- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In Asian honeybees, virgin queens typically only mate during a single nuptial flight before founding a colony. This behavior is controlled by the queen-released mandibular pheromone
(QMP). 9-oxo-(_E_)-2-decenoic acid (9-ODA), a key QMP component, acts as sex pheromone and attracts drones. However, how the queens prevent additional mating remains elusive. Here, we show
that the secondary QMP component methyl _p_-hydroxybenzoate (HOB) released by mated queens inhibits male attraction to 9-ODA. Results from electrophysiology and in situ hybridization assay
indicated that HOB alone significantly reduces the spontaneous spike activity of 9-ODA-sensitive neurons, and _AcerOr11_ is specifically expressed in sensilla placodea from the drone’s
antennae, which are the sensilla that narrowly respond to both 9-ODA and HOB. Deorphanization of _AcerOr11_ in _Xenopus_ oocyte system showed 9-ODA induces robust inward (regular) currents,
while HOB induces inverse currents in a dose-dependent manner. This suggests that HOB potentially acts as an inverse agonist against _AcerOr11_. SIMILAR CONTENT BEING VIEWED BY OTHERS
IDENTIFICATION OF AN ADDITIONAL PERIPLANONE RECEPTOR FAMILY GENE PREFERENTIALLY EXPRESSED IN THE MALE ANTENNAE OF THE AMERICAN COCKROACH Article Open access 31 January 2025 A MODULAR CIRCUIT
COORDINATES THE DIVERSIFICATION OF COURTSHIP STRATEGIES Article Open access 09 October 2024 MALE FIRE ANT NEUROTRANSMITTER PRECURSORS TRIGGER REPRODUCTIVE DEVELOPMENT IN FEMALES AFTER
MATING Article Open access 15 December 2021 INTRODUCTION A Virgin honeybee queen usually mates only once with several drones, and for the rest of her prolific life, she will not engage in
subsequent mating events1,2. The queen mandibular pheromone (QMP) plays a key role in regulating colony reproduction. QMP directly triggers the mating behavior and provides information about
the mating status of queens. It also inhibits the development of worker ovaries by changing relevant gene expression3. Several studies have focused on _Apis mellifera_ QMP, which consists
of four main components: 9-oxo-(_E_)-2-decenoic acid (9-ODA), (_R_, _S_)-9-hydroxy-(_E_)-2-decenoic acid (9-HDA), methyl _p_-hydroxybenzoate (HOB), and 4-hydroxy-3-methoxyphenyl-ethanol
(HVA)4. To date, only 9-ODA has been shown to have an attractive effect on drones3. In _A. cerana_, 9-ODA, 9-HDA, and HOB are also the dominant component of QMP, while HVA is absent4,5.
Behavioral evidence suggests that the QMP mixture lacking HVA in _A. cerana_ is sufficient to elicit retinue behavior in workers4, indicating a similar function of QMP in _A. cerana_ to that
in _A. mellifera_. However, the role of HOB remains poorly understood, as single secondary QMP components are not attractive to drones6,7. So far, the mechanism by which HOB alone regulates
mating behavior in _A. cerana_ has remained unknown. Olfactory sensing of QMP has been extensively studied in _A. mellifera_. At the peripheral olfactory system level, the primary QMP
component, 9-ODA, is detected by the placoid sensilla located on the drone antenna, which expresses the _A. mellifera_ odorant receptor 11 gene (_AmelOr11_)8. When expressed in the _Xenopus
laevis_ system, _AmelOr11_ exhibits a narrow response to 9-ODA and does not respond to other QMP components9. This suggests the involvement of other olfactory proteins in detecting QMP
secondary components. The predominant role of 9-ODA in mating is further supported by calcium imaging experiments at the antennal lobe level, where the _A. mellifera_ macroglomerulus MG2 in
drones is activated by 9-ODA10. Although _A. mellifera_ and _A. cerana_ share overall similar morphology and social behavior, they differ in their olfactory systems, as well as QMP
composition, the number of odorant receptors (Ors), and antennal lobe topology11. The QMP olfactory sensing in _A. cerana_ has received less attention compared to _A. mellifera_. In _A.
cerana_, odorant binding protein 11 (AcerOBP11) demonstrates strong binding affinities for both 9-ODA and HOB12. However, the specific ORs responsible for detecting 9-ODA and HOB in _A.
cerana_ remain unclear. Our transcriptome data showed that the 9-ODA receptor ortholog also exists in _A. cerana_, which shed a light on discovery the olfactory pathway on sex pheromone
sensing in Asian honeybees13. In this study, we found that HOB, released only by mated queens, significantly reduces the attraction of drones to 9-ODA. This inverse effect of HOB was further
validated by in vivo electrophysiological assays, electroantennography (EAG), and single sensillum recording (SSR). Lastly, we uncovered that, similar to the _A. mellifera_ orthologs,
_AcerOr11_ is robustly activated by 9-ODA, moreover, the secondary component HOB elicited reverse current fluxes, implying the existence of a dual coding mechanism at the QMP receptor,
_AcerOr11_. This study aimed to explore how QMP regulates reproduction at the olfactory sensing level of _A. cerana_. RESULTS QUEEN-RELEASED HOB REDUCES 9-ODA ATTRACTION TO DRONES To measure
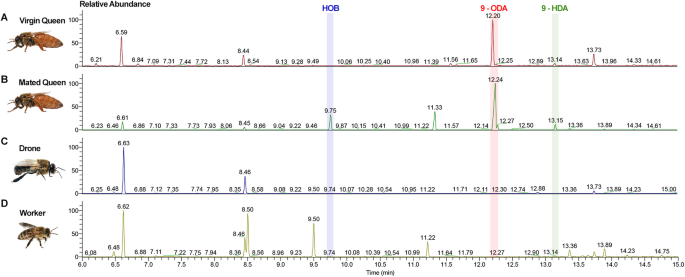
the contents of QMPs in _A. cerana_, we performed GC-MS analysis on 12- to 15-day-old virgin queens, mated queens as well as drones and workers. 9-ODA and 9-HDA, two main components in
QMPs, were detectable in both virgin and mated queens (Fig. 1A, B, Supplementary Fig. 1). However, interestingly, HOB was only detected in the mated queens and not in virgin queens (Fig.
1B). This inspired us that HOB might exercise some functions on the post-mating regulation. Notably, the amount of HOB released by mated queens was significantly smaller than that of 9-ODA
(Supplementary Table 1). Thus, we aimed to test the behavioral effects of HOB on drones, using the Y-tube olfactometer assay. First, we used 9-ODA as a stimulus and found that it has a
significant attractive effect on drones only at the highest concentration (100 μg) (_p_ < 0.05, two-tailed, T-test). At a lower concentration of 0.1–10 µg, the attraction effect of 9-ODA
was not significant (_p_ = 0.5614 for 0.1 μg; _p_ = 0.9580 for 1 μg; _p_ = 0.0668 for 10 μg) (Fig. 2A). While HOB alone did not elicit any effect on drones at any of the tested doses
(0.1–100 μg) (Fig. 2B). When we mixed 100 µg of 9-ODA with different concentrations of HOB (0.1–100 μg), we found that 9-ODA’s attraction was suppressed by HOB at concentration beyond 1 µg
(_p_ = 0.2302 for 1 μg; _p_ = 0.3771 for 10 μg; _p_ = 0.9414 for 100 μg) (Fig. 2C). This suggested that queens only released HOB after mating, which compromises the attraction to drones. HOB
INHIBITED 9-ODA NEURONS IN SENSILLA PLACODES To explore the physiological role of HOB in vivo, we conducted an EAG assay. First, we tested the olfactory response in the antenna to 9-ODA or
HOB alone in different castes. 9-ODA elicited significant EAG responses in drones at 100 µg (Fig. 3A); the normalized EAG response was 64.63 ± 2.08, which was nearly 64 times higher than
that of the negative control group (paraffin oil). The antenna of workers and queens only exhibited a mild response to 9-ODA (_N_ = 5–9, _p_ < 0.05, Wilcoxon signed-ranked test) (Fig. 3C,
Supplementary Fig. 2A, B). On the contrary, HOB did not elicit any significant antennal response in any castes (Fig. 3B and Supplementary Fig. 2C, D). We next stimulated the antenna with
9-ODA-HOB mixtures. The 9-ODA concentration was fixed to 100 µg for a saturated EAG response. We observed an inhibitory effect of HOB on the EAG response to 9-ODA (Supplementary Fig. 3) in a
dose-dependent manner. For mixtures with 0.1 and 1 µg HOB, the normalized EAG responses to 9-ODA decreased to 33.33 ± 4.34 and 20.05 ± 2.94, respectively (_N_ = 6–7, _p_ < 0.05, One-way
ANOVA followed by Turkey’s test), and the effects were significantly lower than those from 9-ODA alone (Fig. 3D). Intriguingly, HOB at 100 µg eliminated the effect of all tested 9-ODA
concentrations (1–100 µg; _N_ = 7, _p_ < 0.05) (Fig. 3E). To examine the in vivo effect of HOB on the ORN response, we conducted single sensillum recordings (SSRs). Three types of
chemosensory sensilla were observed in the _A. cerana_ drone’s antenna, including the sensilla trichodea, placodea, and basiconica. Sensilla placodea outnumbers the other two types of
sensilla. Of over 150 chemosensory sensilla tested with 9-ODA and HOB, only sensilla placodea showed responses to 9-ODA and HOB, and no response were detected in sensilla trichodea and
sensilla basiconica. Consistent with _A. mellifera_ studies8, three types of spike amplitudes were observed in _A. cerana_ sensilla placodea, suggesting the presence of three ORNs (neurons
A, B, and C) (Fig. 4A). When stimulated with 9-ODA, the activity of the A neurons was significantly increased compared to the negative control (_N_ = 5, _p_ < 0.001, two-tailed, T-test)
(Fig. 4B, C), when stimulated with HOB, the spontaneous activity of the A neuron was significantly reduced (_N_ = 5, _p_ < 0.001). This indicates the inverse effect of HOB. Intriguingly,
neither 9-ODA nor HOB changed the spontaneous activity of B and C neuron (Fig. 4D), suggesting that they were specifically acting on A neuron. We next tested the effect of the 9-ODA-HOB
mixture on A neurons. The presence of HOB inhibited 9-ODA-induced activity in A neurons (_N_ = 5, _p_ < 0.05) (Fig. 4E). Taken together, these results indicate that HOB inhibits not only
the antennal response to 9-ODA, but also the spontaneous firing in the absence of 9-ODA. Overall, these results implicating that HOB could act as an inverse agonist at the ORNs level.
_ACEROR11_ IS ABUNDANTLY EXPRESSED IN SENSILLA PLACODEA To check the expression profile of _AcerOr11_ underlying the physiological response to 9-ODA and HOB in the antenna, we conducted an
RT-qPCR survey of the _AmelOr11_ ortholog in _A. cerana_ in different tissues and castes. We found that _AcerOr11_ is abundantly expressed in drone antennae, while little expression was also
detected in the queen and worker antennae (Supplementary Fig. 4). To further determine the expression level and location of _AcerOr11_, we conducted in situ hybridization experiment. The
DIG-labeled riboprobes for _AcerOr11_ were applied to transversal antennal sections of bees from three castes. We found that many cells in the drone antenna expressed _AcerOr11_, which was
uniformly distributed from the F1 to the F11 segments (Fig. 5A, B). Only a few cells in workers’ antennae expressed _AcerOr11_, while _AcerOr11_ was fully undetectable in queens’ antennae
(Fig. 5C, D, L, M). No labeled cells were observed in the negative control group (Supplementary Fig. 5). _AcerOr11_-labeled areas were mainly distributed in dendrite-like structures of ORNs
housed in sensilla placodea (Fig. 5K). The _AcerOr11_ and 4’,6-diamidino-2-phenylindole (DAPI) labeled areas were neatly separated (Fig. 5N), suggesting that _AcerOr11_ is expressed in the
ORN cytoplasm instead of the nucleus. DUAL CODING OF _ACEROR11_ AGAINST 9-ODA AND HOB To identify the QMP ORs in _A. cerana_, we cloned _AcerOr11_, which is the 1:1 ortholog of _AmelOr11_
(9-ODA receptor in _A. mellifera_) and specifically expressed in sensilla placodea from drones9. We functionally expressed _AcerOr11_ in the _Xenopus laevi_s system and screened it with a
163-compound panel. AcerOr11 produced robust regular currents in response to increasing concentrations of 9-ODA (EC50 = 0.35 nM). Meanwhile, HOB elicited inverse currents (Supplementary Fig.
6) in a dose-dependent manner (EC50 = 150 nM) (Fig. 6A–D). To further confirm the inverse response of _AcerOr11_ to HOB, a current-voltage (I–V) curve was constructed using 9-ODA and HOB as
stimuli. The results showed that in the range of −80 to +40 mV, the slope of the 9-ODA-elicited I–V curves was much higher (9.729) than that of the baseline (4.015) (Fig. 6E). On the
contrary, HOB-generated I–V curves had a much lower slope (2.689) compared with the baseline (4.032) (Fig. 6F). These results validated that the presence of the agonist 9-ODA dramatically
increases the conductivity of the cell membrane, and channels are massively opened. Contrarily, the presence of HOB decreases the conductivity as much as in the resting state. We next
measured the TEVC response of the 9-ODA-HOB mixture under different dose ratios. We fixed the concentration of 9-ODA to 10−5 M for a saturated response while increasing the HOB concentration
from 10−6 M to 10−3 M. We found a clear dose-dependent inhibitory effect of HOB on 9-ODA activity (Supplementary Fig. 7). At 10−4 M or higher, HOB significantly inhibited 9-ODA-elicited
currents (_N_ = 4, _p_ < 0.05, One-way ANOVA followed by Turkey’s test). At 10−3 M HOB concentration, the AcerOr11 response to the 9-ODA-HOB mixture was 208.4 ± 11.8 nA, which was nearly
half of that induced by 9-ODA alone (445.6 ± 29.9 nA). Subsequently, we used 9-ODA alone for a series of concentration tests and found that the response to 9-ODA was recovered (Fig. 6G).
These results suggest that HOB inhibits the Or11 response to 9-ODA. Likewise, when HOB was at 10−3 M and 9-ODA was at lower doses (10−8 M and 10−7 M), the mixture still induced
depolarization (inverse) currents. However, once 9-ODA was raised to 10−6 M or higher, the response turned to inverse currents (Fig. 6H). DISCUSSION HOB, a pheromone signal only released by
mated queens, exerts a “stop mating” function on drones. While a few studies have also reported the presence of tiny levels of HOB in virgin queens14, most of the QMP studies, as well as
ours, showed HOB is fully absent in virgin queens4,15. To begin with, GC-MS analysis indicated that HOB was only released by mated queens in _A. cerana_, which is consistent with a report
about HOB in _A. mellifera_ queens4,7. In our case, HOB alone did not elicit any behavioral effect on drones, even at the highest doses, in the binary choice assay. However, it significantly
reduced the attraction of drones to 9-ODA. Therefore, HOB takes an inverse behavioral effect compared with 9-ODA in _A. cerana_. Previous study showed that the antennal-specific protein 1
(_Asp1_), has a high affinity for HOB, and the _Asp1_ expression level is positively correlated with colony sizes in both _A. cerana_ and _A. mellifera_16, these results support that HOB
alone could be detected by _Apis_ honeybees at a peripheral olfactory sensing level, and might play a crucial role in stop mating in honeybees. Our behavioral assay results indicate that HOB
directly inhibits the mating behavior of drones and might prevent the queens from consuming the energy for redundant mating. In insects, excitatory and inverse chemical signals are equally
important for maintaining the population dynamic. In _Helicoverpa armigera_, female moths release (Z)-11-Hexadecen-1-ol (Z11-16: OH) to repel males and avoid non-optimal mating17. Likewise,
in _Drosophila_, Gr8-associated alkenes inhibit courtship behaviors18. Collectively, these findings suggest that the inverse agonist may be widespread in insects. With a few exceptions,
honeybee queens do not remate in their lifetime19. Mated queens release HOB to prevent drones from multiple mating, which can help in avoiding resource waste due to multiple mating and
subsequent breeding. Interreceptor inhibition by semiochemicals at the sensillum level, a well-established phenomenon in _D. melanogaster_, is mediated by non-synaptic “lateral inhibitions”
between neurons located in the same sensilla, termed ephaptic coupling20,21. In mosquitoes, high concentrations of ammonia elicit atypical bursts of action potentials, followed by inhibition
in multiple adjacent ORNs22. In _Culex. quinquefasciatus_, eucalyptol significantly decreases the number of spikes in the ab7 sensillum23. Besides the interreceptor inhibition, here, we
show that HOB reduces the activity of 9-ODA at the same ORNs, supporting the hypothesis of the intrareceptor inhibition. Notably, HOB alone did not elicit any “normal” EAG response, which
negates the possibility of any HOB-activated ORs, i.e., HOB does not act as an agonist. Since HOB reduces spontaneous spikes, it had the opposite effect to 9-ODA, which induced the firing of
ORNs. We speculate that HOB acts as an inverse agonist to modulate mating behavior at the ORNs level. The _A. cerana AcerOr11_ gene is expressed in neurons specifically tuned to 9-ODA and
HOB. However, how those two opposite ligands affect the _AcerOr11_ receptor is unknown. Therefore, we cloned and expressed _AcerOr11_ in the _Xenopus_ oocyte system, which exhibited robust
responses to 9-ODA while HOB evoked a reverse, concentration-dependent current. These results again indicated that HOB acts as an inverse agonist of _AcerOr11_. Inverse agonists have been
identified in various receptor-ligand interactions, including GABAA, melanocortin receptors, mu-opioid receptors, adrenoceptors, and histamine receptors24,25,26,27. Currently, the concept of
inverse agonists in insect ORs is poorly understood and much less studied. Some experimental evidence suggests that insect ORs have specific inverse agonists. For example, in _C.
quinquefasciatus_, OR32 produces regular currents when stimulated with methyl salicylate, while eucalyptol elicits inverse currents, suggesting that eucalyptol might be an inverse agonist23.
In _Aedes aegypti_, _AaegOR8_ was found to be sensitively tuned to (_R_)‑1‑octen‑3‑ol while the structurally unrelated odorant indole inhibited octenol-activated _OR8_ and repelled
mosquitoes28. The potential mechanism of inverse currents can be speculated in three stages: when the receptor is challenged, the agonist interacts with the ligand binding site, triggering
an inward current, while the antagonist blocks the binding site to prevent the agonist binding while maintaining spontaneous activity, and inverse agonist “snare” receptor to inactiveness.
Our results suggest that HOB to _AcerOr11_ generates an additional signal coding, which may execute more complex regulation instead of a simple “on-off” function. Agonist-inverse agonist
combinations may elicit opposite physiological effects. For example, the mushroom psychoactive compound muscimol induces a relaxing effect by activating the GABAA receptor. On the other
hand, beta-carbolines act as inverse agonists and cause convulsive or anxiogenic effects24. Similarly, 9-ODA promotes mating behavior, while HOB inhibits mating. In summary, our results
suggest that mating in honeybees is modulated by the two key QMP components, 9-ODA and HOB. _AcerOr11_ is not merely an on/off switch but rather functions as a molecular olfactory dimmer.
METHODS HONEYBEES Honeybees (_A. cerana_) used in this study were provided by the Jilin Provincial Institute of Apicultural Sciences (JLAS), China. The bee colony was originally collected
from Dunhua, China (43° 51′ 46′′ N, 128° 20′ 30′′ E) and has been reared since 2020 in the conservation area of JLAS in a natural environment. Before beginning the experiments, the honeybees
were cultured in an artificial incubator (Boxun, China) at 30 °C, 70% humidity, with a 16-h photoperiod. Adult bees were fed with a 10% sucrose solution. GAS CHROMATOGRAPHIC-MASS
SPECTROMETRY (GC-MS) ANALYSIS Heads from the virgin queens, mated queens (mated on day 6 or 7), drones, and workers were collected from 12- to 15-day-old honeybees. The compounds of the
mandibular gland were extracted by placing the heads in 200 μL dichloromethane for at least 24 h. The extracts were then dried under a stream of nitrogen, and the remainders were dissolved
in 20 μL internal standard solution (octanoic acid and tetradecane in dichloromethane) and 20 μL N,O-Bis (trimethylsilyl) trifluoroacetamide. The mandibular gland pheromone mixes were
separated by a GC-MS system (Thermo Fisher Scientific, USA), which was equipped with an HP-INNOWax capillary column, in the split-less mode on a methyl silicone-coated fused silica column
(HP - 1MS, 25 m × 0.20 mm × 0.33 µm). Helium gas was used as a carrier gas at a constant flow rate of 1 mL/min. The oven temperature was set to 100 °C for 2 min and then increased to 250 °C
at a rate of 10 °C per minute. The final temperature was maintained for 10 min. The compounds were identified by comparing their retention times and mass fragmentation with the known
reference compounds. 9-ODA and HOB were further quantified by injecting corresponding standard compounds. BEHAVIORAL ASSAY To explore the biological effect of 9-ODA and HOB on drones, we
designed a binary-choice Y-tube olfactometer assay. Briefly, 10 μL of a test compound solution was applied to a 25 × 15 mm filter paper and then placed in one arm of the olfactometer (15 cm
base, 10 cm arm length, and 2 cm diameter) as the odorant source. The solvent in the other arm was the mock control. Bee responses within 5 min were scored as “made a choice” when an
individual moved at least 2/3 into one arm. More than thirty honeybees were used in each behavioral assay for a series of test compound concentrations. ELECTROANTENNOGRAPHY RECORDING In the
EAG assay, the tip of the honeybee antennae was cut and covered with a conductive gel (Parker Laboratories Inc., USA), and the honeybee head was attached to the reference electrode. In a
preliminary test, we found that both 9-ODA and HOB are difficult to dissolve in hexane. Thus, we first dissolved them in ethanol and then diluted them with paraffin oil to the desired dose.
Ethanol alone, diluted with paraffin oil, was used as a negative control. A 10 μL stimulus was loaded onto a 5.0 × 0.5 cm filter paper strip and then inserted into a syringe with a
continuous flow of 500 mL/min and an air humidity of 60–70%. The pulse flow duration was 0.2 s, and the antenna response was recorded for 5 s. To ensure EAG sensitivity restoration, we had
1-min gaps between two stimulations. For the EAG inhibition test, 9-ODA and HOB were first separated, and then the two pulse flows were mixed at the end of the tube before puffing against
the _A. cerana_ antennae. The negative control was performed both at the beginning and the end of each preparation. The EAG data were normalized using the negative control data. SINGLE
SENSILLUM RECORDING In the SSR test, 12- to 15-day-old drones were wedged into a 1 mL plastic pipette tip, and the protruding head was fixed to the rim of the pipette tip with dental wax.
One of the exposed antennae was stuck to a coverslip with double-sided tape under a microscope (LEICA Z16 APO, Germany). The reference tungsten electrode was inserted into the eye, and
spikes were recorded by inserting the tungsten electrode into the base of a sensillum until a stable electrical signal with a high signal-to-noise ratio was achieved. For stimulus delivery,
10 μL of the QMP component was added on a 1 cm × 2.5 cm filter paper strip and then inserted into a Pasteur pipette. A flow of purified and humidified air (2 L/min) was continuously
maintained on the antennae through a 14-cm-long metal tube controlled (Syntech Hilversum, Netherlands) by a Syntech stimulus controller (CS-55 model, Syntech, Germany). The two antennae were
exposed to a stimulus for 500 ms with airflow of 0.6 L/min through a Pasteur pipette. The action potential signals were amplified using a pre-amplifier (IDAC-4 USB System, Syntech, Germany)
and visualized by the Autospike 32 software (Syntech, Germany). The number of induced spikes were calculated as the subtraction from the firing spike number by spontaneous spikes number
before the stimulus. IN SITU HYBRIDIZATION Antisense and sense digoxigenin- and biotin-labeled riboprobes of _AcerOr11_ were synthesized using linearized pGEMHE plasmids containing
appropriate insertion sequences as a template using the DIG and Biotin RNA Labeling Mix (Roche, Germany) and T7 RNA Polymerase (Roche, Germany). Subsequently, the probe was digested into
approximately 400 base fragments by incubating in carbonate buffer (80 mM NaHCO3, 120 mM Na2CO3, pH 10.2). Antennae of 12- to 15-day-old honeybees of three castes were collected and then
embedded in a Tissue-Tek optimal cutting temperature compound (Sakura Finetek, USA). Longitudinal and transverse sections (10 µm thick) through antennae were prepared using the Cryostar NX50
cryostat (ThermoFisher, USA) at −25 °C. The sections were thaw-mounted on adhesive microscope slides (Citotest, China) and immediately utilized for in situ hybridization experiments.
Tissues were fixed in 4% paraformaldehyde, and slides were washed with phosphate-buffered saline (PBS) buffer and 0.6% HCl respectively. For pre-hybridization, slides were immersed in 50%
formamide with 2× saline-sodium citrate (SSC) for 1 h at 60 °C. Afterward, the slides were added with 100 μL of the hybridization buffer containing the labeled probe for _AcerOr11_ and
incubated at 60 °C for a minimum of 16 h. After hybridization, slides were washed in 0.2× SSC, followed by treatment with a 1% blocking solution (Roche, Germany) prepared in tris-buffered
saline (TBS) buffer with 0.03% Triton X-100. Anti-Digoxigenin-AP, Fab fragments (catalog number 11093274910, Roche, Germany) and NBT/BCIP (Roche, Germany) were used to detect the DIG-labeled
probe under an Upright Microscope BX51 (Olympus, Japan). Anti-Digoxigenin-Fluorescein, Fab fragments (catalog number 11207741910, Roche, Germany) were used to detect the biotin-labeled
probe, and the fluorescence signals were visualized under a Zeiss LSM 880 confocal microscope (Zeiss, Jena, Germany) using excitation at 550 nm. RNA EXTRACTION, GENE CLONING, AND
QUANTITATIVE PCR RNA samples from different tissues, including the chemosensory organs (antenna, proboscis) and non-chemosensory body parts (thorax, abdomen, and legs), were collected from
15 bees per caste. Total RNA was extracted using the TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocols. The concentration and purity of the extracted RNA were measured
by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and 1% agarose gel electrophoresis, respectively. For PCRs, we used gene-specific primers for _AcerOrco_ and _AcerOr11_
(Supplementary Table 2). First-strand cDNA synthesis was performed using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China). PCR was
performed with the TSINGKE TSE101 PCR enzyme mix (TsingKe Biotech, Beijing, China) at the following conditions: 2 min at 98 °C; followed by 35 cycles of 98 °C for 10 s, 50–60 °C for 10 s,
and 72 °C for 20 s; and final extension for 5 min at 72 °C. PCR-amplified products were examined and gel purified using the SanPrep Column DNA Gel Extraction Kit (Sangon Bio, Shanghai,
China). The purified PCR products were subcloned into a pGEMHE vector between the BamHI and HindIII restriction sites using the pEASY-Uni Seamless Cloning and Assembly Kit (Transgen Biotech,
Beijing, China). For the qPCR assay, the cDNA sample was quantified using 1 μg of total RNA, and _β-actin_ (GenBank accession: HM640276.1) was used as an internal control gene. Primers for
_AcerOr11_ and _AcerOrco_ were designed using Primer 3 (Supplementary Table 2). RT-qPCR was conducted on a LightCycler 480 II Detection System (Roche, Switzerland) with TransStar Tip Top
Green qPCR Supermix (Transgen Biotech, China) at the following conditions: 94 °C for 30 s, followed by 45 cycles of 94 °C for 5 s, 55 °C for 15 s, and 72 °C for 10 s. qPCR data were analyzed
by the 2-ΔΔCT method. DEORPHANIZATION OF ACERORS IN THE _XENOPUS_ OOCYTE SYSTEM The cRNAs with the templates, the linearized pGEMHE vector containing of _AcerOrco_ and _AcerOr11_, using the
mMESSAGE mMACHINE T7 Kit (Ambion, USA) following the manufacturer’s instructions. The cRNAs were adjusted concentration of 200 ng/μL in nuclease-free water and 18.4 nL of _AcerOr11_ with
same amount of _AcerOrco_ cRNAs were microinjected into _Xenopus laevis_ oocytes at vegetal pole in stages V or VI using a NanoLiter 2000 injector (World Precision Instruments, Sarasota,
USA). Subsequently, oocytes were incubated at 18 °C for 2–8 days in Barth’s solution (96 mM NaCl, 2 mM KCl, 5 mM MgCl2, 0.8 mM CaCl2, and 5 mM HEPES; pH 7.6) supplemented with 50 μg/mL
tetracycline, 100 μg/mL streptomycin, and 500 μg/mL sodium pyruvate. A two-electrode voltage-clamp (TEVC) technique was used to record the ion channel-induced currents in _Xenopus_ oocytes
at a holding potential of −80 mV. For I–V curves, the holding potentials were held between −80 and +40 mV. Signals were amplified with an Axonclamp 900 A amplifier (Molecular Devices, San
Jose, USA). Data acquisition and analysis were performed using Axon Digidata 1550B and pCLAMP10 software, using 50 Hz low-pass filters and digitization at 1 kHz (Molecular Devices, USA). The
stock solutions (1 M) of all compounds were prepared in DMSO and then diluted with Ringer buffer. Data collected in TEVC were analyzed by Clampfit 10 software. _AcerOr11_ expressed ORs were
deorphanized against a panel of 163 odorants, including honeybee pheromones and plant volatiles (Supplementary Table 3). The I–V curves were measured by applying a series of voltages, −80,
−60, −40, −20, 0, +20, and +40 mV to the tested eggs and current changes were observed. STATISTICS AND REPRODUCIBILITY The significant difference of behavioral assay and SSR were analyzed by
using T-test (two-tailed), Wilcoxon signed-ranked test was used for dose-dependent curve in EAG, the inhibitory effect of HOB in TEVC and EAG were analyzed by one-way ANOVA followed by
Tukey’s multiple comparison test (_p_ < 0.05) after checking the normality and homogeneity of variance. All the statistic were performed by SPSS v25.0 (IBM) and visualized by GraphPad
Prism v8.0 (GraphPad Software). REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All
relavant data linked to manuscript are available in Supplementary materials and the raw data are available from corresponding author on reasonable request. REFERENCES * Butler, C. G. The
mating behavior of the honeybee (_Apis mellifera_ L.). _J. Entomol._ 46, 1–11 (2009). Google Scholar * Gary, N. E. & Marston, J. Mating behaviour of drone honey bees with queen models
(_Apis mellifera_ L.). _Animal Behav._ 19, 299–304 (1971). Article Google Scholar * Sandoz, J. C., Deisig, N., de Brito Sanchez, M. G. & Giurfa, M. Understanding the logics of
pheromone processing in the honeybee brain: from labeled-lines to across-fiber patterns. _Front. Behav. Neurosci._ 1, 5 (2007). Article PubMed PubMed Central Google Scholar * Plettner,
E. et al. Species- and caste-determined mandibular gland signals in honeybees (_Apis_). _J. Chem. Ecol._ 23, 363–377 (1997). Article CAS Google Scholar * Keeling, C. I., Otis, G. W.,
Hadisoesilo, S. & Slessor, K. N. Mandibular gland component analysis in the head extracts of _Apis cerana_ and _Apis nigrocincta_. _Apidologie_ 32, 243–252 (2001). Article CAS Google
Scholar * Pankiw, T. et al. Mandibular gland components of European and Africanized honey bee queens (_Apis mellifera_ L.). _J. Chem. Ecol._ 22, 605–615 (1996). Article CAS PubMed Google
Scholar * Keeling, C. I., Slessor, K. N., Higo, H. A. & Winston, M. L. New components of the honey bee (_Apis mellifera_ L.) queen retinue pheromone. _Proc. Natl Acad. Sci. USA_ 100,
4486–4491 (2003). Article CAS PubMed PubMed Central Google Scholar * Kaissling, K. E. & Renner, M. Antennale Rezeptoren für Queen Substance und Sterzelduft bei der Honigbiene. _Z.
Vgl. Physiol._ 59, 357–361 (1968). Article Google Scholar * Wanner, K. W. et al. A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. _Proc. Natl Acad. Sci. USA_
104, 14383–14388 (2007). Article CAS PubMed PubMed Central Google Scholar * Sandoz, J. C. Odour-evoked responses to queen pheromone components and to plant odours using optical imaging
in the antennal lobe of the honey bee drone _Apis mellifera_ L. _J. Exp. Biol._ 209, 3587–3598 (2006). Article CAS PubMed Google Scholar * McKenzie, S. K., Fetter-Pruneda, I., Ruta, V.
& Kronauer, D. J. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. _Proc. Natl Acad. Sci. USA_ 113,
14091–14096 (2016). Article CAS PubMed PubMed Central Google Scholar * Song, X. et al. Various bee pheromones binding affinity, exclusive chemosensillar localization, and key amino acid
sites reveal the distinctive characteristics of odorant-binding protein 11 in the eastern honey bee, _Apis cerana_. _Front. Physiol._ 9, 422 (2018). Article PubMed PubMed Central Google
Scholar * Ke, H. et al. Odorant receptors expressing and antennal lobes architecture are linked to caste dimorphism in Asian Honeybee, _Apis cerana_ (Hymenoptera: Apidae). _Int. J. Mol.
Sci_. 25, 3934 (2024). * Strauss, K. et al. The role of the queen mandibular gland pheromone in honeybees (_Apis mellifera_): honest signal or suppressive agent? _Behav. Ecol. Sociobiol._
62, 1523–1531 (2008). Article Google Scholar * Villar, G., Hefetz, A. & Grozinger, C. M. Evaluating the effect of honey bee (_Apis mellifera_) queen reproductive state on
pheromone-mediated interactions with male drone bees. _J. Chem. Ecol._ 45, 588–597 (2019). Article CAS PubMed Google Scholar * Wu, F. et al. Differences in ASP1 expression and binding
dynamics to queen mandibular pheromone HOB between _Apis mellifera_ and _Apis cerana_ workers reveal olfactory adaptation to colony organization. _Int. J. Biol. Macromol._ 217, 583–591
(2022). Article CAS PubMed Google Scholar * Chang, H. et al. A pheromone antagonist regulates optimal mating time in the moth _Helicoverpa armigera_. _Curr. Biol._ 27, 1610–1615 (2017).
Article CAS PubMed Google Scholar * Vernier, C. L. et al. A pleiotropic chemoreceptor facilitates the production and perception of mating pheromones. _iScience_ 26, 105882 (2023).
Article CAS PubMed Google Scholar * Boomsma, J. J., Baer, B. & Heinze, J. The evolution of male traits in social insects. _Annu. Rev. Entomol._ 50, 395–420 (2005). Article CAS
PubMed Google Scholar * Su, C., Menuz, K., Reisert, J. & Carlson, J. Non-synaptic inhibition between grouped neurons in an olfactory circuit. _Nature_ 492, 66–72 (2012). Article CAS
PubMed PubMed Central Google Scholar * Zhang, Y. et al. Asymmetric ephaptic inhibition between compartmentalized olfactory receptor neurons. _Nat. Commun._ 10, 1560 (2019). Article
PubMed PubMed Central Google Scholar * Clark, J. T. et al. Chemosensory detection of aversive concentrations of ammonia and basic volatile amines in insects. _iScience_ 26, 105777 (2023).
Article CAS PubMed Google Scholar * Xu, P. et al. Odorant inhibition in mosquito olfaction. _iScience_ 19, 25–38 (2019). Article CAS PubMed PubMed Central Google Scholar *
Sieghart, W. Pharmacology of benzodiazepine receptors: an update. _J. Psychiatry Neurosci._ 19, 24–29 (1994). CAS PubMed PubMed Central Google Scholar * Ollmann, M. M., Lamoreux, M. L.,
Wilson, B. D. & Barsh, G. S. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. _Genes Dev._ 12, 316–330 (1998). Article CAS PubMed PubMed Central
Google Scholar * Wang, D., Raehal, K., Bilsky, E. & Sadee, W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic
dependence. _J. Neurochem._ 77, 1590–1600 (2001). Article CAS PubMed Google Scholar * Khilnani, G. & Khilnani, A. K. Inverse agonism and its therapeutic significance. _Indian J.
Pharmacol._ 43, 492–501 (2011). Article CAS PubMed PubMed Central Google Scholar * Dekel, A., Sar-Shalom, E., Vainer, Y., Yakir, E. & Bohbot, J. D. The ovipositor cue indole
inhibits animal host attraction in _Aedes aegypti_ (Diptera: Culicidae) mosquitoes. _Parasit. Vectors_ 15, 422 (2022). Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by the National Key Research and Development Program (2023YFE0113600), Israel Science Foundation grants (No. 719/21). We thank Dr. Pingxi
Xu (Department of MCB, UC Davis) for critically reading an earlier manuscript draft; Dr. Yuanhong Wang (School of Chemistry, Northeast Normal University) for the help with GC-MS analysis;
Xiuli Wang and Fan Zhang (School of Life Sciences, Northeast Normal Univerisity) for assistance in in situ hybridization; Dr. Baiwei Ma (Chinese Academy of Agricultural Sciences), Dr. Shuai
Liu (Department of Plant Protection, Jilin Agricultural University) and Cuiwei Liu for the guidance in SSR experiments. AUTHOR INFORMATION Author notes * These authors contributed equally:
Haoqin Ke, Jonathan D. Bohbot. AUTHORS AND AFFILIATIONS * Key Laboratory of Vegetation Ecology, MOE, Northeast Normal University, Changchun, China Haoqin Ke, Shiwen Duan, Xiaomei Ma,
Bingzhong Ren & Yinliang Wang * Department of Entomology, The Hebrew University of Jerusalem, The Robert H. Smith Faculty of Agriculture, Food and Environment, Rehovot, Israel Jonathan
D. Bohbot * Apiculture Science Institute of Jilin Province, Jilin, China Yongjuan Chi Authors * Haoqin Ke View author publications You can also search for this author inPubMed Google Scholar
* Jonathan D. Bohbot View author publications You can also search for this author inPubMed Google Scholar * Yongjuan Chi View author publications You can also search for this author
inPubMed Google Scholar * Shiwen Duan View author publications You can also search for this author inPubMed Google Scholar * Xiaomei Ma View author publications You can also search for this
author inPubMed Google Scholar * Bingzhong Ren View author publications You can also search for this author inPubMed Google Scholar * Yinliang Wang View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W. and J.D.B. designed this study; H.K., S.D., and X.M. performed this research; C.X. provided and reared the honeybee colony;
Y.W. and H.K. analyzed the data; Y.W., J.D.B., and B.R. wrote and revised manuscript. CORRESPONDING AUTHORS Correspondence to Bingzhong Ren or Yinliang Wang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Robert Hanus and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. Primary Handling Editor: Luke R. Grinham. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ke, H., D. Bohbot,
J., Chi, Y. _et al._ The dual coding of a single sex pheromone receptor in Asian honeybee _Apis cerana_. _Commun Biol_ 7, 502 (2024). https://doi.org/10.1038/s42003-024-06206-5 Download
citation * Received: 27 December 2023 * Accepted: 17 April 2024 * Published: 25 April 2024 * DOI: https://doi.org/10.1038/s42003-024-06206-5 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative