- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
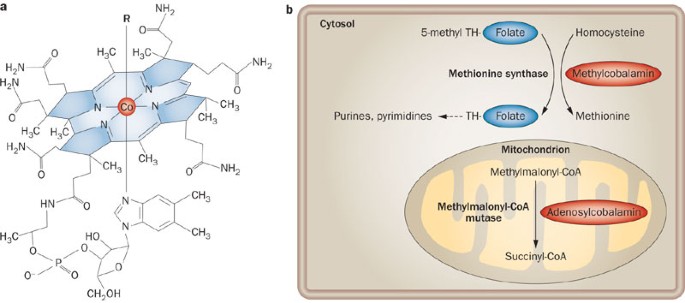
ABSTRACT Vitamin B12 (B12; also known as cobalamin) is a cofactor in many metabolic processes; deficiency of this vitamin is associated with megaloblastic anaemia and various neurological
disorders. In contrast to many prokaryotes, humans and other mammals are unable to synthesize B12. Instead, a sophisticated pathway for specific uptake and transport of this molecule has
evolved. Failure in the gastrointestinal part of this pathway is the most common cause of nondietary-induced B12 deficiency disease. However, although less frequent, defects in cellular
processing and further downstream steps in the transport pathway are also known culprits of functional B12 deficiency. Biochemical and genetic approaches have identified novel proteins in
the B12 transport pathway—now known to involve more than 15 gene products—delineating a coherent pathway for B12 trafficking from food to the body's cells. Some of these gene products
are specifically dedicated to B12 transport, whereas others embrace additional roles, which explains the heterogeneity in the clinical picture of the many genetic disorders causing B12
deficiency. This Review describes basic and clinical features of this multistep pathway with emphasis on gastrointestinal transport of B12 and its importance in clinical medicine. KEY POINTS
* A coherent vitamin B12 (B12) transport pathway from food to the body's cells has now been delineated; the pathway includes an ABC transporter for cellular B12 efflux and a receptor
for uptake of B12-bound transcobalamin * More than 15 gene products are involved in B12 transport and/or processing; several new genes encoding intracellular proteins (including a potential
lysosomal transporter of B12) have been identified * Gastrointestinal uptake of B12 is via cubam, the complex of cubilin and amnionless * Novel genetic causes of B12 deficiency disease have
been clarified; many of the new proteins have been identified by positional cloning of the genes harbouring the disease-causing mutations * New diagnostic assays for B12 deficiency are being
developed; plasma level of holo-transcobalamin is a promising biomarker in combination with existing markers Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CLYBL AVERTS VITAMIN B12
DEPLETION BY REPAIRING MALYL-COA Article 19 March 2025 AMINO ACID METABOLISM IN KIDNEY HEALTH AND DISEASE Article 28 August 2024 IRON FROM NANOSTRUCTURED FERRIC PHOSPHATE: ABSORPTION AND
BIODISTRIBUTION IN MICE AND BIOAVAILABILITY IN IRON DEFICIENT ANEMIC WOMEN Article Open access 18 February 2022 REFERENCES * McLean, E., de Benoist, B. & Allen, L. H. Review of the
magnitude of folate and vitamin B12 deficiencies worldwide. _Food Nutr. Bull._ 29 (2 Suppl.), S38–S51 (2008). Article PubMed Google Scholar * Dali-Youcef, N. & Andres, E. An update on
cobalamin deficiency in adults. _QJM_ 102, 17–28 (2009). Article CAS PubMed Google Scholar * Reynolds, E. Vitamin B12, folic acid, and the nervous system. _Lancet Neurol._ 5, 949–960
(2006). Article CAS PubMed Google Scholar * Stubbe, J. Binding site revealed of nature's most beautiful cofactor. _Science_ 266, 1663–1664 (1994). Article CAS PubMed Google
Scholar * Rickes, E. L., Brink, N. G., Koniuszy, F. R., Wood, T. R. & Folkers, K. Crystalline vitamin B12. _Science_ 107, 396–397 (1948). Article CAS PubMed Google Scholar * Rickes,
E. L., Brink, N. G., Koniuszy, F. R., Wood, T. R. & Folkers, K. Vitamin B12, a cobalt complex. _Science_ 108, 134 (1948). Article CAS PubMed Google Scholar * Smith, E. L.
Purification of anti-pernicious anaemia factors from liver. _Nature_ 161, 638 (1948). Article CAS PubMed Google Scholar * Kamper, M. J. & Hodgkin, D. C. Some observations on the
crystal structure of a chlorine-substituted vitamin B12. _Nature_ 176, 551–553 (1955). Article CAS PubMed Google Scholar * Lindstrand, K. Isolation of methylcobalamin from natural source
material. _Nature_ 10, 188–189 (1964). Article Google Scholar * Hodgkin, D. C. _ et al_. Structure of vitamin B12. _Nature_ 178, 64–66 (1956). Article CAS PubMed Google Scholar *
Martens, J. H., Barg, H., Warren, M. J. & Jahn, D. Microbial production of vitamin B12. _Appl. Microbiol. Biotechnol._ 58, 275–285 (2002). Article CAS PubMed Google Scholar *
Mathews, F. S. _ et al_. Crystal structure of human intrinsic factor: cobalamin complex at 2.6-A resolution. _Proc. Natl Acad. Sci. USA_ 104, 17311–17316 (2007). Article PubMed PubMed
Central Google Scholar * Wuerges, J. _ et al_. Structural basis for mammalian vitamin B12 transport by transcobalamin. _Proc. Natl Acad. Sci. USA_ 103, 4386–4391 (2006). Article CAS
PubMed PubMed Central Google Scholar * Birn, H. _ et al_. Characterization of an epithelial approximately 460 kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and
binds receptor-associated protein. _J. Biol. Chem._ 272, 26497–26504 (1997). Article CAS PubMed Google Scholar * Kozyraki, R. _ et al_. The human intrinsic factor-vitamin B12 receptor,
cubilin: molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. _Blood_ 91, 3593–3600 (1998). CAS PubMed
Google Scholar * Hurlimann, J. & Zuber, C. Vitamin B12-binders in human body fluids. I. Antigenic and physico-chemical characteristics. _Clin. Exp. Immunol._ 4, 125–140 (1969). CAS
PubMed PubMed Central Google Scholar * Gordon, M. M., Hu, C., Chokshi, H., Hewitt, J. E. & Alpers, D. H. Glycosylation is not required for ligand or receptor binding by expressed rat
intrinsic factor. _Am. J. Physiol._ 260, G736–G742 (1991). CAS PubMed Google Scholar * Drennan, C. L., Huang, S., Drummond, J. T., Matthews, R. G. & Lidwig, M. L. How a protein binds
B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. _Science_ 266, 1669–1674 (1994). Article CAS PubMed Google Scholar * Mancia, F. _ et al_. How coenzyme B12
radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 A resolution. _Structure_ 4, 339–350 (1996). Article CAS PubMed Google Scholar * Schwartz, M.
Intrinsic factor antibody in serum from patients with pernicious anaemia. _Lancet_ 2, 1263–1267 (1960). Article CAS PubMed Google Scholar * Tanner, S. M. _ et al_. Hereditary juvenile
cobalamin deficiency caused by mutations in the intrinsic factor gene. _Proc. Natl Acad. Sci. USA_ 102, 4130–4133 (2005). Article CAS PubMed PubMed Central Google Scholar * Castle, W.
B. The etiology of pernicious and related macrocytic anemias. _Science_ 82, 159–164 (1935). Article CAS PubMed Google Scholar * Li, N., Rosenblatt, D. S. & Seetharam, B. Nonsense
mutations in human transcobalamin II deficiency. _Biochem. Biophys. Res. Commun._ 204, 1111–1118 (1994). Article CAS PubMed Google Scholar * Li, N., Rosenblatt, D. S., Kamen, B. A.,
Seetharam, S. & Seetharam, B. Identification of two mutant alleles of transcobalamin II in an affected family. _Hum. Mol. Genet._ 3, 1835–1840 (1994). Article CAS PubMed Google
Scholar * Namour, F. _ et al_. Transcobalamin deficiency due to activation of an intra exonic cryptic splice site. _Br. J. Haematol._ 123, 915–920 (2003). Article CAS PubMed Google
Scholar * Nissen, P. H., Nordwall, M., Hoffmann-Lucke, E., Sorensen, B. S. & Nexo, E. Transcobalamin deficiency caused by compound heterozygosity for two novel mutations in the TCN2
gene: a study of two affected siblings, their brother, and their parents. _J. Inherit. Metab. Dis._ http://dx.doi.org/10.1007/s10545-010-9145-z. * Qian, L., Quadros, E. V., Regec, A.,
Zittoun, J. & Rothenberg, S. P. Congenital transcobalamin II deficiency due to errors in RNA editing. _Blood Cells Mol. Dis._ 28, 134–142 (2002). Article PubMed Google Scholar *
Hygum, K. _ et al_. Mouse transcobalamin has features resembling both human transcobalamin and haptocorrin. _PLoS ONE_ 6, e20638 (2011). Article CAS PubMed PubMed Central Google Scholar
* Morkbak, A. L., Poulsen, S. S. & Nexo, E. Haptocorrin in humans. _Clin. Chem. Lab. Med._ 45, 1751–1759 (2007). Article CAS PubMed Google Scholar * Allen, R. H. & Stabler, S.
P. Identification and quantitation of cobalamin and cobalamin analogues in human feces. _Am. J. Clin. Nutr._ 87, 1324–1335 (2008). Article CAS PubMed Google Scholar * Hardlei, T. F.
& Nexo, E. A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. _Clin. Chem._ 55, 1002–1010 (2009). Article CAS
PubMed Google Scholar * Green, R. Ins and outs of cellular cobalamin transport. _Blood_ 115, 1476–1477 (2010). Article CAS PubMed Google Scholar * Quadros, E. V. Advances in the
understanding of cobalamin assimilation and metabolism. _Br. J. Haematol._ 148, 195–204 (2010). Article CAS PubMed Google Scholar * Nexo, E. & Hoffmann-Lucke, E. Holotranscobalamin,
a marker of vitamin B-12 status: analytical aspects and clinical utility. _Am. J. Clin. Nutr._ 94, 359S–365S (2011). Article CAS PubMed PubMed Central Google Scholar * Hvas, A. M. &
Nexo, E. Diagnosis and treatment of vitamin B12 deficiency—an update. _Haematologica_ 91, 1506–1512 (2006). CAS PubMed Google Scholar * Green, R. Indicators for assessing folate and
vitamin B12 status and for monitoring the efficacy of intervention strategies. _Food Nutr. Bull._ 29, S52–S63 (2008). Article PubMed Google Scholar * Refsum, H. _ et al_. Facts and
recommendations about total homocysteine determinations: an expert opinion. _Clin. Chem._ 50, 3–32 (2004). Article CAS PubMed Google Scholar * Savage, D. G., Lindenbaum, J., Stabler, S.
P. & Allen, R. H. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. _Am. J. Med._ 96, 239–246 (1994).
Article CAS PubMed Google Scholar * Allen, R. H., Seetharam, B., Podell, E. & Alpers, D. H. Effect of proteolytic enzymes on the binding of cobalamin to R protein and intrinsic
factor. _In vitro_ evidence that a failure to partially degrade R protein is responsible for cobalamin malabsorption in pancreatic insufficiency. _J. Clin. Invest._ 61, 47–54 (1978). Article
CAS PubMed PubMed Central Google Scholar * Seetharam, B., Levine, J. S., Ramasamy, M. & Alpers, D. H. Purification, properties, and immunochemical localization of a receptor for
intrinsic factor-cobalamin complex in the rat kidney. _J. Biol. Chem._ 263, 4443–4449 (1988). CAS PubMed Google Scholar * Seetharam, B., Christensen, E. I., Moestrup, S. K., Hammond, T.
G. & Verroust, P. J. Identification of rat yolk sac target protein of teratogenic antibodies, gp280, as intrinsic factor-cobalamin receptor. _J. Clin. Invest._ 99, 2317–2322 (1997).
Article CAS PubMed PubMed Central Google Scholar * Moestrup, S. K. _ et al_. The intrinsic factor—vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding
peripheral membrane protein with homology to developmental proteins. _J. Biol. Chem._ 273, 5235–5242 (1998). Article CAS PubMed Google Scholar * Fyfe, J. C. _ et al_. The functional
cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. _Blood_ 103, 1573–1579 (2004). Article CAS PubMed Google Scholar * Sahali, D. _ et al_.
Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal
antibodies. _J. Exp. Med._ 167, 213–218 (1988). Article CAS PubMed Google Scholar * He, Q. _ et al_. Amnionless function is required for cubilin brush-border expression and intrinsic
factor-cobalamin (vitamin B12) absorption _in vivo_. _Blood_ 106, 1447–1453 (2005). Article CAS PubMed PubMed Central Google Scholar * Kalantry, S. _ et al_. The amnionless gene,
essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. _Nat. Genet._ 27, 412–416 (2001). Article CAS PubMed Google
Scholar * Strope, S., Rivi, R., Metzger, T., Manova, K. & Lacy, E. Mouse amnionless, which is required for primitive streak assembly, mediates cell-surface localization and endocytic
function of cubilin on visceral endoderm and kidney proximal tubules. _Development_ 131, 4787–4795 (2004). Article CAS PubMed Google Scholar * Tanner, S. M. _ et al_. Amnionless,
essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. _Nat. Genet._ 33, 426–429 (2003). Article CAS PubMed Google Scholar * Kristiansen, M. _ et al_.
Molecular dissection of the intrinsic factor-vitamin B12 receptor, cubilin, discloses regions important for membrane association and ligand binding. _J. Biol. Chem._ 274, 20540–20544
(1999). Article CAS PubMed Google Scholar * Andersen, C. B., Madsen, M., Storm, T., Moestrup, S. K. & Andersen, G. R. Structural basis for receptor recognition of
vitamin-B(12)-intrinsic factor complexes. _Nature_ 464, 445–448 (2010). Article CAS PubMed Google Scholar * Birn, H. _ et al_. Cubilin is an albumin binding protein important for renal
tubular albumin reabsorption. _J. Clin. Invest._ 105, 1353–1361 (2000). Article CAS PubMed PubMed Central Google Scholar * Kozyraki, R. _ et al_. The intrinsic factor—vitamin B12
receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. _Nat. Med._ 5, 656–661 (1999). Article CAS PubMed Google Scholar *
Zhai, X. Y. _ et al_. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. _Kidney Int._ 58, 1523–1533 (2000). Article CAS PubMed Google
Scholar * Pedersen, G. A., Chakraborty, S., Steinhauser, A. L., Traub, L. M. & Madsen, M. AMN directs endocytosis of the intrinsic factor-vitamin B(12) receptor cubam by engaging ARH or
Dab2. _Traffic_ 11, 706–720 (2010). Article CAS PubMed PubMed Central Google Scholar * Coudroy, G. _ et al_. Contribution of cubilin and amnionless to processing and membrane targeting
of cubilin-amnionless complex. _J. Am. Soc. Nephrol._ 16, 2330–2337 (2005). Article CAS PubMed Google Scholar * Fyfe, J. C., Ramanujam, K. S., Ramaswamy, K., Patterson, D. F. &
Seetharam, B. Defective brush-border expression of intrinsic factor-cobalamin receptor in canine inherited intestinal cobalamin malabsorption. _J. Biol. Chem._ 266, 4489–4494 (1991). CAS
PubMed Google Scholar * Namour, F. _ et al_. Luminal expression of cubilin is impaired in Imerslund-Grasbeck syndrome with compound AMN mutations in intron 3 and exon 7. _Haematologica_
96, 1715–1719 (2011). Article CAS PubMed PubMed Central Google Scholar * Storm, T. _ et al_. A patient with cubilin deficiency. _N. Engl. J. Med._ 364, 89–91 (2011). Article CAS
PubMed Google Scholar * Xu, D., Kozyraki, R., Newman, T. C. & Fyfe, J. C. Genetic evidence of an accessory activity required specifically for cubilin brush-border expression and
intrinsic factor-cobalamin absorption. _Blood_ 94, 3604–3606 (1999). CAS PubMed Google Scholar * Grasbeck, R., Gordin, R., Kantero, I. & Kuhlback, B. Selective vitamin B12
malabsorption and proteinuria in young people. A syndrome. _Acta Med. Scand._ 167, 289–296 (1960). Article CAS PubMed Google Scholar * Imerslund, O. Idiopathic chronic megaloblastic
anemia in children. _Acta Paediatr. Suppl._ 49 (Suppl. 119), 1–115 (1960). Google Scholar * Aminoff, M. _ et al_. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor,
cubilin, cause hereditary megaloblastic anaemia 1. _Nat. Genet._ 21, 309–313 (1999). Article CAS PubMed Google Scholar * He, Q. _ et al_. Canine Imerslund-Grasbeck syndrome maps to a
region orthologous to HSA14q. _Mamm. Genome_ 14, 758–764 (2003). Article CAS PubMed Google Scholar * Kristiansen, M. _ et al_. Cubilin P1297L mutation associated with hereditary
megaloblastic anemia 1 causes impaired recognition of intrinsic factor-vitamin B(12) by cubilin. _Blood_ 96, 405–409 (2000). CAS PubMed Google Scholar * Rutsch, F. _ et al_.
Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. _Nat. Genet._ 41, 234–239 (2009). Article CAS PubMed Google Scholar *
Rosenblatt, D. S., Hosack, A., Matiaszuk, N. V., Cooper, B. A. & Laframboise, R. Defect in vitamin B12 release from lysosomes: newly described inborn error of vitamin B12 metabolism.
_Science_ 228, 1319–1321 (1985). Article CAS PubMed Google Scholar * Froese, D. S. & Gravel, R. A. Genetic disorders of vitamin B metabolism: eight complementation groups—eight
genes. _Expert Rev. Mol. Med._ 12, e37 (2010). Article CAS PubMed PubMed Central Google Scholar * Banerjee, R., Gherasim, C. & Padovani, D. The tinker, tailor, soldier in
intracellular B12 trafficking. _Curr. Opin. Chem. Biol._ 13, 484–491 (2009). Article CAS PubMed PubMed Central Google Scholar * Hannibal, L. _ et al_. Processing of alkylcobalamins in
mammalian cells: a role for the MMACHC (cblC) gene product. _Mol. Genet. Metab._ 97, 260–266 (2009). Article CAS PubMed PubMed Central Google Scholar * Lerner-Ellis, J. P. _ et al_.
Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. _Nat. Genet._ 38, 93–100 (2006). Article CAS PubMed Google Scholar * Kim, J., Gherasim,
C. & Banerjee, R. Decyanation of vitamin B12 by a trafficking chaperone. _Proc. Natl Acad. Sci. USA_ 105, 14551–14554 (2008). Article PubMed PubMed Central Google Scholar * Kim, J.,
Hannibal, L., Gherasim, C., Jacobsen, D. W. & Banerjee, R. A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. _J. Biol. Chem._
284, 33418–33424 (2009). Article CAS PubMed PubMed Central Google Scholar * Coelho, D. _ et al_. Gene identification for the cblD defect of vitamin B12 metabolism. _N. Engl. J. Med._
358, 1454–1464 (2008). Article CAS PubMed Google Scholar * Miousse, I. R. _ et al_. Clinical and molecular heterogeneity in patients with the cblD inborn error of cobalamin metabolism.
_J. Pediatr._ 154, 551–556 (2009). Article CAS PubMed Google Scholar * Suormala, T. _ et al_. The cblD defect causes either isolated or combined deficiency of methylcobalamin and
adenosylcobalamin synthesis. _J. Biol. Chem._ 279, 42742–42749 (2004). Article CAS PubMed Google Scholar * Beedholm-Ebsen, R. _ et al_. Identification of multidrug resistance protein 1
(MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. _Blood_ 115, 1632–1639 (2010). Article CAS PubMed Google Scholar * Bakos, E. & Homolya, L. Portrait of multifaceted
transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). _Pflugers Arch._ 453, 621–641 (2007). Article CAS PubMed Google Scholar * Cole, S. P. _ et al_. Overexpression
of a transporter gene in a multidrug-resistant human lung cancer cell line. _Science_ 258, 1650–1654 (1992). Article CAS PubMed Google Scholar * Flens, M. J. _ et al_. Tissue
distribution of the multidrug resistance protein. _Am. J. Pathol._ 148, 1237–1247 (1996). CAS PubMed PubMed Central Google Scholar * Peng, K. C. _ et al_. Tissue and cell distribution of
the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. _J. Histochem. Cytochem._ 47, 757–768 (1999). Article CAS PubMed Google Scholar * Ostray, F. & Gams,
R. A. Cellular fluxes of vitamin B12. _Blood_ 50, 877–888 (1977). CAS PubMed Google Scholar * Ramasamy, M., Alpers, D. H., Tiruppathi, C. & Seetharam, B. Cobalamin release from
intrinsic factor and transfer to transcobalamin II within the rat enterocyte. _Am. J. Physiol._ 257, G791–G797 (1989). CAS PubMed Google Scholar * Seetharam, B. & Yammani, R. R.
Cobalamin transport proteins and their cell-surface receptors. _Expert Rev. Mol. Med._ 5, 1–18 (2003). Article PubMed Google Scholar * Schilling, R. F. Intrinsic factor studies II. The
effect of gastric juice on the urinary excretion of radioactivity after the oral administration of radioactive vitamin B12. _J. Lab. Clin. Med._ 42, 860–866 (1953). CAS PubMed Google
Scholar * Shah, N. P., Beech, C. M., Sturm, A. C. & Tanner, S. M. Investigation of the ABC transporter MRP1 in selected patients with presumed defects in vitamin B12 absorption. _Blood_
117, 4397–4398 (2011). Article CAS PubMed Google Scholar * Deeley, R. G., Westlake, C. & Cole, S. P. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding
cassette multidrug resistance proteins. _Physiol. Rev._ 86, 849–899 (2006). Article CAS PubMed Google Scholar * Hall, C. A. The carriers of native vitamin B12 in normal human serum.
_Clin. Sci. Mol. Med._ 53, 453–457 (1977). CAS PubMed Google Scholar * Quadros, E. V., Nakayama, Y. & Sequeira, J. M. The protein and the gene encoding the receptor for the cellular
uptake of transcobalamin-bound cobalamin. _Blood_ 113, 186–192 (2009). Article CAS PubMed PubMed Central Google Scholar * Bose, S., Seetharam, S. & Seetharam, B. Membrane expression
and interactions of human transcobalamin II receptor. _J. Biol. Chem._ 270, 8152–8157 (1995). Article CAS PubMed Google Scholar * Nexo, E. & Hollenberg, M. D. Characterization of
the particulate and soluble acceptor for transcobalamin II from human placenta and rabbit liver. _Biochim. Biophys. Acta_ 628, 190–200 (1980). Article CAS PubMed Google Scholar *
Seligman, P. A. & Allen, R. H. Characterization of the receptor for transcobalamin II isolated from human placenta. _J. Biol. Chem._ 253, 1766–1772 (1978). CAS PubMed Google Scholar *
Amagasaki, T., Green, R. & Jacobsen, D. W. Expression of transcobalamin II receptors by human leukemia K562 and HL-60 cells. _Blood_ 76, 1380–1386 (1990). CAS PubMed Google Scholar *
Cho, W. _ et al_. Expression of CD320 in human B cells in addition to follicular dendritic cells. _BMB Rep._ 41, 863–867 (2008). Article CAS PubMed Google Scholar * Park, H. J., Kim, J.
Y., Jung, K. I. & Kim, T. J. Characterization of a novel nene in the nxtended MHC region of mouse, NG29/Cd320, a homolog of the human CD320. _Immune Netw._ 9, 138–146 (2009). Article
PubMed PubMed Central Google Scholar * Arendt, J. F., Quadros, E. V. & Nexo, E. Soluble transcobalamin receptor, sCD320, is present in human serum and relates to serum
cobalamin—establishment and validation of an ELISA. _Clin. Chem. Lab Med._ http://dx/doi.org/10.1515/cclm.2011.810. * Quadros, E. V. & Jacobsen, D. W. The dynamics of cobalamin
utilization in L-1210 mouse leukemia cells: a model of cellular cobalamin metabolism. _Biochim. Biophys. Acta_ 1244, 395–403 (1995). Article CAS PubMed Google Scholar * Youngdahl-Turner,
P., Rosenberg, L. E. & Allen, R. H. Binding and uptake of transcobalamin II by human fibroblasts. _J. Clin. Invest._ 61, 133–141 (1978). Article CAS PubMed PubMed Central Google
Scholar * Quadros, E. V. _ et al_. Positive newborn screen for methylmalonic aciduria identifies the first mutation in TCblR/CD320, the gene for cellular uptake of transcobalamin-bound
vitamin B(12). _Hum. Mutat._ 31, 924–929 (2010). Article CAS PubMed PubMed Central Google Scholar * Birn, H., Nexo, E., Christensen, E. I. & Nielsen, R. Diversity in rat tissue
accumulation of vitamin B12 supports a distinct role for the kidney in vitamin B12 homeostasis. _Nephrol. Dial. Transplant._ 18, 1095–1100 (2003). Article CAS PubMed Google Scholar *
Moestrup, S. K. _ et al_. Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. _Proc. Natl Acad. Sci. USA_ 93,
8612–8617 (1996). Article CAS PubMed PubMed Central Google Scholar * Burger, R. L., Schneider, R. J., Mehlman, C. S. & Allen, R. H. Human plasma R-type vitamin B12-binding proteins.
II. The role of transcobalamin I, transcobalamin III, and the normal granulocyte vitamin B12-binding protein in the plasma transport of vitamin B12. _J. Biol. Chem._ 250, 7707–7713 (1975).
CAS PubMed Google Scholar * Birn, H. _ et al_. Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin-B(12). _Am. J. Physiol. Renal Physiol._ 282,
F408–F416 (2002). Article CAS PubMed Google Scholar * Birn, H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier
proteins. _Am. J. Physiol. Renal Physiol._ 291, F22–F36 (2006). Article CAS PubMed Google Scholar * Newmark, P., Newman, G. E. & O'Brien, J. R. Vitamin B12 in the rat kidney.
Evidence for an association with lysosomes. _Arch. Biochem. Biophys._ 141, 121–130 (1970). Article CAS PubMed Google Scholar * Hammad, S. M., Barth, J. L., Knaak, C. & Argraves, W.
S. Megalin acts in concert with cubilin to mediate endocytosis of high density lipoproteins. _J. Biol. Chem._ 275, 12003–12008 (2000). Article CAS PubMed Google Scholar * Kozyraki, R. _
et al_. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. _Proc. Natl Acad. Sci. USA_ 98, 12491–12496 (2001).
Article CAS PubMed PubMed Central Google Scholar * Leheste, J. R. _ et al_. Megalin knockout mice as an animal model of low molecular weight proteinuria. _Am. J. Pathol._ 155, 1361–1370
(1999). Article CAS PubMed PubMed Central Google Scholar * Nagai, J. _ et al_. Mutually dependent localization of megalin and Dab2 in the renal proximal tubule. _Am. J. Physiol. Renal
Physiol._ 289, F569–F576 (2005). Article CAS PubMed Google Scholar * Green, R. Is it time for vitamin B-12 fortification? What are the questions? _Am. J. Clin. Nutr._ 89, 712S–716S
(2009). Article CAS PubMed PubMed Central Google Scholar * Hvas, A. M., Morkbak, A. L., Hardlei, T. F. & Nexo, E. The vitamin B12 absorption test, CobaSorb, identifies patients not
requiring vitamin B12 injection therapy. _Scand. J. Clin. Lab. Invest._ 71, 432–438 (2011). Article CAS PubMed Google Scholar * Fedosov, S. N. _ et al_. Human intrinsic factor expressed
in the plant Arabidopsis thaliana. _Eur. J. Biochem._ 270, 3362–3367 (2003). Article CAS PubMed Google Scholar * Kuzminski, A. M., Del Giacco, E. J., Allen, R. H., Stabler, S. P. &
Lindenbaum, J. Effective treatment of cobalamin deficiency with oral cobalamin. _Blood_ 92, 1191–1198 (1998). CAS PubMed Google Scholar * Clardy, S. M., Allis, D. G., Fairchild, T. J.
& Doyle, R. P. Vitamin B12 in drug delivery: breaking through the barriers to a B12 bioconjugate pharmaceutical. _Expert Opin. Drug Deliv._ 8, 127–140 (2011). Article CAS PubMed
Google Scholar * Quadros, E. V., Nakayama, Y. & Sequeira, J. M. Targeted delivery of saporin toxin by monoclonal antibody to the transcobalamin receptor, TCblR/CD320. _Mol. Cancer
Ther._ 9, 3033–3040 (2010). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by the Novo Nordisk Foundation, the Lundbeck
Foundation, the Danish Medical Research Council and the European Research Council. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedicine, Aarhus University, Ole Worms Allé
3, Building 1170, Aarhus, 8000, Denmark Marianne J. Nielsen, Mie R. Rasmussen, Christian B. F. Andersen & Søren K. Moestrup * Department of Clinical Biochemistry, Aarhus University
Hospital, Norrebrogade, Aarhus, 8000, Denmark Ebba Nexø Authors * Marianne J. Nielsen View author publications You can also search for this author inPubMed Google Scholar * Mie R. Rasmussen
View author publications You can also search for this author inPubMed Google Scholar * Christian B. F. Andersen View author publications You can also search for this author inPubMed Google
Scholar * Ebba Nexø View author publications You can also search for this author inPubMed Google Scholar * Søren K. Moestrup View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS M. J. Nielsen contributed to the writing and reviewing/editing of the manuscript. M. R. Rasmussen and C. B. F. Andersen researched data. E. Nexø
researched data and contributed to reviewing/editing the manuscript. S. K. Moestrup contributed to all aspects of this manuscript. CORRESPONDING AUTHOR Correspondence to Søren K. Moestrup.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nielsen,
M., Rasmussen, M., Andersen, C. _et al._ Vitamin B12 transport from food to the body's cells—a sophisticated, multistep pathway. _Nat Rev Gastroenterol Hepatol_ 9, 345–354 (2012).
https://doi.org/10.1038/nrgastro.2012.76 Download citation * Published: 01 May 2012 * Issue Date: June 2012 * DOI: https://doi.org/10.1038/nrgastro.2012.76 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative