- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
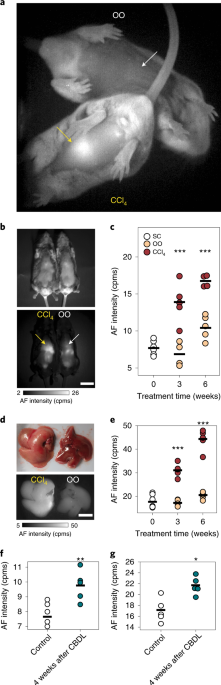
ABSTRACT Monitoring the progression of non-alcoholic fatty liver disease is hindered by a lack of suitable non-invasive imaging methods. Here, we show that the endogenous pigment lipofuscin
displays strong near-infrared and shortwave-infrared fluorescence when excited at 808 nm, enabling label-free imaging of liver injury in mice and the discrimination of pathological processes
from normal liver processes with high specificity and sensitivity. We also show that the near-infrared and shortwave-infrared fluorescence of lipofuscin can be used to monitor the
progression and regression of liver necroinflammation and fibrosis in mouse models of non-alcoholic fatty liver disease and advanced fibrosis, as well as to detect non-alcoholic
steatohepatitis and cirrhosis in biopsied samples of human liver tissue. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A NANOPARTICLE PROBE FOR THE IMAGING OF AUTOPHAGIC FLUX IN LIVE MICE VIA
MAGNETIC RESONANCE AND NEAR-INFRARED FLUORESCENCE Article 11 July 2022 LONG-TERM MONITORING OF INTRAVITAL BIOLOGICAL PROCESSES USING FLUORESCENT PROTEIN-ASSISTED NIR-II IMAGING Article Open
access 04 November 2022 NEAR-INFRARED II FLUORESCENCE IMAGING Article 04 April 2024 DATA AVAILABILITY The main data supporting the results in this study are available within the paper and
its Supplementary Information. The raw and analysed datasets generated during the study are too big to be publicly shared but are available for research purposes from the corresponding
authors on reasonable request. CHANGE HISTORY * _ 12 FEBRUARY 2021 A Correction to this paper has been published: https://doi.org/10.1038/s41551-020-00664-y _ REFERENCES * Younossi, Z. M. et
al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. _Hepatology_ 64, 73–84 (2016). Article PubMed Google Scholar
* Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. _Nat. Rev. Gastroenterol. Hepatol._ 15, 11–20 (2018). Article PubMed Google Scholar
* Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. _N. Engl. J. Med._ 381, 2440–2450 (2019). Article PubMed Google Scholar * Satapathy, S.
K. & Sanyal, A. J. Epidemiology and natural history of nonalcoholic fatty liver disease. _Semin. Liver Dis._ 35, 221–235 (2015). Article PubMed Google Scholar * Brunt, E. M. et al.
Nonalcoholic fatty liver disease. _Nat. Rev. Dis. Primers_ 1, 15080 (2015). * Kleiner, D. E. On beyond staging and grading: liver biopsy evaluation in a posttreatment world. _Hepatology_ 65,
1432–1434 (2017). Article PubMed Google Scholar * Rockey, D. C., Caldwell, S. H., Goodman, Z. D., Nelson, R. C. & Smith, A. D. Liver biopsy. _Hepatology_ 49, 1017–1044 (2009).
Article PubMed Google Scholar * Dietrich, C. F. et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography. _Ultraschall Med._ 38, e16–e47 (2017).
PubMed Google Scholar * Masarone, M. et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. _Oxid. Med. Cell. Longev._ 2018, 9547613 (2018). Article
PubMed PubMed Central CAS Google Scholar * Koyama, Y. & Brenner, D. A. Liver inflammation and fibrosis. _J. Clin. Invest_. 127, 55–64 (2017). * Marcellin, P. & Kutala, B. K.
Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. _Liver Int._ 38(Suppl. 1), 2–6 (2018). Article PubMed Google Scholar *
Vishwanath, K. & Ramanujam, N. in _Encyclopedia of Analytical Chemistry_ (ed. Meyers, R.A.) 20–56 (John Wiley & Sons, 2011). * Croce, A. C., Ferrigno, A., Bottiroli, G. &
Vairetti, M. Autofluorescence-based optical biopsy: an effective diagnostic tool in hepatology. _Liver Int._ 38, 1160–1174 (2018). Article CAS PubMed Google Scholar * Monici, M. Cell and
tissue autofluorescence research and diagnostic applications. _Biotechnol. Annu. Rev._ 11, 227–256 (2005). Article CAS PubMed Google Scholar * Frangioni, J. V. In vivo near-infrared
fluorescence imaging. _Curr. Opin. Chem. Biol._ 7, 626–634 (2003). Article CAS PubMed Google Scholar * Ntziachristos, V., Ripoll, J. & Weissleder, R. Would near-infrared fluorescence
signals propagate through large human organs for clinical studies? _Opt. Lett._ 27, 333–335 (2002). Article PubMed Google Scholar * Lim, Y. T. et al. Selection of quantum dot wavelengths
for biomedical assays and imaging. _Mol. Imaging_ 2, 50–64 (2003). Article CAS PubMed Google Scholar * Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically
approved near-infrared dye indocyanine green. _Proc. Natl Acad. Sci. USA_ 115, 4465–4470 (2018). Article CAS PubMed PubMed Central Google Scholar * Bruns, O. T. et al. Next-generation
in vivo optical imaging with short-wave infrared quantum dots. _Nat. Biomed. Eng._ 1, 0056 (2017). Article CAS PubMed PubMed Central Google Scholar * Iwaisako, K. et al. Origin of
myofibroblasts in the fibrotic liver in mice. _Proc. Natl Acad. Sci. USA_ 111, E3297–E3305 (2014). Article CAS PubMed PubMed Central Google Scholar * Delire, B., Stärkel, P. &
Leclercq, I. Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. _J. Clin. Transl. Hepatol._ 3, 53–66 (2015). Article PubMed PubMed
Central Google Scholar * Scholten, D., Trebicka, J., Liedtke, C. & Weiskirchen, R. The carbon tetrachloride model in mice. _Lab. Anim._ 49, 4–11 (2015). Article CAS PubMed Google
Scholar * Geerts, A. M. et al. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. _Int. J. Exp. Pathol._ 89,
251–263 (2008). Article CAS PubMed PubMed Central Google Scholar * Tag, C. et al. Induction of experimental obstructive cholestasis in mice. _Lab. Anim._ 49, 70–80 (2015). Article CAS
PubMed Google Scholar * Majno, G. & Joris, I. in _Cells, Tissues, and Disease: Principles of General Pathology_ (eds Majno, G. & Joris, I.) 74–128 (Oxford University Press,
2004). * Terman, A. & Brunk, U. T. Lipofuscin. _Int. J. Biochem. Cell Biol._ 36, 1400–1404 (2004). Article CAS PubMed Google Scholar * Rantakari, P. et al. Stabilin-1 expression
defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. _Proc. Natl Acad. Sci. USA_ 113, 9298–9303 (2016). Article CAS PubMed PubMed
Central Google Scholar * Barden, H. The intragranular location of carboxyl groups in neuromelanin and lipofuscin in human brain and in meningeal melanosomes in mouse brain. _J. Histochem.
Cytochem._ 34, 1271–1279 (1986). Article CAS PubMed Google Scholar * Lillie, R. D. A Nile blue staining technic for the differentiation of melanin and lipofuscins. _Stain Technol._ 31,
151–153 (1956). Article CAS PubMed Google Scholar * Evangelou, K. & Gorgoulis, V. G. in _Oncogene-Induced Senescence: Methods and Protocols, Methods in Molecular Biology_ Vol. 1534
(ed. Nikiforov, M.) 111–119 (Humana Press, 2017). * Everson Pearse, A. G. in _Histochemistry Theoretical and Applied_ Vol. 2, 898–928 (Churchill Livingstone, 1985). * Terman, A., Kurz, T.,
Navratil, M., Arriaga, E. A. & Brunk, U. T. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial–lysosomal axis theory of aging. _Antioxid. Redox Signal._
12, 503–535 (2010). Article CAS PubMed PubMed Central Google Scholar * Seehafer, S. S. & Pearce, D. A. You say lipofuscin, we say ceroid: defining autofluorescent storage material.
_Neurobiol. Aging_ 27, 576–588 (2006). Article CAS PubMed Google Scholar * Schnell, S. A., Staines, W. A. & Wessendorf, M. W. Reduction of lipofuscin-like autofluorescence in
fluorescently labeled tissue. _J. Histochem. Cytochem._ 47, 719–730 (1999). Article CAS PubMed Google Scholar * Erben, T., Ossig, R., Naim, H. Y. & Schnekenburger, J. What to do with
high autofluorescence background in pancreatic tissues—an efficient Sudan black B quenching method for specific immunofluorescence labelling. _Histopathology_ 69, 406–422 (2016). Article
PubMed Google Scholar * Nazeer, S. S., Saraswathy, A., Shenoy, S. J. & Jayasree, R. S. Fluorescence spectroscopy as an efficient tool for staging the degree of liver fibrosis: an in
vivo comparison with MRI. _Sci. Rep._ 8, 10967 (2018). Article PubMed PubMed Central CAS Google Scholar * Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during
regression of liver fibrosis. _Proc. Natl Acad. Sci. USA_ 109, 9448–9453 (2012). Article CAS PubMed PubMed Central Google Scholar * Duffield, J. S. et al. Selective depletion of
macrophages reveals distinct, opposing roles during liver injury and repair. _J. Clin. Invest._ 115, 56–65 (2005). Article CAS PubMed PubMed Central Google Scholar * Liu, C. et al.
Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. _Lab. Invest._ 90, 1805–1816 (2010). Article CAS PubMed Google Scholar *
Beljaars, L. et al. Hepatic localization of macrophage phenotypes during fibrogenesis and resolution of fibrosis in mice and humans. _Front. Immunol._ 5, 430 (2014). Article PubMed PubMed
Central CAS Google Scholar * Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. _Int. J. Exp. Pathol._ 94, 93–103 (2013).
Article CAS PubMed PubMed Central Google Scholar * Giannessi, F., Giambelluca, M. A., Scavuzzo, M. C. & Ruffoli, R. Ultrastructure of testicular macrophages in aging mice. _J.
Morphol._ 263, 39–46 (2005). Article PubMed Google Scholar * Jara, M., Carballada, R. & Esponda, P. Age-induced apoptosis in the male genital tract of the mouse. _Reproduction_ 127,
359–366 (2004). Article CAS PubMed Google Scholar * Brunk, U. T. & Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. _Free Radic. Biol.
Med._ 33, 611–619 (2002). Article CAS PubMed Google Scholar * Softic, S. et al. Lipodystrophy due to adipose tissue-specific insulin receptor knockout results in progressive NAFLD.
_Diabetes_ 65, 2187–2200 (2016). Article CAS PubMed PubMed Central Google Scholar * Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD
development and therapeutic strategies. _Nat. Med._ 24, 908–922 (2018). Article CAS PubMed PubMed Central Google Scholar * Alonso, C. et al. Metabolomic identification of subtypes of
nonalcoholic steatohepatitis. _Gastroenterology_ 152, 1449–1461 (2017). Article PubMed Google Scholar * Brunt, E. M., Janney, C. G., Bisceglie, A. M. Di, Neuschwander-Tetri, B. A. &
Bacon, B. R. Nonalcoholic steatohepatitis—a proposal for grading and staging the histological lesions. _Am. J. Gastroenterol._ 94, 2467–2474 (1999). Article CAS PubMed Google Scholar *
Orchard, G.E., in _Bancroft’s Theory and Practice of Histological Techniques_ (eds Suvarna, S. K., Layton, C. et al.) 239–270 (Elsevier, 2013). * Schmittgen, T. D. & Livak, K. J.
Analyzing real-time PCR data by the comparative _C_T method. _Nat. Protoc._ 3, 1101–1108 (2008). Article CAS PubMed Google Scholar * Casteilla, L., Pénicaud, L., Cousin, B. & Calise,
D. Choosing an adipose tissue depot for sampling: factors in selection and depot specificity. _Methods Mol. Biol._ 456, 23–38 (2008). Article PubMed Google Scholar * Schneider, C. A.,
Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. _Nat. Methods_ 9, 671–675 (2012). Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS We thank S. Roberge for excellent technical support, W. Ho for RT-qPCR support and T. Reiberger for early discussions. This work received financial support, in
part, from the National Institutes of Health through the Laser Biomedical Research Center (grant no. 9-P41-EB015871-26A1 to M.G.B.) and the US Army Research Office through the Institute for
Soldier Nanotechnologies at MIT, under cooperative agreement no. W911NF-18-2-0048 (to M.G.B.). M.S. was supported by the National Science Foundation Graduate Research Fellowship Program
(grant no. 1122374). J.A.C. was supported by a National Defense Science and Engineering Graduate Fellowship 32 CFR 168a. K.v.L., Y.Z. and M.G.B. were supported by a Grand Challenges Grant
from the MGH/MIT Strategic Initiative. This work was also in part supported by grants from the National Foundation for Cancer Research, the Ludwig Center at Harvard, the US National Cancer
Institute (grant nos. P01-CA080124, R35-CA197743, R01-CA208205 and U01-CA224173 to R.K.J.). J.M.P. was supported by a NIH Training Grant (grant no. T32HL007627). This work was further
supported in part by the NIH (grant nos. R01 DK031036 and R01 DK033201 to C.R.K., and grant nos. K12 HD000850 and P30 DK40561) and a NASPGHAN Young Investigator/Nestle Nutrition Award to
S.S. M.P. was supported by an Erwin Schroedinger Fellowship from the Austrian Science Fund (FWF; project number J 3747-B28). S.C. was supported by an ABTA Basic Research Fellowship, MGH
Tosteon and Fund for Medical Discovery Fellowship and PCRF Emerging Investigator Fellowship Grant. O.T.B. was supported by a European Molecular Biology Organization long-term fellowship.
AUTHOR INFORMATION Author notes * Matthias Pinter Present address: Division of Gastroenterology and Hepatology, Department of Internal Medicine III, Medical University of Vienna, Vienna,
Austria * Oliver T. Bruns Present address: Helmholtz Pioneer Campus, Helmholtz Zentrum München, Neuherberg, Germany * These authors contributed equally: Mari Saif, Wilhelmus J. Kwanten,
Jessica A. Carr, Ivy X. Chen. AUTHORS AND AFFILIATIONS * Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, USA Mari Saif, Jessica A. Carr, Juanye Zhang, Oliver
T. Bruns & Moungi G. Bawendi * Edwin L. Steele Laboratories of Tumor Biology, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Wilhelmus J. Kwanten, Ivy X. Chen, Jessica M. Posada, Matthias Pinter, Sampurna Chatterjee & Rakesh K. Jain * Laboratory of Experimental Medicine and Pediatrics (LEMP)—Gastroenterology
and Hepatology, University of Antwerp, Wilrijk, Belgium Wilhelmus J. Kwanten * Department of Pathology, Brigham and Women’s Hospital, Boston, MA, USA Jessica M. Posada & Amitabh
Srivastava * Neuroprotection Research Laboratory, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA Yi Zheng & Klaus van Leyen * Joslin Diabetes Center,
Harvard Medical School, Boston, MA, USA Samir Softic & C. Ronald Kahn * Division of Gastroenterology, Boston Children’s Hospital, Boston, MA, USA Samir Softic * Division of
Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, University of Kentucky College of Medicine and Kentucky Children’s Hospital, Lexington, KY, USA Samir Softic Authors *
Mari Saif View author publications You can also search for this author inPubMed Google Scholar * Wilhelmus J. Kwanten View author publications You can also search for this author inPubMed
Google Scholar * Jessica A. Carr View author publications You can also search for this author inPubMed Google Scholar * Ivy X. Chen View author publications You can also search for this
author inPubMed Google Scholar * Jessica M. Posada View author publications You can also search for this author inPubMed Google Scholar * Amitabh Srivastava View author publications You can
also search for this author inPubMed Google Scholar * Juanye Zhang View author publications You can also search for this author inPubMed Google Scholar * Yi Zheng View author publications
You can also search for this author inPubMed Google Scholar * Matthias Pinter View author publications You can also search for this author inPubMed Google Scholar * Sampurna Chatterjee View
author publications You can also search for this author inPubMed Google Scholar * Samir Softic View author publications You can also search for this author inPubMed Google Scholar * C.
Ronald Kahn View author publications You can also search for this author inPubMed Google Scholar * Klaus van Leyen View author publications You can also search for this author inPubMed
Google Scholar * Oliver T. Bruns View author publications You can also search for this author inPubMed Google Scholar * Rakesh K. Jain View author publications You can also search for this
author inPubMed Google Scholar * Moungi G. Bawendi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization/evolution: W.J.K.,
J.A.C., I.X.C., M.P., K.v.L., O.T.B., R.K.J. and M.G.B. Formal analysis: M.S., W.J.K., J.A.C. and I.X.C. Funding acquisition: S.S., C.R.K., K.v.L., R.K.J. and M.G.B.
Investigation/experiments: M.S., W.J.K., J.A.C., I.X.C., J.M.P., J.Z., Y.Z., M.P. and S.C. Methodology: M.S., W.J.K., J.A.C., I.X.C., J.M.P., A.S., R.K.J. and M.G.B. Project management:
M.S., W.J.K. and J.A.C. Resources: S.S., C.R.K., K.v.L., R.K.J. and M.G.B. Supervision: R.K.J. and M.G.B. Validation: M.S., W.J.K. and J.A.C. Visualization: M.S. and J.A.C. Writing of the
original draft: M.S., W.J.K. and J.A.C. Writing, review and editing: all authors. CORRESPONDING AUTHORS Correspondence to Rakesh K. Jain or Moungi G. Bawendi. ETHICS DECLARATIONS COMPETING
INTERESTS M.P. is an investigator for Bayer, BMS and Lilly; a consultant for Bayer, BMS, Ipsen, Eisai and Lilly; and has received speaker honoraria from Bayer, BMS, Eisai and MSD as well as
travel support from Bayer and BMS. R.K.J. received honorarium from Amgen; consultant fees from Chugai, Merck, Ophthotech, Pfizer, SPARC, SynDevRx and XTuit; owns equity in Enlight,
Ophthotech, SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund.
M.G.B. is a consultant and owns equity in Lumicell Inc. Neither any reagent nor any funding from these organizations was used in this study. MIT and MGH have filed a patent (United States
application no. 62/654,665; international application no. PCT/US2019/026550) based on some of the findings described in this manuscript. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary methods,
figures and references. REPORTING SUMMARY SUPPLEMENTARY DATASET 1 Supplementary Tables 1–11. SUPPLEMENTARY DATASET 2 Statistical details. SUPPLEMENTARY VIDEO 1 Real-time and non-invasive
imaging of quantifiable autofluorescent pigments in rodent liver with progressive fibrosis. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saif, M.,
Kwanten, W.J., Carr, J.A. _et al._ Non-invasive monitoring of chronic liver disease via near-infrared and shortwave-infrared imaging of endogenous lipofuscin. _Nat Biomed Eng_ 4, 801–813
(2020). https://doi.org/10.1038/s41551-020-0569-y Download citation * Received: 21 June 2019 * Accepted: 13 May 2020 * Published: 22 June 2020 * Issue Date: August 2020 * DOI:
https://doi.org/10.1038/s41551-020-0569-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative