- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Immunological and inflammatory reactions have been suggested to have a role in the development of schizophrenia, a hypothesis that has recently been supported by genetic data. The
aim of our study was to perform an unbiased search for autoantibodies in patients with a first psychotic episode, and to explore the association between any seroreactivity and the
development of a Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) disorder characterized by chronic or relapsing psychotic symptoms. We collected plasma samples
from 53 patients when they were treated for their first-episode psychosis, and 41 non-psychotic controls, after which the patients were followed for a mean duration of 7 years. Thirty
patients were diagnosed with schizophrenia, delusional disorder, schizoaffective disorder, bipolar disorder or a long-term unspecified nonorganic psychosis during follow-up, whereas 23
patients achieved complete remission. At the end of follow-up, plasma samples were analyzed for IgG reactivity to 2304 fragments of human proteins using a multiplexed affinity proteomic
technique. Eight patient samples showed autoreactivity to the N-terminal fragment of the PAGE (P antigen) protein family (PAGE2B/PAGE2/PAGE5), whereas no such autoreactivity was seen among
the controls. PAGE autoreactivity was associated with a significantly increased risk of being diagnosed with schizophrenia during follow-up (odds ratio 6.7, relative risk 4.6). An
immunohistochemistry analysis using antisera raised against the N-terminal fragment stained an unknown extracellular target in human cortical brain tissue. Our findings suggest that
autoreactivity to the N-terminal portion of the PAGE protein family is associated with schizophrenia in a subset of patients with first-episode psychosis. SIMILAR CONTENT BEING VIEWED BY
OTHERS AUTOANTIBODY PROFILES ASSOCIATED WITH CLINICAL FEATURES IN PSYCHOTIC DISORDERS Article Open access 13 September 2021 EXPLORING AUTOANTIBODY SIGNATURES IN BRAIN TISSUE FROM PATIENTS
WITH SEVERE MENTAL ILLNESS Article Open access 18 November 2020 SCREENING FOR PATHOGENIC NEURONAL AUTOANTIBODIES IN SERUM AND CSF OF PATIENTS WITH FIRST-EPISODE PSYCHOSIS Article Open access
05 November 2021 INTRODUCTION Psychiatric diagnostics is based almost exclusively on observed behavioral characteristics and self-reporting, with few existing biological correlates that are
clinically useful.1 The lack of refined diagnostic tools makes it difficult to distinguish clinically relevant subgroups of psychiatric disorders, although such stratification would
possibly improve treatment outcomes. In the case of schizophrenia, it is well established that aberrations in the dopamine system play an important role in the pathogenesis, in addition to a
strong underlying genetic component, and stress.2 Further, a growing body of literature suggests that immunology has a role in the development of the disease, at least in a subgroup of
patients.3 Some autoimmune diseases, including diabetes mellitus type 1, Grave’s disease and systemic lupus erythematosus, are more prevalent in patients with schizophrenia compared with the
general population.4 Others, such as rheumatoid arthritis, are rarer than would be expected.5 In addition, both positive and negative associations between human leukocyte antigen
polymorphisms and schizophrenia have been reported.3, 6, 7 To our knowledge, the first article on autoantibodies in patients with schizophrenia was published in 1937.8 Since then, many
studies have investigated autoreactivity and its possible association with psychosis. In conclusion, a majority of these studies show higher frequencies of autoantibodies in psychotic
patients compared with controls.7 Specifically, autoantibodies in serum directed to the N-methyl-D-aspartate receptor and the voltage-gated potassium channel have been reported in patients
with first-episode psychosis.9 However, a recent review article concluded that autoantibodies towards brain antigens may be equally prevalent in healthy controls.10 Hence, it remains unclear
whether autoimmunity has an etiopathological role in psychosis, and, if so, in all cases or in a subgroup of patients. In this study, we utilized a unique collection of thousands of protein
fragments generated within the Human Protein Atlas (www.proteinatlas.org), together with protein microarrays, to search for autoantibody reactivity in plasma samples. Analyzing 53 patients
with first-episode psychosis and 41 non-psychotic controls, our aims were to explore the association between autoantibodies and psychosis and to study whether any certain autoantibody
reactivity was associated with the severity of psychotic symptoms and/or the eventual establishment of any DSM-IV-defined disorder characterized by chronic or relapsing psychotic symptoms.
We hypothesized that a subgroup of patients would have a higher level of autoreactivity compared with the non-psychotic controls, and that pronounced autoreactivity would be associated with
increased symptom severity and chronification. MATERIALS AND METHODS EXPERIMENTAL STUDY DESIGN The study was carried out in four steps. First, we performed a cross-sectional study analyzing
plasma samples from 53 patients with first-episode psychosis and 41 controls, assessing differences in autoimmune reactivity by using an untargeted protein microarray set-up, and secondly,
confirming the results with a full-length protein in an enzyme-linked immunosorbent assay (ELISA) analysis. Thirdly, we conducted a clinical follow-up study including all 53 patients,
investigating whether autoimmune reactivity at admission was associated with symptom severity or could predict subsequent development of schizophrenia, delusional disorder, schizoaffective
disorder, bipolar disorder or long-term unspecified nonorganic psychosis. Fourthly, we performed a complementary immunohistochemistry analysis using rabbit antisera against the N-terminal
and C-terminal ends of the PAGE2B protein in 44 different human tissues. STUDY POPULATION Consecutive patients with first-episode psychosis were recruited from the Department of Psychiatry
(Psykiatri Sydväst), Karolinska University Hospital Huddinge, Sweden, between 2002 and 2010. Eligible patients were examined by a senior consultant psychiatrist. To be included in the study,
patients had to suffer from a first-episode psychosis based on the consensus of board-certified psychiatrists and have no previous history of psychosis, nor of any severe somatic illness
such as diabetes or heart failure. Psychotic symptoms as well as general psychiatric symptoms were evaluated through the Positive and Negative Syndrome Scale (PANSS) for schizophrenia.11 In
addition, blood samples were drawn and plasma was stored at −70 °C until use.12 The large majority of the patients with first-episode psychosis had been treated with antipsychotics for 1–3
weeks at the time of blood drawing. During the same time period, as the patient samples were collected, control subjects were recruited from a medical drop-in center located in the same
hospital and open to patients with non-severe injuries. A senior consultant psychiatrist examined the recruited control subjects by thorough clinical interviews, to verify that these had no
actual signs of psychosis nor history thereof, after which blood samples were collected and stored at −70 °C until use. All study participants gave informed written consent to participate
and the study was approved in 2001 by the local ethics committee in Stockholm, Sweden (reference number: 471/01). FOLLOW-UP The included psychosis patients were followed from the date of
study inclusion until the end of the study period (April 28, 2012), or death, at which point information on their current mental health status and psychiatric diagnoses determined during
follow-up was collected from medical records. Individuals were classified according to whether or not they had been diagnosed with an established DSM-IV disorder characterized by chronic or
relapsing psychotic symptoms during follow-up, defined as having a registered diagnosis of schizophrenia, delusional disorder, schizoaffective disorder, bipolar disorder or several
subsequent diagnoses of unspecified nonorganic psychosis. At the end of the study period, the stored plasma samples from patients (obtained during first-episode psychosis) and controls were
analyzed (see below). EXPERIMENTAL PROCEDURES PROTEOMIC ANALYSIS OF PLASMA SAMPLES USING PROTEIN FRAGMENTS The Human Protein Atlas13 hosts information about protein expression of the large
majority of all human protein-coding genes (www.proteinatlas.org). The database includes protein fragments designed based on bioinformatics analyses of each protein-coding gene, for which
one or several regions of 30–120 (average 82) amino acids have been selected because of their low sequence identity to other human proteins. The selected sequences were cloned, expressed,
purified and spotted on protein microarrays.14 For this study, we used 2304 protein fragments, representing 1812 protein-coding genes, from the Human Protein Atlas for the initial screening
for IgG autoantibody reactivity in plasma samples from the included patients and controls. As previously discussed,15 protein fragment on the antigen arrays was selected in an untargeted
manner in the order they were produced in the Human Protein Atlas project, where they are utilized for the generation of polyclonal rabbit antibodies and used also to validate the
specificity and selectivity. They were initially chosen based on their low sequence identity to other human proteins. The number of protein-coding genes per chromosome represented by the
antigens on the array correlate with the total number of protein-coding genes per chromosome and could be assumed to represent a small untargeted sample from the human proteome
(Supplementary Figure S1). In addition, another 92 protein fragments were selected based on findings from previous in-house profiling efforts and studies on neuroimmunology.16, 17, 18, 19,
20, 21, 22, 23 SCREENING FOR AUTOANTIBODIES ON PLANAR MICROARRAYS The use of planar microarrays for profiling of IgG autoantibody repertoires in each plasma sample was performed in
accordance with previous studies.14, 15, 24 In brief, each microarray glass slide was created by immobilizing 384 different protein fragments in 21 identical subarrays, enabling the analysis
of 21 samples in parallel on each slide. In total, six different collections of microarray slides were utilized, allowing binding analysis of 2304 human protein fragments. Plasma samples
were diluted 1:250 in assay buffer (1x phosphate-buffered saline (PBS) supplemented with 3% w/v bovine serum albumin Cohn fraction V (Saveen Werner, Limhamn, Sweden) and 5% w/v non-fat milk
powder (Semper, Sundbyberg, Sweden)), and 60 μl of each diluted sample was applied on the microarray slides for 75 min. After 75 min, the arrays were washed three times in PBST (1x PBS
supplemented with 0.1% v/v Tween-20) and a fluorescently labeled detection antibody goat anti-human IgG Alexa 647 (Invitrogen, Carlasbad, CA, USA) diluted 1:60 000 in PBST was applied for
another 75 min. Thereafter, the arrays were washed and dried before being scanned with a microarray scanner (G2565BA, Agilent, Santa Clara, CA, USA). The scanned image was analyzed using
GenePix Pro 5.1 (Molecular Devices, Sunnyvale, CA, USA). VALIDATION OF AUTOANTIBODIES BY SUSPENSION BEAD ARRAYS On the basis of the results from the screening on planar microarrays, 29
protein fragments were selected for further analysis and validation by suspension bead arrays. An additional set of 26 protein fragments, covering different regions of the same selected
protein targets, were included to extend the epitope coverage of the proteins of interest. Further, 92 protein fragments representing proteins that are suggested to be autoantibody targets
in previous neuroimmunology studies16, 17, 18, 19, 20, 21, 22, 23 or during previous in-house profiling efforts were included in the analysis for explorative purposes. Each protein fragment
was coupled to different color-coded carboxylated beads (MagPlex Microspheres, Luminex, Austin, TX, USA).15, 24, 25 The suspension bead array with covalently coupled protein fragments was
then transferred to the wells of a microtiter plate, incubated with 50 μl plasma diluted 1:250 in assay buffer (as described above) and supplemented with 160 μg ml−1 His6-ABP (fusion tag
present in all fragments). The plate was incubated on rotation for 1 h in dark and at room temperature. After 1 h, the plate was washed three times in 100 μl PBST (0.05% v/v), after which 50
μl detection antibody, R-phycoerythrin-conjugated goat anti-human IgG (Moss, Pasadena, MD, USA), at 1 μg ml−1, was added to each well and incubated for 30 min. The microtiter plate was
subsequently washed three more times, and fluorescent signals were measured using an Lx200 instrument (Luminex). EPITOPE MAPPING OF ANTIBODIES TOWARD PAGE2B On the basis of our previous
attempts of epitope-mapping seroreactivity to PAGE2B (data not shown), 15-mer peptides with one amino-acid lateral shift, covering the amino acids 5–32 of PAGE2B, were designed and obtained
(PEPscreen, Sigma-Aldrich, St Louis, MO, USA). The peptides were synthesized with biotin and a six-carbon linker in the N terminus. Coupling of peptides to color-coded carboxylated beads and
the epitope-mapping assay were performed as previously described.26, 27 In brief, neutravidin (ImmunoPure NeutrAvidin, Fisher Scientific, Hampton, NH, USA) was covalently coupled to
color-coded carboxylated beads. The biotinylated peptides were dissolved according to the manufacturer’s instructions and added to the color-coded carboxylated beads. Beads were then stored
at 4 °C until use. Upon analysis of the epitopes, all the color-coded carboxylated beads (with neutravidin) were pooled together, and 5 μl were added to plasma samples that had been
previously thawed, diluted 1:50 and pre-incubated in assay buffer (PBS supplemented with 5% bovine serum albumin and 10 μg μl−1 neutravidin) for 1 h. For the rabbit antisera toward the
N-terminal fragment of PAGE2B (HPA045952, www.proteinatlas.org), the dilution of the antibody was 1:3000. After an additional 1 h incubation, the beads were washed three times in 100 μl PBST
and incubated with anti-human IgG-PE (H10104, Invitrogen) and anti-rabbit IgG-PE (111-116-144, Jackson ImmunoResearch, West Grove, PA, USA), respectively, for 30 min and thereafter washed
three more times and fluorescent signals were measured using a FLEXMAP3D instrument (Luminex). VALIDATION BY ELISA WITH FULL-LENGTH PAGE2B PROTEIN VECTOR CONSTRUCTION FOR EXPRESSION OF
PAGE2B PROTEIN The full-length complementary DNA clone for PAGE2B was obtained as vector synthetic gene (pCMV6-PAGE2B-MycDDK, Prod No. RC220466, Origene, Rockville, MD, USA) and recloned
into the high-expression SFV-replicon vector, DREP-E2A-EGFP.28, 29 First, the PAGE2B segment of pCMV6-PAGE2B-MycDDK was PCR-amplified with primers adding the restriction sites _Xma_I and
_Spe_I at the 5′ and 3′ ends, respectively. The PCR product and the vector DREP-E2A-EGFP were digested using _Xma_I and _Spe_I, and the resulting fragments purified on agarose gel (GeneJet
gel extraction kit, ThermoFisher). The digested PCR product and vector were ligated using Quick Ligation (New England Biolabs, Ipswich, MA, USA), resulting in the vector DREP-E2A-PAGE2B. In
a second step, a double Strep-tag was added by PCR amplification from the plasmid pcScFv(XN)-1:7,30 and inserted at the _Xho_I and _Spe_I sites in the DREP-E2A-PAGE2B plasmid. The final
vector was called DREP-E2A-PAGE2B-Strep, coding for the peptide sequence of PAGE2B fused to double Strep-tags. EXPRESSION AND PURIFICATION OF PAGE2B PROTEIN Freestyle HEK 293 cells (Life
Technologies, Carlsbad, CA, USA) were grown at 37 °C, 8% CO2, 125 r.p.m. shaking. The cells were transfected with the DREP-E2A-PAGE2b-Strep vector using the 293fectin Transfection Reagent
(Life Technologies) using the manufacturer's protocol. Forty-eight hours post transfection, cells were harvested by centrifugation, followed by lysis for 40 min at room temperature in
200 μl Cell-lytic M (Sigma-Aldrich) per ml cell suspension. The lysate was spun down 1500 _g_ for 20 min and sterilized by 0.4 μm filtration (Merck Millipore, Billerica, MA, USA). For
affinity purification, the filtrate was loaded onto a StrepTrap HP 1 ml column (GE Healthcare, Little Chalfont, UK), and the protein was eluted with 2.5 mM desthiobiotin in PBS. The purity
and size of the protein was analyzed and confirmed by gel electrophoresis using the NuPAGE system with pre-cast 4–12% Bis-Tris gels (Life Technologies) in MOPS buffer, followed by Coomassie
staining and western blot. In the western blot analysis, two different rabbit antisera raised and purified against the C-terminal fragment of the PAGE2B protein (HPA052619,
www.proteinatlas.org) and the N-terminal fragment of the PAGE2B protein (HPA045952, www.proteinatlas.org) were used, and diluted 1:800 in PBST (0.05% v/v). Secondary detection reagents were
goat anti-rabbit Fab-AP (Pierce, Waltham, MA, USA) diluted to 2 μg ml−1 in PBST (0.05% v/v), or antibody directed toward the Strep-tag, Streptactin-AP (IBA, Göttingen, Germany) diluted
1:1000 in PBST (0.05% v/v). ELISA ANALYSIS The recombinant purified full-length PAGE2B protein was used to establish an ELISA. Each well was coated with 0.6 μg purified protein diluted in
50-μl 0.05 M carbonate–bicarbonate buffer (pH 9.6) and incubated overnight at 4 oC. The plate was washed once with PBST (0.05% v/v) and blocked for 1.5 h with 5% (w/v) bovine serum albumin
in PBS, and then washed three times with PBST (0.05% v/v) before incubation with the diluted plasma samples. Crude plasma samples were centrifuged at 3000 _g_ for 5 min and diluted
1:40–1:5120 in eight steps in PBS supplemented with 2.5% (w/v) bovine serum albumin in PBST (0.05% v/v). The diluted plasma samples were added to the wells coated with PAGE2B (as above), and
incubated at 4 °C overnight. The next day, three washings with PBST were performed, and alkaline phosphatase-conjugated goat anti-human Fc antibody (2 μg ml−1 in PBST, Pierce) added, and
the plates incubated for 2 h at room temperature. After three additional washes, 50 μl of the substrate Phos (Microwell Phosphatase Substrate System) was added and signals measured at 620
nm. IMMUNOHISTOCHEMISTRY ANALYSIS Expression of the PAGE2B protein was explored in 44 different normal human tissues within the Human Protein Atlas,31 using the two rabbit antisera
previously described (one raised against the C-terminal fragment of PAGE2B (HPA052619, www.proteinatlas.org) and the other raised against the N-terminal fragment (HPA045952)). Briefly,
formalin-fixed and paraffin-embedded tissue material was used, from which regions of interest were selected and collected into tissue microarrays and sectioned 4 μm thin. After baking, the
slides were deparaffinized and treated with heat-induced antigen retrieval (pH 6). Subsequently, the immunohistochemical protocol was performed as previously described,32 the primary
antibody incubated 30 min and the secondary antibody incubated 20 min, followed by 5 min 3,3'-diaminobenzidine. All reagents used for immunohistochemistry were manufactured by
ThermoFisher Scientific (Waltham, CA, USA) and Lab Vision (Fremont, CA, USA), and immunohistocehmistry was performed in Autostainer (ThermoFisher Scientific). Mayer’s Hematoxylin was used
for counterstaining (Histolab, Västra Frölunda, Sweden), and the results were digitalized with a scanning (Aperio Scanscope XT, Richmond, IL, USA). DATA ANALYSIS Two different statistical
approaches were applied to define the presence of seroreactivity in plasma samples analyzed on planar microarrays. First, each sample was evaluated by its median signal intensity in all
targets plus 5, 10, 15 and 20 times the quartile deviation, generating sample-specific cutoffs with varied stringency. Reactivities were then converted to binary signals and reactivity
frequencies were compared between samples using Fisher’s exact test. The second approach was based on the comparison of the median-normalized signals for each sample. The signals were
log2-transformed and compared between samples using the Mann–Whitney _U_-test. Antigens with significantly higher levels of seroreactivity across samples (the _P_-value cutoff set to 0.05
for Fisher’s exact test and 0.01 for the Mann–Whitney _U_-test) were selected for further analysis on suspension bead arrays. A similar approach was applied for analysis of the suspension
bead array data. In this case, we analyzed one well at a time, using the median signal intensity and the quartile deviation of each well as comparison. Significant differences were explored
using Fisher’s exact test. Linear epitopes were characterized from the suspension bead arrays, and the epitope sequences were searched for sequence similarity by BLASTP 2.2.29+ (ref. 33)
with default settings (see Supplementary Methods for further details). In the PAGE2B ELISA assay, the cutoff for positive samples was determined by the background-subtracted signals of the
11 negative control samples (C1–11) plus four times the s.d. at 1:40 dilution. One of the patient samples (P20) that showed a consistently strong reactivity to PAGE2B was used as internal
control to normalize between the different plates. Descriptive statistics was used to summarize characteristics of the included patients and controls. Differences in PANSS scores between
patients who subsequently achieved complete remission versus those who were diagnosed with different forms of established DSM-IV disorders characterized by chronic or relapsing psychotic
symptoms were analyzed by Welch’s _t_-test (the _P_-value set to 0.01). Odds ratios as well as relative risks for developing schizophrenia or any of the other DSM-IV disorders listed in
Table 1 were calculated in patients with autoreactivity to the PAGE protein family (PAGE2B/PAGE2/PAGE5). The significance level was set to 0.05. All analyses were performed in R (v. 3.3.1),
a language and software for statistical computing.34 RESULTS In this study, we performed an untargeted autoantibody profiling in 53 patients with first-episode psychosis and 41 non-psychotic
controls. After assessing autoreactivity at admission, patients were followed for a mean of 7 years with regard to disease progression, allowing us to study the association between
autoreactivity at admission and subsequent chronification of symptoms. Plasma autoreactivity was assessed in the following three steps: first, we screened for antibody reactivity toward 2304
protein fragments, representing 1812 human protein-coding genes, by using planar protein microarrays. Second, protein fragments to which the autoantibody reactivity was significantly
different in the patient- and control groups were used to generate a suspension bead array, in which the binding to these specific protein fragments was reassessed. Third, ELISA confirmed
binding with full-length protein. On the basis of the results, we performed an additional immunohistochemistry analysis of 44 different human tissues. CHARACTERISTICS OF STUDY PARTICIPANTS
AND RESULTS FROM FOLLOW-UP Clinical results are summarized in Table 1. The mean age at study inclusion was 28 and 33 years for patients and controls, respectively. Men were more prevalent in
both groups. The mean follow-up time was 7 years (range 2–12 years). Thirty out of fifty-three patients were diagnosed with a DSM-IV disorder characterized by chronic or relapsing psychotic
symptoms during follow-up, of which 14 individuals were diagnosed with schizophrenia. The mean PANSS score at study entry was 65. A subsequent diagnosis of schizophrenia was associated with
higher PANSS scores at study entry (Welch’s _t_-test _P_-value<0.01, Table 1). Further, a diagnosis of delusional disorder was associated with a lower score on the Negative Scale at
study entry (Welch’s _t_-test _P_-value<0.01, Table 1). RESULTS FROM AUTOANTIBODY SCREENING Autoantibodies were detected in the plasma samples of all study participants. The majority of
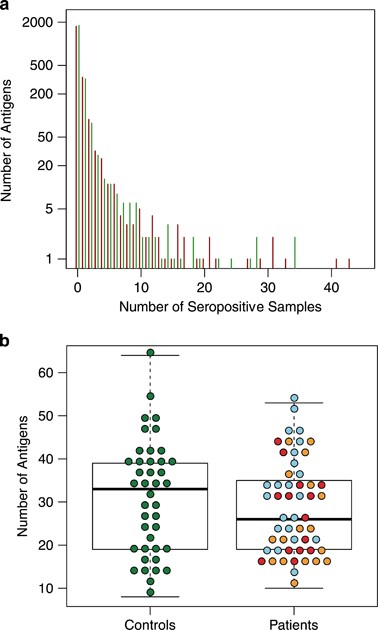
the 2304 screened protein fragments were targets for seroreactivity in single or very few individuals, whereas a few protein fragments were targets for seroreactivity in almost all plasma
samples (Figure 1a). The median level of seropositivity per individual was slightly higher in control subjects compared with patients with first-episode psychosis, although not significantly
(Figure 1b). In the screening on planar microarrays, we identified 29 antigens with differential seroreactivity in patients and controls. RESULTS FROM SUSPENSION BEAD ARRAYS Validation by
suspension bead array assays confirmed a significantly different pattern of seroreactivity in patients versus controls for three of the 29 antigens (Fisher’s test _P_-value<0.05; Figure
2a). Autoantibodies toward a fragment of the PAGE2B (P antigen family, member 2B) protein, representing its N-terminal region, were observed in eight patients with first-episode psychosis,
whereas no control subject presented with seropositivity toward the N-terminal fragment of PAGE2B (Figure 2b). Out of the mentioned eight patients, five were diagnosed with schizophrenia
during follow-up, whereas three achieved complete remission. The mean age at admission for first-episode psychosis of the five patients who developed schizophrenia was 23 years. For the two
other antigens, DST (dystonin) and AIDA (axin interactor, dorsalization associated), seroreactivity was more prevalent in the non-psychotic controls compared to patients with first-episode
psychosis (Figure 2a). SEQUENCE ALIGNMENT OF THE PAGE2B PROTEIN In an effort to explore potential targets of autoantibodies reactive to the N-terminal fragment of the PAGE2B protein, we
compared the sequence of PAGE2B to other human proteins. A high sequence identity to two other human proteins, PAGE2 and PAGE5, was found (Figure 3a). Both the N-terminal and the C-terminal
ends of PAGE2B matched PAGE2 and PAGE5 with high sequence identity. In fact, the N-terminal part of PAGE2B, which was the protein fragment used in our proteomic analyses, was found to be
identical to PAGE2, and only differed with regard to two amino acids compared with PAGE5. Furthermore, by epitope-mapping the eight patient samples that were seropositive for the N-terminal
fragment of PAGE2B (PAGE-N), we could identify linear epitopes, with the common sequence NDQESS (Figure 3a and Supplementary Figure S2a). Sequence similarity searches from BLASTP resulted in
only three matches with perfect identity match (Supplementary Table S1): PAGE2, PAGE2B and PAGE5. In addition, antisera toward the N-terminal region of PAGE2B (HPA045952,
www.proteinatlas.org) was epitope-mapped with the minimal epitope DQESSQP (Figure 3a and Supplementary Figure S2b), resulting in very similar results from the BLASTP searches (Supplementary
Table S2). Hence, it is important to note that these antibodies are likely also to be directed to PAGE2 and PAGE5. RESULTS FROM CONFIRMATORY ANALYSES IN ELISA WITH FULL-LENGTH PAGE2B PROTEIN
Full-length protein was successfully expressed and purified (Supplementary Figure S3), and used to establish an ELISA. Seven patients and one control (C30) were seropositive for the
full-length PAGE2B protein in the ELISA (Figure 3b). One patient (P47) who had not shown seropositivity to the N-terminal fragment of the PAGE2B protein in the array analyses was
seropositive to full-length PAGE2B protein. Further, two patients (P7 and P41) who had been seropositive to the N-terminal fragment of the PAGE2B protein in the previous experiments were
negative to full-length PAGE2B protein. The degree of binding correlated well between the suspension bead array assays and the ELISA (Figures 2b and 3b). Notably, the two plasma samples
positive to the whole protein, but not to the N-terminal fragment of PAGE2B (P47 and C30), were both positive to the C-terminal fragment of PAGE2B (Supplementary Figure S4). Whereas the
pattern of seroreactivity toward the N-terminal fragment of PAGE2B differed significantly between patients and controls, the pattern of seroreactivity toward the C-terminal fragment was
comparable in the two groups (data not shown). SEROREACTIVITY TO THE N-TERMINAL PORTION OF THE PAGE PROTEIN FAMILY Patients with first-episode psychosis who presented with autoantibodies
reactive to the N-terminal fragment of PAGE2B (representative for the PAGE2B, PAGE2 and/or PAGE5 proteins) at study inclusion had an increased risk of being diagnosed with a DSM-IV disorder
characterized by chronic or relapsing psychotic symptoms (odds ratio (OR) 1.33), in particular schizophrenia (OR 6.7, relative risk 4.6; Figures 4a and b). RESULTS FROM THE
IMMUNOHISTOCHEMISTRY ANALYSIS Polyclonal rabbit antisera raised to the N-terminal fragment of the PAGE2B protein and the C-terminal fragment of the PAGE2B protein, respectively, were applied
to a total of 44 different human tissues. We found that the antisera to the N-terminal fragment of PAGE2B mainly were reactive to unknown extracellular structures in the cerebral cortex,
whereas antisera to the C-terminal fragment of PAGE2B stained intracellular components of testicular cells (Figure 5). DISCUSSION We have assessed autoantibody repertoires in patients with
first-episode psychosis, and performed an untargeted screening toward thousands of autoantigens in this population of patients. We found that all study participants, including all
non-psychotic controls, showed some level of seroreactivity toward the 2304 examined protein fragments, representing 1812 human proteins. Although there was a trend toward a higher level of
seroreactivity in the control group, with two antigens with significant higher seroreactivity, DST and AIDA, the most significant finding was the antigen represented by the N-terminal
portion of the PAGE protein group (PAGE2B/PAGE2/PAGE5), detected in eight patients with first-episode psychosis. Autoantibodies toward this protein were associated with a fourfold increased
risk of a future diagnosis of schizophrenia. Immunohistochemistry studies showed that rabbit antisera raised to the N-terminal fragment of the PAGE2B protein mainly were reactive to
structures in the human cerebral cortex. Additional epitope-mapping experiments showed that the identified linear epitopes map to the PAGE protein family, and subsequent sequence similarity
searches showed highest match to PAGE protein family among the human proteins. Whereas the global screening for novel autoantibodies has long been hampered by the limited access to broad
representations of the proteome, we were able to use the unique collection of protein fragments generated within the Human Protein Atlas to perform an exploratory and unbiased screening for
novel autoantibody targets in psychosis. The long and complete clinical follow-up over an average period of 7 years allowed us to draw firm conclusions on disease progression, and to assess
the potential prognostic properties of the identified autoantibodies. There are, however, some important limitations to consider. Even if our cohort of patients with first-episode psychosis
has a reasonable size for a pilot study, 53 patients is still a limited number. As previously described,15 the different immobilization strategies of the antigens on the different protein
microarrays are likely to affect epitope recognition. The use of fragments from human proteins rather than full-length proteins could affect the observed autoantibody reactivities because of
the obvious risk of missing out potential interesting epitopes. This could be the reason for why the level of seroreactivity to included protein fragments that represented proteins
previously described in the literature as potential autoantigens in schizophrenia9, 17 was similar in patients and controls. The lack of stringent matching of patients and controls is
another limitation of this study, although the groups had similar age- and sex distributions and were all living in the same metropolitan area of Stockholm, Sweden. Furthermore, as we did
not control for socioeconomic status, differences in social and physical environments between patients and controls could have affected the outcome of this study.35 The mean age for the
patient group, 28 years, can look high, given that many psychotic disorders have their onset in late teens or early 20-ies. We note, however, that the five PAGE-N-seropositive patients who
developed schizophrenia had a mean age of 23 years, and a recently cohort study from another Scandinavian country reported that the peak age of schizophrenia onset was between 18 and 31
years, with the upper age limit stretching up to 55.36 Still, the mean age at onset in our patient cohort is high, for which we do not have a clear explanation. Lastly, it should be
acknowledged that the classification of patients as having achieved complete remission versus having developed a DSM-IV disorder characterized by chronic or relapsing psychotic symptoms, and
the subclassification into different diagnostic categories, was made based on diagnoses decided by the attending physician and thus retrieved from medical records, which may have impaired
the diagnostic reliability. In concordance with recent findings indicating that autoantibodies may be equally prevalent in healthy individuals,10 we found no significant difference in the
general level of seroreactivity in patients versus controls. Indeed, certain antibody specificities occurred primarily in the control group. This finding will warrant further studies of
whether some autoantibodies may be 'protective' or associated with better health. However, it can also be seen as a reflection of the overall high degree of individual
heterogeneity in human IgG reactivity profiles.37 Among the three types of autoantibodies with a significantly different distribution pattern in patients versus controls, autoantibodies
toward the N-terminal portion of the PAGE protein family (PAGE2B/PAGE2/PAGE5) were exclusively seen in patients with first-episode psychosis. The subset of patients who were seropositive for
the PAGE protein family had a significantly increased risk of being diagnosed with schizophrenia (relative risk 4.6 and OR 6.7). Although based on a relatively small set of patients, this
is an intriguing finding, especially considering that this observed association is considerably stronger than most known risk associations for single genetic elements implicated in the
development of schizophrenia.3, 38 The PAGE protein family is a broad but closely related group of proteins, mainly studied as tumor-associated molecules in the testis, ovaries and mammary
glands.39 Expression and immunohistochemistry data indicate the presence of PAGE proteins in malignancies in these tissues, but some reports have also suggested a low degree presence of
PAGE2B in the human cerebellum and cerebral cortex.40, 41 Previous immunohistochemistry analyses on normal tissue have only used rabbit antisera against the C-terminal fragment of PAGE2B,
which has stained an intracellular protein of testicular cells, the small intestine and other peripheral tissues (www.proteinatlas.org). Our immunohistochemistry analysis confirmed binding
to testicular cells by antisera to the C-terminal fragment of PAGE2B. However, we found that rabbit antisera immunized with the N-terminal fragment of PAGE2B mainly stained unknown
extracellular structures in the human cerebral cortex. We consider this an interesting finding, as we performed an unbiased screening for antigens without focusing on antigens of the central
nervous system (CNS). However, the data presented in this study are insufficient to claim that the PAGE2B protein is located in the CNS, as there is a possibility that the antisera are
staining a different antigen in the CNS, cross-reactive with the N-terminal fragment of PAGE2B. Autoantibodies to antigens in the CNS or peripheral neurons have been reported in several
neurological and psychiatric conditions. However, it is often unknown to what extent these antibodies are the direct cause for induction of symptoms.42 In myastenia gravis, autoantibodies
have a clear disease-causing effect by attacking the acetylcholine receptor,43 whereas in neuromyelitis optica, specific T-cell reactivity is thought to be required for the disease
development, in addition to antibodies to aquaporin-4.44, 45 Accordingly, putative autoantibodies may be markers for a more general immune reaction rather than representing specific
disease-causing agents. Several genetic elements with a role in inflammation and/or immunological reactions have previously been linked to schizophrenia.3, 6, 38 Similar to these risk genes,
disease-associated autoantibodies may exist in a subgroup of psychotic patients for whom the immunological component has a larger role in the etiopathology than for the remainder of
individuals with psychosis. Possibly, this subgroup would benefit from immune-suppressive treatments in addition to the standard neuroleptic regimen. Indeed, clozapine, the neuroleptic drug
recommended for patients who fail to respond to standard neuroleptics, has immunosuppressive effects.46 In conclusion, we found that a subgroup of patients with first-episode psychosis was
seropositive to the N-terminal portion of the PAGE protein family (PAGE2B/PAGE2/PAGE5), and that such seropositivity was associated with the development of schizophrenia. Our findings
indicate that these autoantibodies possibly could, if confirmed in larger cohorts, be used as biomarkers for the probability of disease progression in patients with first-episode psychosis,
and support the hypothesis that immune aberrations may contribute to the development of schizophrenia in a subgroup of patients. Additional studies are necessary to confirm these findings
and to elucidate whether PAGE2B, PAGE2, PAGE5 or another protein cross-reactive to the N-terminal end of PAGE2B is the critical autoantibody target, and to further disentangle its role in
the etiopathology of psychosis. We note that affinity proteomic tools can provide novel discoveries and bring important new knowledge in psychiatry. REFERENCES * Weickert CS, Weickert TW,
Pillai A, Buckley PF . Biomarkers in schizophrenia: a brief conceptual consideration. _Dis Markers_ 2013; 35: 3–9. Article Google Scholar * Howes OD, McCutcheon R, Owen MJ, Murray RM . The
role of genes, stress, and dopamine in the development of schizophrenia. _Biol Psychiatry_ 2016; 81: 9–20. Article Google Scholar * Schizophrenia Working Group of the Psychiatric Genomics
C. Biological insights from 108 schizophrenia-associated genetic loci. _Nature_ 2014; 511: 421–427. Article Google Scholar * Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M,
Mortensen PB . A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. _Am J Psychiatry_ 2014; 171:
218–226. Article Google Scholar * Sellgren C, Frisell T, Lichtenstein P, Landen M, Askling J . The association between schizophrenia and rheumatoid arthritis: a nationwide population-based
Swedish study on intraindividual and familial risks. _Schizophr Bull_ 2014; 40: 1552–1559. Article Google Scholar * Jones AL, Mowry BJ, Pender MP, Greer JM . Immune dysregulation and
self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? _Immunol Cell Biol_ 2005; 83: 9–17. Article CAS Google Scholar * Goldsmith CA, Rogers DP . The
case for autoimmunity in the etiology of schizophrenia. _Pharmacotherapy_ 2008; 28: 730–741. Article CAS Google Scholar * Lehmann-Facius H . Uber die liquordiagnose der schizophrenien.
_Klin Wochenschr_ 1937; 16: 1646–1648. Article Google Scholar * Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P _et al_. Disease-relevant autoantibodies in first episode
schizophrenia. _J Neurol_ 2011; 258: 686–688. Article Google Scholar * Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S _et al_. Seroprevalence of autoantibodies against
brain antigens in health and disease. _Anna Neurol_ 2014; 76: 82–94. Article CAS Google Scholar * Kay SR, Fiszbein A, Opler LA . The positive and negative syndrome scale (PANSS) for
schizophrenia. _Schizophr Bull_ 1987; 13: 261–276. Article CAS Google Scholar * Owe-Larsson B, Ekdahl K, Edbom T, Osby U, Karlsson H, Lundberg C _et al_. Increased plasma levels of
thioredoxin-1 in patients with first episode psychosis and long-term schizophrenia. _Progr Neuropsychopharmacol Biol Psychiatry_ 2011; 35: 1117–1121. Article CAS Google Scholar * Ponten
F, Jirstrom K, Uhlen M . The Human Protein Atlas—a tool for pathology. _J Pathol_ 2008; 216: 387–393. Article CAS Google Scholar * Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg
M, Andersson AC _et al_. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. _Proteomics_ 2005; 5: 4327–4337. Article CAS Google
Scholar * Ayoglu B, Haggmark A, Khademi M, Olsson T, Uhlen M, Schwenk JM _et al_. Autoantibody profiling in multiple sclerosis using arrays of human protein fragments. _Mol Cell Proteomics_
2013; 12: 2657–2672. Article CAS Google Scholar * Ganzinelli S, Borda T, Sterin-Borda L . Regulation of m1 muscarinic receptors and nNOS mRNA levels by autoantibodies from schizophrenic
patients. _Neuropharmacology_ 2006; 50: 362–371. Article CAS Google Scholar * Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT . Losing your nerves? Maybe it's the antibodies.
_Nat Rev Immunol_ 2009; 9: 449–456. Article CAS Google Scholar * Loukaides P, Schiza N, Pettingill P, Palazis L, Vounou E, Vincent A _et al_. Morvan's syndrome associated with
antibodies to multiple components of the voltage-gated potassium channel complex. _J Neurol Sci_ 2012; 312: 52–56. Article Google Scholar * Hanly JG, Urowitz MB, Siannis F, Farewell V,
Gordon C, Bae SC _et al_. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. _Arthritis
Rheum_ 2008; 58: 843–853. Article CAS Google Scholar * Schneider AS . Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. _Baillieres Best Pract Res Clin
Haematol_ 2000; 13: 119–140. Article CAS Google Scholar * Kimura A, Kanoh Y, Sakurai T, Koumura A, Yamada M, Hayashi Y _et al_. Antibodies in patients with neuropsychiatric systemic lupus
erythematosus. _Neurology_ 2010; 74: 1372–1379. Article CAS Google Scholar * Eber T, Chapman J, Shoenfeld Y . Anti-ribosomal P-protein and its role in psychiatric manifestations of
systemic lupus erythematosus: myth or reality? _Lupus_ 2005; 14: 571–575. Article CAS Google Scholar * Pritchard DB, Harris B . Aspects of perinatal psychiatric illness. _Br J Psychiatry_
1996; 169: 555–562. Article CAS Google Scholar * Haggmark-Manberg A, Zandian A, Forsstrom B, Khademi M, Lima Bomfim I, Hellstrom C _et al_. Autoantibody targets in vaccine-associated
narcolepsy. _Autoimmunity_ 2016; 49: 421–433. Article Google Scholar * Ayoglu B, Mitsios N, Kockum I, Khademi M, Zandian A, Sjoberg R _et al_. Anoctamin 2 identified as an autoimmune
target in multiple sclerosis. _Proc Natl Acad Sci USA_ 2016; 113: 2188–2193. Article CAS Google Scholar * Ayoglu B, Szarka E, Huber K, Orosz A, Babos F, Magyar A _et al_. Bead arrays for
antibody and complement profiling reveal joint contribution of antibody isotypes to C3 deposition. _PLoS ONE_ 2014; 9: e96403. Article Google Scholar * Zandian A, Forsstrom B,
Haggmark-Manberg A, Schwenk JM, Uhlen M, Nilsson P _et al_. Whole-proteome peptide microarrays for profiling autoantibody repertoires within multiple sclerosis and narcolepsy. _J Proteome
Res_ 2017; 16: 1300–1314. Article CAS Google Scholar * Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljestrom P . Enhancing immune responses using suicidal DNA vaccines. _Nat
Biotechnol_ 1998; 16: 562–565. Article CAS Google Scholar * Knudsen ML, Mbewe-Mvula A, Rosario M, Johansson DX, Kakoulidou M, Bridgeman A _et al_. Superior induction of T cell responses
to conserved HIV-1 regions by electroporated alphavirus replicon DNA compared to that with conventional plasmid DNA vaccine. _J Virol_ 2012; 86: 4082–4090. Article CAS Google Scholar *
Gilmartin AA, Lamp B, Rumenapf T, Persson MA, Rey FA, Krey T . High-level secretion of recombinant monomeric murine and human single-chain Fv antibodies from Drosophila S2 cells. _Protein
Eng Des Sel_ 2012; 25: 59–66. Article CAS Google Scholar * Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A _et al_. Tissue-based map of the human proteome.
_Science_ 2015; 347: 1260419. Article Google Scholar * Kampf C, Olsson I, Ryberg U, Sjöstedt E, Ponten F . Production of tissue microarrays, immunohistochemistry staining and
digitalization within the human protein atlas. _J Vis Exp_ 2012; 63: e3620. Google Scholar * Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W _et al_. Gapped BLAST and
PSI-BLAST: a new generation of protein database search programs. _Nucleic Acids Res_ 1997; 25: 3389–3402. Article CAS Google Scholar * Ihaka R, Gentleman R . R: a language for data
analysis and graphics. _J Comput Graph Stat_ 1996; 5: 299–314. Google Scholar * Calixto OJ, Anaya JM . Socioeconomic status. The relationship with health and autoimmune diseases. _Autoimmun
Rev_ 2014; 13: 641–654. Article Google Scholar * Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM _et al_. Is an early age at illness onset in schizophrenia
associated with increased genetic susceptibility? Analysis of data from the Nationwide Danish Twin Register. _EBioMedicine_ 2017; 18: 320–326. Article Google Scholar * Brodin P, Jojic V,
Gao T, Bhattacharya S, Angel CJ, Furman D _et al_. Variation in the Human Immune System Is Largely Driven by Non-Heritable. _Cell_ 2015; 160: 37–47. Article CAS Google Scholar *
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D _et al_. Common variants conferring risk of schizophrenia. _Nature_ 2009; 460: 744–747. Article CAS Google Scholar
* Gjerstorff MF, Ditzel HJ . An overview of the GAGE cancer/testis antigen family with the inclusion of newly identified members. _Tissue Antigens_ 2008; 71: 187–192. Article CAS Google
Scholar * Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA _et al_. An anatomically comprehensive atlas of the adult human brain transcriptome. _Nature_ 2012; 489:
391–399. Article CAS Google Scholar * Jones AR, Overly CC, Sunkin SM . The Allen Brain Atlas: 5 years and beyond. _Nat Rev Neurosci_ 2009; 10: 821–828. Article CAS Google Scholar *
Buckley C, Vincent A . Autoimmune channelopathies. _Nat Clin Pract Neurol_ 2005; 1: 22–33. Article CAS Google Scholar * Toyka KV, Brachman DB, Pestronk A, Kao I . Myasthenia gravis:
passive transfer from man to mouse. _Science_ 1975; 190: 397–399. Article CAS Google Scholar * Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR . IgG marker of optic-spinal
multiple sclerosis binds to the aquaporin-4 water channel. _J Exp Med_ 2005; 202: 473–477. Article CAS Google Scholar * Mitsdoerffer M, Kuchroo V, Korn T . Immunology of neuromyelitis
optica: a T cell-B cell collaboration. _Ann N Y Acad Sci_ 2013; 1283: 57–66. Article CAS Google Scholar * Leykin I, Mayer R, Shinitzky M . Short and long-term immunosuppressive effects of
clozapine and haloperidol. _Immunopharmacology_ 1997; 37: 75–86. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr Jan Mulder for his critical comments on this
manuscript and Professor Erik Jönsson for valuable discussions. This study was supported by grants from the Swedish Research Council (Medicine and Health Project, 521-2014-3857), KTH Center
of Applied Precision Medicine funded by the Erling-Persson Familiy Foundation, ProNova VINN Excellence Centre for Protein Technology (VINNOVA), Knut and Alice Wallenberg Foundation, IN-SENS
(FP7-PEOPLE-2013-ITN-607616) and the Stanley Medical Research Institute. AUTHOR INFORMATION Author notes * L Wingård Present address: 8Present address: Department of Medicine, Centre for
Pharmacoepidemiology, Karolinska Institutet, Karolinska University Hospital Solna, Stockholm, Sweden., * A Zandian and L Wingård: Co-first authors. * P Nilsson and M A A Persson: Shared
senior authors. AUTHORS AND AFFILIATIONS * Affinity Proteomics, SciLifeLab, School of Biotechnology, KTH—Royal Institute of Technology, Stockholm, Sweden A Zandian, D Just, C Hellström, M
Uhlén, J M Schwenk, A Häggmark-Månberg & P Nilsson * Department of Clinical Neuroscience, Karolinska Institutet at Center for Molecular Medicine, L8:01, Karolinska University Hospital
Solna, Stockholm, Sweden L Wingård, H Nilsson, D X Johansson & M A A Persson * Psykiatri Nordväst, SLSO, Karolinska University Hospital Solna, Stockholm, Sweden L Wingård & M A A
Persson * Department of Immunology, SciLifeLab, Genetics and Pathology, Uppsala University, Uppsala, Sweden E Sjöstedt * Department of Medicine, Karolinska Institutet at Center for Molecular
Medicine, L8:01, Karolinska University Hospital Solna, Stockholm, Sweden O Norbeck * Department of Clinical Neuroscience, Centre for Psychiatric Research, Karolinska Institutet, Section of
Psychiatry at Karolinska University Hospital Huddinge, Stockholm, Sweden O Norbeck & B Owe-Larsson Authors * A Zandian View author publications You can also search for this author
inPubMed Google Scholar * L Wingård View author publications You can also search for this author inPubMed Google Scholar * H Nilsson View author publications You can also search for this
author inPubMed Google Scholar * E Sjöstedt View author publications You can also search for this author inPubMed Google Scholar * D X Johansson View author publications You can also search
for this author inPubMed Google Scholar * D Just View author publications You can also search for this author inPubMed Google Scholar * C Hellström View author publications You can also
search for this author inPubMed Google Scholar * M Uhlén View author publications You can also search for this author inPubMed Google Scholar * J M Schwenk View author publications You can
also search for this author inPubMed Google Scholar * A Häggmark-Månberg View author publications You can also search for this author inPubMed Google Scholar * O Norbeck View author
publications You can also search for this author inPubMed Google Scholar * B Owe-Larsson View author publications You can also search for this author inPubMed Google Scholar * P Nilsson View
author publications You can also search for this author inPubMed Google Scholar * M A A Persson View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHORS Correspondence to P Nilsson or M A A Persson. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary
Information accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOCX 1788 KB) RIGHTS AND PERMISSIONS This work is licensed
under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the
material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zandian, A., Wingård, L., Nilsson,
H. _et al._ Untargeted screening for novel autoantibodies with prognostic value in first-episode psychosis. _Transl Psychiatry_ 7, e1177 (2017). https://doi.org/10.1038/tp.2017.160 Download
citation * Received: 25 May 2017 * Accepted: 01 June 2017 * Published: 25 July 2017 * Issue Date: July 2017 * DOI: https://doi.org/10.1038/tp.2017.160 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative






