- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
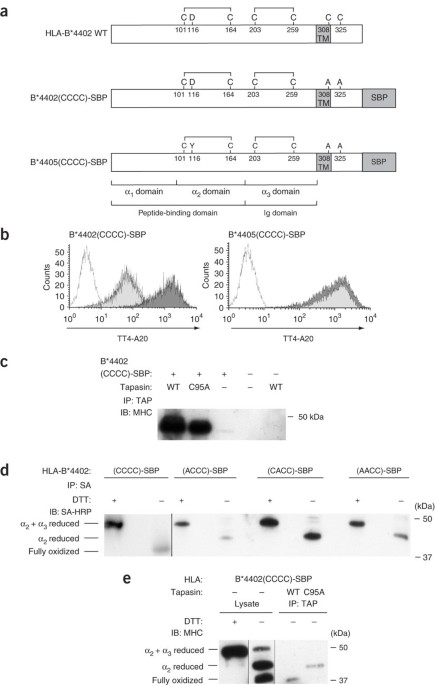
ABSTRACT The function of the oxidoreductase ERp57 in the major histocompatibility complex (MHC) class I peptide-loading complex has remained elusive. Here we show that in the absence of
tapasin, the α2 disulfide bond in the MHC class I peptide-binding groove was rapidly reduced. Covalent sequestration of ERp57 by tapasin was needed to protect the α2 disulfide bond against
reduction and thus to maintain the binding groove in a peptide-receptive state. Allelic variations in MHC class I tapasin dependency reflected their susceptibility to reduction of the α2
disulfide bond. In the absence of sequestration, ERp57 acted directly on the α2 disulfide bond. Our work provides insight into how the immune system customizes 'quality control' in
the endoplasmic reticulum to fit the needs of antigen presentation. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURE OF AN MHC I–TAPASIN–ERP57 EDITING COMPLEX DEFINES CHAPERONE
PROMISCUITY Article Open access 14 September 2022 EXCHANGE CATALYSIS BY TAPASIN EXPLOITS CONSERVED AND ALLELE-SPECIFIC FEATURES OF MHC-I MOLECULES Article Open access 09 July 2021 MOLECULAR
BASIS OF MHC I QUALITY CONTROL IN THE PEPTIDE LOADING COMPLEX Article Open access 10 August 2022 REFERENCES * Pamer, E. & Cresswell, P. Mechanisms of MHC class I–restricted antigen
processing. _Annu. Rev. Immunol._ 16, 323–358 (1998). Article CAS Google Scholar * Bouvier, M. Accessory proteins and the assembly of human class I MHC molecules: a molecular and
structural perspective. _Mol. Immunol._ 39, 697–706 (2003). Article CAS Google Scholar * Bjorkman, P.J. et al. Structure of the human class I histocompatibility antigen, HLA-A2. _Nature_
329, 506–512 (1987). Article CAS Google Scholar * Dick, T.P. Assembly of MHC class I peptide complexes from the perspective of disulfide bond formation. _Cell. Mol. Life Sci._ 61, 547–556
(2004). Article CAS Google Scholar * Cresswell, P., Ackerman, A.L., Giodini, A., Peaper, D.R. & Wearsch, P.A. Mechanisms of MHC class I-restricted antigen processing and
cross-presentation. _Immunol. Rev._ 207, 145–157 (2005). Article CAS Google Scholar * Momburg, F. & Tan, P. Tapasin-the keystone of the loading complex optimizing peptide binding by
MHC class I molecules in the endoplasmic reticulum. _Mol. Immunol._ 39, 217–233 (2002). Article CAS Google Scholar * Garbi, N., Tanaka, S., van den Broek, M., Momburg, F. &
Hammerling, G.J. Accessory molecules in the assembly of major histocompatibility complex class I/peptide complexes: how essential are they for CD8+ T-cell immune responses? _Immunol. Rev._
207, 77–88 (2005). Article CAS Google Scholar * Ribaudo, R.K. & Margulies, D.H. Independent and synergistic effects of disulfide bond formation, β2-microglobulin, and peptides on
class I MHC folding and assembly in an in vitro translation system. _J. Immunol._ 149, 2935–2944 (1992). CAS PubMed Google Scholar * Wang, H., Capps, G.G., Robinson, B.E. & Zuniga,
M.C. Ab initio association with β2-microglobulin during biosynthesis of the H-2Ld class I major histocompatibility complex heavy chain promotes proper disulfide bond formation and stable
peptide binding. _J. Biol. Chem._ 269, 22276–22281 (1994). CAS PubMed Google Scholar * Smith, J.D., Solheim, J.C., Carreno, B.M. & Hansen, T.H. Characterization of class I MHC folding
intermediates and their disparate interactions with peptide and β2-microglobulin. _Mol. Immunol._ 32, 531–540 (1995). Article CAS Google Scholar * Tector, M., Zhang, Q. & Salter,
R.D. Beta 2-microglobulin and calnexin can independently promote folding and disulfide bond formation in class I histocompatibility proteins. _Mol. Immunol._ 34, 401–408 (1997). Article CAS
Google Scholar * Lewis, J.W. & Elliott, T. Evidence for successive peptide binding and quality control stages during MHC class I assembly. _Curr. Biol._ 8, 717–720 (1998). Article
CAS Google Scholar * Antoniou, A.N. et al. The oxidoreductase ERp57 efficiently reduces partially folded in preference to fully folded MHC class I molecules. _EMBO J._ 21, 2655–2663
(2002). Article CAS Google Scholar * Dick, T.P., Bangia, N., Peaper, D.R. & Cresswell, P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. _Immunity_
16, 87–98 (2002). Article CAS Google Scholar * Peaper, D.R., Wearsch, P.A. & Cresswell, P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I
peptide-loading complex. _EMBO J._ 24, 3613–3623 (2005). Article CAS Google Scholar * Williams, A.P., Peh, C.A., Purcell, A.W., McCluskey, J. & Elliott, T. Optimization of the MHC
class I peptide cargo is dependent on tapasin. _Immunity_ 16, 509–520 (2002). Article CAS Google Scholar * Keefe, A.D., Wilson, D.S., Seelig, B. & Szostak, J.W. One-step purification
of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. _Protein Expr. Purif._ 23, 440–446 (2001). Article CAS Google Scholar * Grandea, A.G.,
Lehner, P.J., Cresswell, P. & Spies, T. Regulation of MHC class I heterodimer stability and interaction with TAP by tapasin. _Immunogenetics_ 46, 477–483 (1997). Article CAS Google
Scholar * Steinle, A. & Schendel, D.J. HLA class I alleles of LCL 721 and 174 x CEM.T2 (T2). _Tissue Antigens_ 44, 268–270 (1994). Article CAS Google Scholar * Zernich, D. et al.
Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. _J. Exp. Med._ 200, 13–24 (2004). Article CAS Google Scholar * Park, B.
et al. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. _Cell_ 127, 369–382 (2006). Article CAS Google Scholar * Stam, N., Spits, H. &
Ploegh, H. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. _J. Immunol._ 137, 2299–2306 (1986).
CAS PubMed Google Scholar * Sadasivan, B., Lehner, P.J., Ortmann, B., Spies, T. & Cresswell, P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC
class I molecules with TAP. _Immunity_ 5, 103–114 (1996). Article CAS Google Scholar * Ortmann, B., Androlewicz, M.J. & Cresswell, P. MHC class I/β2-microglobulin complexes associate
with TAP transporters before peptide binding. _Nature_ 368, 864–867 (1994). Article CAS Google Scholar * Dick, T.P. & Cresswell, P. Thiol oxidation and reduction in major
histocompatibility complex class I-restricted antigen processing and presentation. _Methods Enzymol._ 348, 49–54 (2002). Article CAS Google Scholar * Garbi, N., Tanaka, S., Momburg, F.
& Hammerling, G.J. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. _Nat. Immunol._ 7, 93–102
(2006). Article CAS Google Scholar * Lindquist, J.A., Jensen, O.N., Mann, M. & Hammerling, G.J. ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I
assembly. _EMBO J._ 17, 2186–2195 (1998). Article CAS Google Scholar * Solda, T., Garbi, N., Hammerling, G.J. & Molinari, M. Consequences of ERp57 deletion on oxidative folding of
obligate and facultative clients of the calnexin cycle. _J. Biol. Chem._ 281, 6219–6226 (2006). Article CAS Google Scholar * Helenius, A. & Aebi, M. Roles of N-linked glycans in the
endoplasmic reticulum. _Annu. Rev. Biochem._ 73, 1019–1049 (2004). Article CAS Google Scholar * Jessop, C.E. & Bulleid, N.J. Glutathione directly reduces an oxidoreductase in the
endoplasmic reticulum of mammalian cells. _J. Biol. Chem._ 279, 55341–55347 (2004). Article CAS Google Scholar * Mezghrani, A. et al. Manipulation of oxidative protein folding and PDI
redox state in mammalian cells. _EMBO J._ 20, 6288–6296 (2001). Article CAS Google Scholar * Ellgaard, L. & Helenius, A. ER quality control: towards an understanding at the molecular
level. _Curr. Opin. Cell Biol._ 13, 431–437 (2001). Article CAS Google Scholar * Howarth, M., Williams, A., Tolstrup, A.B. & Elliott, T. Tapasin enhances MHC class I peptide
presentation according to peptide half-life. _Proc. Natl. Acad. Sci. USA_ 101, 11737–11742 (2004). Article CAS Google Scholar * Zhang, Y., Baig, E. & Williams, D.B. Functions of ERp57
in the folding and assembly of major histocompatibility complex class I molecules. _J. Biol. Chem._ 281, 14622–14631 (2006). Article CAS Google Scholar * Zacharias, M. & Springer, S.
Conformational flexibility of the MHC class I α1-α2 domain in peptide bound and free states: a molecular dynamics simulation study. _Biophys. J._ 87, 2203–2214 (2004). Article CAS Google
Scholar * Elliott, T. & Williams, A. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. _Immunol. Rev._ 207, 89–99 (2005). Article CAS
Google Scholar * Santos, S.G. et al. MHC class I-ERp57-tapasin interactions within the peptide-loading complex. _J. Biol. Chem._ 282, 17587–17593 (2007). Article CAS Google Scholar *
Kanaseki, T., Blanchard, N., Hammer, G.E., Gonzalez, F. & Shastri, N. ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the
endoplasmic reticulum. _Immunity_ 25, 795–806 (2006). Article CAS Google Scholar * Greenwood, R., Shimizu, Y., Sekhon, G.S. & DeMars, R. Novel allele-specific, post-translational
reduction in HLA class I surface expression in a mutant human B cell line. _J. Immunol._ 153, 5525–5536 (1994). CAS PubMed Google Scholar * Peh, C.A. et al. HLA-B27-restricted antigen
presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. _Immunity_ 8, 531–542 (1998). Article CAS Google Scholar * Diedrich, G., Bangia,
N., Pan, M. & Cresswell, P. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. _J. Immunol._ 166, 1703–1709 (2001). Article CAS Google
Scholar * Lutz, P.M. & Cresswell, P. An epitope common to HLA class I and class II antigens, Ig light chains, and β2-microglobulin. _Immunogenetics_ 25, 228–233 (1987). Article CAS
Google Scholar * Tahara, T. et al. HLA antibody responses in HLA class I transgenic mice. _Immunogenetics_ 32, 351–360 (1990). Article CAS Google Scholar * Call, M.E., Pyrdol, J.,
Wiedmann, M. & Wucherpfennig, K.W. The organizing principle in the formation of the T cell receptor-CD3 complex. _Cell_ 111, 967–979 (2002). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We thank C. Mader for expert technical assistance; F. Momburg, N. Garbi and N. Bangia (Roswell Park Cancer Institute) for discussions; and F. Momburg and P.
Cresswell (Yale University) for reagents. Supported by the Deutsche Forschungsgemeinschaft (DI 731/2-1) and the European Commission (Marie Curie Excellence Grant). AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Redox Regulation Research Group, German Cancer Research Center, Heidelberg, D-69120, Germany Alexandra Kienast, Marc Preuss, Monique Winkler & Tobias P Dick Authors *
Alexandra Kienast View author publications You can also search for this author inPubMed Google Scholar * Marc Preuss View author publications You can also search for this author inPubMed
Google Scholar * Monique Winkler View author publications You can also search for this author inPubMed Google Scholar * Tobias P Dick View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS T.P.D. designed the research, supervised the experiments, analyzed data and wrote the manuscript; A.K. designed experiments, did most
experiments, analyzed data and contributed to the writing of the manuscript; M.P. did PDI experiments; and M.W. contributed to ERp57 depletion experiments. CORRESPONDING AUTHOR
Correspondence to Tobias P Dick. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES
Supplementary Figures 1–5 (PDF 1890 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kienast, A., Preuss, M., Winkler, M. _et al._ Redox regulation of
peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. _Nat Immunol_ 8, 864–872 (2007). https://doi.org/10.1038/ni1483 Download citation * Received:
26 October 2006 * Accepted: 30 May 2007 * Published: 01 July 2007 * Issue Date: August 2007 * DOI: https://doi.org/10.1038/ni1483 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative