubMed Google Scholar * Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D 2001 Direct neural fate specification from embryonic stem cells: a primitive
mammalian neural stem cell stage acquired through a default mechanism. _Neuron_ 30: 65–78 Article CAS PubMed Google Scholar * Besser D 2004 Expression of nodal, lefty-a, and lefty-B in
undifferentiated human embryonic stem cells requires activation of Smad2/3. _J Biol Chem_ 279: 45076–45084 Article CAS PubMed Google Scholar * James D, Levine AJ, Besser D,
Hemmati-Brivanlou A 2005 TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. _Development_ 132: 1273–1282 Article CAS PubMed
Google Scholar * Vallier L, Alexander M, Pedersen RA 2005 Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. _J Cell Sci_ 118: 4495–4509
Article CAS PubMed Google Scholar * Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA 2005 Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human.
_ES Cells_ 2: 185–190 CAS Google Scholar * Wilson SI, Edlund T 2001 Neural induction: toward a unifying mechanism. _Nat Neurosci_ 4: 1161–1168 Article CAS PubMed Google Scholar * Varga
AC, Wrana JL 2005 The disparate role of BMP in stem cell biology. _Oncogene_ 24: 5713–5721 Article CAS PubMed Google Scholar * Aubert J, Dunstan H, Chambers I, Smith A 2002 Functional
gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. _Nat Biotechnol_ 20: 1240–1245 Article CAS PubMed Google Scholar * Haegele L, Ingold B,
Naumann H, Tabatabai G, Ledermann B, Brandner S 2003 Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. _Mol Cell
Neurosci_ 24: 696–708 Article CAS PubMed Google Scholar * Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R 2002 Apc
modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. _Nat Genet_ 32: 594–605 Article CAS PubMed Google Scholar * Brustle O, Jones KN,
Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD 1999 Embryonic stem cell-derived glial precursors: a source of myelinating transplants. _Science_ 285: 754–756 Article
CAS PubMed Google Scholar * Munoz-Sanjuan I, Brivanlou AH 2002 Neural induction, the default model and embryonic stem cells. _Nat Rev Neurosci_ 3: 271–280 Article CAS PubMed Google
Scholar * Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, Segal M, Reubinoff BE 2005 Derivation of neural precursors from human embryonic stem cells in the presence of
noggin. _Mol Cell Neurosci_ 30: 24–36 Article CAS PubMed Google Scholar * Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S,
Okano H, Fukuda K 2005 Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. _Nat Biotechnol_ 23: 607–611 Article CAS PubMed
Google Scholar * Czyz J, Wobus A 2001 Embryonic stem cell differentiation: the role of extracellular factors. _Differentiation_ 68: 167–174 Article CAS PubMed Google Scholar * Lang
KJ, Rathjen J, Vassilieva S, Rathjen PD 2004 Differentiation of embryonic stem cells to a neural fate: a route to re-building the nervous system?. _J Neurosci Res_ 76: 184–192 Article CAS
PubMed Google Scholar * Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, Murakami F, Sasai Y 2003 Generation of neural
crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. _Proc Natl Acad Sci USA_ 100: 5828–5833 Article CAS PubMed PubMed Central Google
Scholar * Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y 2005 Directed differentiation of telencephalic precursors from embryonic stem
cells. _Nat Neurosci_ 8: 288–296 Article CAS PubMed Google Scholar * Okada Y, Shimazaki T, Sobue G, Okano H 2004 Retinoic-acid-concentration-dependent acquisition of neural cell identity
during in vitro differentiation of mouse embryonic stem cells. _Dev Biol_ 275: 124–142 Article CAS PubMed Google Scholar * Wichterle H, Lieberam I, Porter JA, Jessell TM 2002 Directed
differentiation of embryonic stem cells into motor neurons. _Cell_ 110: 385–397 Article CAS PubMed Google Scholar * Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jesssell TM, Edlund T 2003
Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. _Nat Neurosci_ 6: 701–707 Article CAS PubMed Google Scholar * Shiotsugu J, Katsuyama Y, Arima K,
Baxter A, Koide T, Song J, Chandraratna RA, Blumberg B 2004 Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. _Development_ 131:
2653–2667 Article CAS PubMed Google Scholar * Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y 2005
Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. _Proc Natl Acad Sci USA_ 102: 11331–11336 Article CAS PubMed PubMed Central Google Scholar * Roessler E,
Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M 1996 Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. _Nat Genet_ 14: 357–360 Article CAS PubMed
Google Scholar * Ruiz i Altaba A, Stecca B, Sanchez P 2004 Hedgehog-Gli signaling in brain tumors: stem cells and paradevelopmental programs in cancer. _Cancer Lett_ 204: 145–157 Article
CAS PubMed Google Scholar * Unden AB, Holmberg E, Lundh-Rozell B, Stahle-Backdahl M, Zaphiropoulos PG, Toftgard R, Vorechovsky I 1996 Mutations in the human homologue of Drosophila
patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: different in vivo mechanisms of PTCH inactivation. _Cancer Res_ 56: 4562–4565 CAS PubMed Google Scholar * Maye P, Becker
S, Kasameyer E, Byrd N, Grabel L 2000 Indian hedgehog signaling in extraembryonic endoderm and ectoderm differentiation in ES embryoid bodies. _Mech Dev_ 94: 117–132 Article CAS PubMed
Google Scholar * Maye P, Becker S, Siemen H, Thorne J, Byrd N, Carpentino J, Grabel L 2004 Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. _Dev Biol_
265: 276–290 Article CAS PubMed Google Scholar * Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF,
Moore MA, Studer L 2003 Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. _Nat Biotechnol_ 21: 1200–1207 Article
CAS PubMed Google Scholar * Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Kang UJ, Isacson O, Kim KS 2002 Genetic engineering of mouse embryonic stem cells by Nurr1
enhances differentiation and maturation into dopaminergic neurons. _Eur J Neurosci_ 16: 1829–1838 Article PubMed PubMed Central Google Scholar * Kim JH, Auerbach JM, Rodriguez-Gomez JA,
Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R 2002 Dopamine neurons derived from embryonic stem cells function in an animal model of
Parkinson's disease. _Nature_ 418: 50–56 Article CAS PubMed Google Scholar * Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS,
Freed WJ 2004 Dopaminergic differentiation of human embryonic stem cells. _Stem Cells_ 22: 925–940 Article CAS PubMed Google Scholar * Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim
JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH 2005 In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. _J Neurochem_
92: 1265–1276 Article CAS PubMed Google Scholar * Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND 2003 FGF-dependent generation of oligodendrocytes by a
hedgehog-independent pathway. _Development_ 130: 6599–6609 Article CAS PubMed Google Scholar * Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW 2000 Embryonic
stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. _Proc Natl Acad Sci USA_ 97: 6126–6131 Article CAS PubMed PubMed Central
Google Scholar * Angelov DN, Arnhold S, Andressen C, Grabsch H, Puschmann M, Hescheler J, Addicks K 1998 Temporospatial relationships between macroglia and microglia during in vitro
differentiation of murine stem cells. _Dev Neurosci_ 20: 42–51 Article CAS PubMed Google Scholar * Billon N, Jolicoeur C, Ying QL, Smith A, Raff M 2002 Normal timing of oligodendrocyte
development from genetically engineered, lineage-selectable mouse ES cells. _J Cell Sci_ 115: 3657–3665 Article CAS PubMed Google Scholar * Xian H, Gottlieb DI 2004 Dividing
Olig2-expressing progenitor cells derived from ES cells. _Glia_ 47: 88–101 Article PubMed Google Scholar * Glaser T, Perez-Bouza A, Klein K, Brustle O 2005 Generation of purified
oligodendrocyte progenitors from embryonic stem cells. _FASEB J_ 19: 112–114 Article CAS PubMed Google Scholar * Johnson AB, Brenner M 2003 Alexander's disease: clinical,
pathologic, and genetic features. _J Child Neurol_ 18: 625–632 Article PubMed Google Scholar * Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A 1999 Subventricular zone
astrocytes are neural stem cells in the adult mammalian brain. _Cell_ 97: 703–716 Article CAS PubMed Google Scholar * Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O,
Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A 2005 Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche
of neural stem cells. _J Comp Neurol_ 494: 415–434 Article Google Scholar * Duncan ID 2005 Oligodendrocytes and stem cell transplantation: their potential in the treatment of
leukoencephalopathies. _J Inherit Metab Dis_ 28: 357–368 Article CAS PubMed Google Scholar * Draper JS, Moore HD, Ruban LN, Gokhale PJ, Andrews PW 2004 Culture and characterization of
human embryonic stem cells. _Stem Cells Dev_ 13: 325–336 Article CAS PubMed Google Scholar * Gu P, LeMenuet D, Chung AC, Mancini M, Wheeler DA, Cooney AJ 2005 Orphan nuclear receptor
GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. _Mol Cell Biol_ 25: 8507–8519 Article CAS PubMed PubMed Central
Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Inserm U676, U676, Paris, F-75019, France Michele Cazillis, Sowmyalakshmi Rasika, Pierre Gressens &
Vincent Leliévre * Université Paris 7, Faculté de Médecine Denis Diderot, Paris, 75251, France Michele Cazillis, Sowmyalakshmi Rasika, Pierre Gressens & Vincent Leliévre * National Brain
Research Centre, Haryana, 122050, India Shyamala Mani * Service de Neurologie Pédiatrique, AP HP Hôpital Robert Debré, Paris, F-75019, France Pierre Gressens Authors * Michele Cazillis View
author publications You can also search for this author inPubMed Google Scholar * Sowmyalakshmi Rasika View author publications You can also search for this author inPubMed Google Scholar *
Shyamala Mani View author publications You can also search for this author inPubMed Google Scholar * Pierre Gressens View author publications You can also search for this author inPubMed
Google Scholar * Vincent Leliévre View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Pierre Gressens. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cazillis, M., Rasika, S., Mani, S. _et al._ _In Vitro_ Induction of Neural Differentiation of Embryonic Stem (ES)
Cells Closely Mimics Molecular Mechanisms of Embryonic Brain Development. _Pediatr Res_ 59 (Suppl 4), 48–53 (2006). https://doi.org/10.1203/01.pdr.0000203566.01600.8c Download citation *
Received: 28 November 2005 * Accepted: 08 December 2005 * Issue Date: 01 April 2006 * DOI: https://doi.org/10.1203/01.pdr.0000203566.01600.8c SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative