- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT microRNAs (miRNAs) are crucial for normal development and physiology. To identify factors that might coordinate with miRNAs to regulate gene expression, we used 2′O-methylated
oligonucleotides to precipitate _Caenorhabditis elegans_ let-7, miR-58, and miR-2 miRNAs and the associated proteins. A total of 211 proteins were identified through mass-spectrometry
analysis of miRNA co-precipitates, which included previously identified interactors of key miRNA pathway components. Gene ontology analysis of the identified interactors revealed an
enrichment for RNA binding proteins, suggesting that we captured proteins that may be involved in mRNA lifecycle. To determine which miRNA interactors are important for miRNA activity, we
used RNAi to deplete putative miRNA co-factors in animals with compromised miRNA activity and looked for alterations of the miRNA mutant phenotypes. Depletion of 25 of 39 tested genes
modified the miRNA mutant phenotypes in three sensitized backgrounds. Modulators of miRNA phenotypes ranged from RNA binding proteins RBD-1 and CEY-1 to metabolic factors such as DLST-1 and
ECH-5, among others. The observed functional interactions suggest widespread coordination of these proteins with miRNAs to ultimately regulate gene expression. This study provides a
foundation for future investigations aimed at deciphering the molecular mechanisms of miRNA-mediated gene regulation. SIMILAR CONTENT BEING VIEWED BY OTHERS ELUCIDATION OF HOW THE
_MIR-23-27-24_ CLUSTER REGULATES DEVELOPMENT AND AGING Article Open access 14 June 2024 IDENTIFICATION OF RNA-BINDING PROTEINS THAT PARTNER WITH LIN28A TO REGULATE DNMT3A EXPRESSION Article
Open access 27 January 2021 MICRORNAS IN ACTION: BIOGENESIS, FUNCTION AND REGULATION Article 28 June 2023 INTRODUCTION Developmental and physiological processes require precise
spatio-temporal regulation of gene expression. One post-transcriptional gene regulatory mechanism is directed by a class of small non-coding RNAs called microRNAs (miRNAs). miRNAs regulate a
wide range of developmental and cellular processes, with dysregulated miRNA activity prevalent in diseases1,2. To exert their regulatory roles, miRNAs are loaded into Argonaute (AGO)
proteins to form a miRNA Induced Silencing Complex (miRISC), which ultimately associates with an effector protein GW182. miRISC binding to the target mRNA via partial sequence
complementarity between a miRNA and 3′ UTR of mRNA triggers a series of gene silencing mechanisms including translation inhibition, decapping, and mRNA decay3,4. miRNAs are produced by a
complex biogenesis process which involves enzymatic processing of miRNA intermediates in the nucleus and cytoplasm. Primary miRNAs are first cleaved by the Microprocessor complex (Drosha and
DGCR8) to form pre-miRNAs5,6. After export from nucleus into cytoplasm, pre-miRNAs are further processed by Dicer to generate a miRNA duplex7,8. The miRNA duplex bound by Argonaute is then
unwound, with the guide miRNA strand retained to form the mature miRISC, and the passenger strand released and degraded9,10. Each of the steps in miRNA biogenesis process can be regulated by
RNA binding and other auxiliary factors thereby modulating the final gene-regulatory impact of miRNAs. These factors could bind miRNA intermediates or miRISC protein components to affect
miRNA activity. For example, several RNA binding proteins including RBFOX3 and HnRNP A1 have been identified to bind to the hairpin structures of primary miRNAs and modulate their
processing11,12. Other proteins, such as NHL-2 and CGH-1, associate with ALG-1 and AIN-1 to promote mRNA targeting13. RNA binding proteins Staufen14 and HuR15,16 indirectly affect
miRNA-mediated gene silencing by competing for binding of the 3′UTRs of target mRNAs. Characterizing miRISC-associated protein complexes followed by functional analyses and mechanistic
studies has high potential to identify additional mechanisms by which miRNA activity may be regulated. Identifying proteins that associate with Argonaute proteins or miRNAs has been a
productive approach to begin to unravel the mechanisms by which aspects of miRNA biogenesis and activity are regulated17,18,19,20. In human cells, investigation of proteomic profiles of AGO
complexes led to identification of common protein interactors of all four AGO proteins, which included heat shock proteins, helicases, and components of translational machinery17. Some
proteins identified in this study, including Hsc70/Hsp90 chaperone machinery, were later characterized for their roles in RISC loading of small RNA duplexes21. In mice, exploration of Dicer
dependent and independent interactions of Ago218 identified proteins that participate in miRISC-mediated decapping22, among other mechanisms. However, while proteomic approaches have
characterized miRNA-associated complexes, it has been challenging to identify which of these co-factors are functionally important for miRNA activity, especially in tissue culture. In
contrast, functional assays that quantitatively assess miRNA activity are available in model organisms such as _C. elegans_. To identify miRNA/miRISC auxiliary cofactors important for miRNA
gene regulatory activity, we took a functional proteomics approach. Specifically, we used 2′O-methylated biotinylated oligonucleotides to pull down three miRNAs of interest (let-7, miR-58,
and miR-2) and subjected the associated protein complexes to proteomic analysis. Comparative analysis of miRNA pulldown and ALG-1 immunoprecipitation precipitates19 identified high
confidence interactors common to all four datasets. In addition, we identified a unique set of interactors in each miRNA pulldown dataset. To assess whether the co-precipitated proteins are
functionally important for miRNA activity, we performed RNAi knockdown of genes encoding for the putative physical interactors in multiple miRNA sensitized genetic backgrounds. Of the 39
interactors tested, depletion of 25 factors modified miRNA reduction of function phenotypes in one or more assays. Overall, we demonstrate that capturing physical interactors of miRNA
machinery followed by in vivo functional assays is an efficient approach to identify novel players in miRNA-mediated gene regulation. While further mechanistic characterizations are
necessary to determine the extent of the physical and functional interactions, this study identifies a functional requirement for a subset of potential ALG-1 and miRNA co-factors. METHODS
_C. ELEGANS_ MAINTENANCE, STRAINS, AND RNAI All _C. elegans_ strains were maintained on NGM and fed with _E. coli_ OP50. Strains were maintained at 20 °C unless otherwise noted. RNAi
knockdown was performed by feeding as previously described23. The following strains were used in this study: N2 (wild type), MT7626 _(let-7(n2853)),_ HW1113 _[Pdpy-30::GFP(PEST)-H2B::lin-41
3’ UTR (xeSi78); Pdpy-30::mCherry::H2B::artificial 3' UTR (xeSi36)]_, HW1114 _[Pdpy-30::GFP (PEST)-H2B::lin-41 3’ UTR (xeSi78); Pdpy-30::mCherry::H2B::artificial 3’ UTR (xeSi36),
let-7(n2853)]_, VT1367 _(col-19::gfp (maIs105)),_ VT1296 (_mir-48 mir-241(nDf51) col-19::gfp (maIs105)),_ BW1932 _[hbl-1p::gfp::NLS::hbl-1 3’ UTR (ctIS39)]_ and UY458 (_mir-48
mir-241(nDf51); hbl-1p::gfp::NLS::hbl-1 3_′_ UTR (ctIS39)]),_ OH812 (_otIs114 [Plim-6-gfp_ + _rol-6(su1006)]),_ OH3646 (_lsy-6(ot150); otIs114 [Plim-6-gfp_ + _rol-6(su1006)])_, PS3662
_(syIs63[cog-1_::_gfp_ + _unc-119(_+_)])_, OH7310 _(otIs193 [cog-1p_::_lsy-6_ + _rol-6(su1006)] syIS63[cog-1_::_gfp_ + _unc-119(_+_)])._ 2′O-METHYL OLIGO PULLDOWNS AND MASS SPEC ANALYSIS All
experiments were performed on mixed-stage animals. Whole worm extracts24 and 2′O-methyl oligo pulldowns25 were performed as previously described. For mass spectrometry, each sample
contained 20 mg of total protein. miRNA pulldowns were performed in two biological replicates using 2′O-methylated oligos with perfect complementation to miR-58, let-7, and miR-2, and
scrambled oligo control (IDT). Sequences of the 2′O-methylated, biotinylated oligonucleotides are as follows: miR-58 oligo (5′-CAUCAUUGCCGUACUGAACGAUCUCAAGUC-3′), miR-2 oligo
(5′-AUUCAGCACAUCAAAGCUGGCUGUGAUAUUCCA-3′), let-7 oligo (5′-UCUUCACUAUACAACCUACUACCUCAACCUU-3′), and scrambled oligo (5′-CAUCACGUACGCGGAAUACUUCGAAAUGUC-3′). Mass spectrometric analysis of
pulldown factors was performed as previously described19. Briefly, DTASelect26 was used to filter the proteins identified by applying a criterion that required proteins to have at least two
unique peptides with total spectral intensities greater or equal to four in both replicates. To determine enrichment of protein association in a miRNA pulldown, the Normalized Spectral
Abundance Factor (NSAF) values in miRNA pulldown were divided by that in control pulldown. NSAF value of zero in control was replaced by 1. Proteins with the pulldown/control ratio of ≥ 4 in
all replicates were considered putative physical interactors. GO TERM AND NETWORK ANALYSIS Gene ontology analysis was performed using Database for Annotation, Visualization and Integrated
Discovery (DAVID)27. Factors that had a fold change ≥ 4 in both replicates of the miRNA pulldowns were used for this analysis. For comparison, we included factors identified in atleast two
replicates of ALG-1 IP for GO term analysis (as previously described19). Protein domain information, domain enrichment analysis, and the associated statistics were retrieved using STRING28.
Enrichment for proteins harboring an RNA binding domain (RBD) among the proteins that passed our criteria was determined against a background set of _C. elegans_ proteins that harbor the
same RNA binding domain. Statistically significant enrichment was determined by applying Benjamini–Hochberg procedure on p-values to correct for multiple-testing. Network analysis was
performed on the top 40 most enriched factors using STRING28 after excluding ribosomal proteins. FUNCTIONAL ASSAYS LET-7(N2853) VULVAL BURSTING ASSAY Vulval bursting assay was performed as
previously described29. Briefly, _let-7(n2853)_ and N2 worms were grown and maintained at 15℃. Embryos obtained through bleaching30 were plated on RNAi plates23 and grown until L4 larval
stage. L4 animals were shifted to new RNAi plates and scored as day 1 adults for vulval bursting using a Leica dissecting microscope. Total number of worms (n) scored for this assay across
two to four independent RNAi experiments ranged from 45 to 330. COL-19::GFP EXPRESSION AND SEAM CELL NUMBER ASSAY _mir-48 mir-241(nDf51) col-19::gfp (maIs105)_ animals were transferred to
RNAi plates as L3 stage larvae and their progeny were scored for heterochronic phenotypes for hypodermal _col-19::gfp_ expression. Worms with seam-only reporter expression were classified as
having “delayed hypodermal _col-19::gfp_ expression”. Seam cell numbers were scored by counting the number of seam cells expressing _col-19::gfp_ between pharynx and anus. For most
candidates, the total number of worms scored (n) across two to four replicates ranged from 22 to 80. For genes whose knockdowns resulted in severe developmental defects such as _snr-4,
snr-6, let-363,_ and _rnp-7,_ the (n) was either 18 or 19. LSY-6(OT150) ASEL CELL FATE ASSAY _lsy-6(ot150); plim-6::gfp_ and _plim-6::gfp_ worms were transferred onto RNAi plates as embryos.
Their progeny were scored as L4s for specification of ASEL cell fate based on _plim-6::gfp_ reporter expression. Worms lacking the reporter expression in ASEL neurons were scored as cell
fate defective. Across two to four replicates, a total of 90 to 286 worms were scored. PDPY-30::GFP::LIN-41 REPORTER ASSAY _pdpy-30::GFP(PEST)-H2B::lin-41 3' UTR (xeSi78); pdpy-30::m
Cherry::H2B::artificial 3' UTR (xeSi36)_ and _pdpy-30::GFP (PEST)-H2B::lin-41 3' UTR (xeSi78); pdpy-30::mCherry::H2B::artificial 3' UTR (xeSi36), let-7(n2853)_ embryos
obtained by bleaching were plated on RNAi plates and grown at 15 °C. Reporter expression was measured in L4 stage animals by imaging the vulva at 63× magnification. To quantify expression
levels in six vulval cells, ROIs were manually drawn and signal intensities within the ROI were measured using the Leica image analysis software. For each vulval cell, GFP signal intensity
was divided by mCherry signal intensity and relative signal intensities were averaged across the six cells imaged in an individual animal. Representative images were equally adjusted after
quantification to make the fluorescence more observable. HBL-1P::GFP REPORTER ASSAY _hbl-1p::gfp::NLS::hbl-1 3′ UTR (ctIS39)_ and _mir-48 mir-241(nDf51); hbl-1p::gfp::NLS::hbl-1 3′ UTR
(ctIS39)_ embryos were obtained by bleaching. Embryos were transferred to RNAi plates and animals were scored for _hbl-1p::gfp_ expression in hypodermal cells at early to mid L3 stage.
Animals were staged by time of development, and gonad size and shape. UTERINE COG-1 REPORTER EXPRESSION _cog-1::gfp_ and _pcog-1::lsy-6; cog-1::gfp_ animals were transferred onto RNAi plates
as embryos and their F1 progeny were scored at L4 stage for _cog-1::gfp_ expression in the uterine cells. Worms expressing _cog-1::gfp_ in both uterine cells and vulval cells were scored as
wild type. Worms that lacked _cog-1::gfp_ reporter expression in either of the two uterine cells were scored as abnormal. FLUORESCENCE MICROSCOPY, IMAGE CAPTURE AND ILLUSTRATIONS
Fluorescence equipped Zeiss Axioplan 2 or Leica DM6 upright microscopes were used for scoring phenotypes. Images were captured using the Leica DM6B camera and processed using the Leica
Application Suite X (3.4.1.17822) software (https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/). Illustrations in Figs. 1a and 6e–g were drawn using BioRender
(biorender.com). STATISTICAL ANALYSIS All statistics were done using GraphPad Prism (9.2.0 (332)) software. Statistical significance was determined using a one-way ANOVA test with
predetermined comparisons. Bonferroni correction was applied as a post hoc analysis. T-test was used to determine statistical significance of _pdpy-30_::_gfp::lin-41, hbl-1p::gfp,_ and
_cog-1::gfp_ reporter assays. RESULTS MIRNA PULLDOWNS (PDS) IDENTIFY OVERLAPPING SETS OF PUTATIVE PHYSICAL INTERACTORS OF MIRNA-CENTERED COMPLEXES To identify factors that may regulate miRNA
activity, we sought to determine the molecular composition of protein complexes associated with let-7_,_ miR-58, and miR-2 miRNAs. _let-7_ is highly conserved across all bilateral animals31
and is required for the larval to adult transition in _C. elegans_32. miR-58 is a highly abundant miRNA that regulates lifespan and dauer formation33, primarily by coordinating with the
TGF-β pathway34,35. miR-2, a neuronal miRNA conserved among invertebrates36, is necessary for proper neuromuscular junction function in _C. elegans_37. We used biotinylated, 2′O-methylated
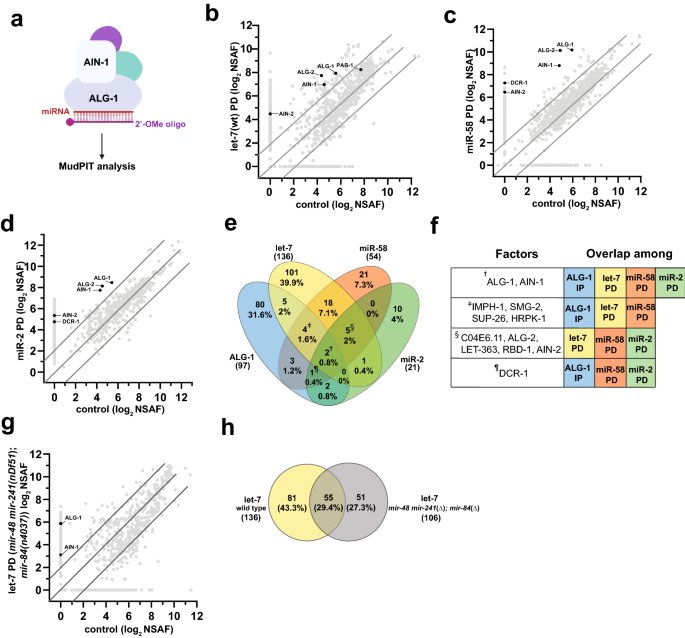
oligonucleotides with perfect sequence complementarity to mature miRNA sequences to pulldown miRNAs of interest and characterized the precipitates using a shotgun proteomics approach (Fig.
1a) to identify proteins associated with miRNAs of interest compared to scrambled control (Fig. 1b–d, Supplementary Table S1). To identify high confidence interactors, we retained only the
proteins that were ≥ fourfold enriched in miRNA pulldowns over the scrambled control, had a minimum NSAF value of 4, and were identified in all replicates. Overall, a total of 211 proteins
passed the criteria we set (Fig. 1e), with 136 factors co-precipitating with let-7, 54 factors co-precipitating with miR-58, and 25 factors co-precipitating with miR-2 (Fig. 1e,
Supplementary Table S1). Among the proteins enriched in miRNA co-precipitates were known miRISC components ALG-1 and ALG-2, the two major miRNA-associated Argonautes in _C. elegans_38 and
AIN-1 and AIN-2, GW182 homologs and miRISC effectors39,40 (Fig. 1b–f). In addition, DCR-1 nuclease, responsible for pre-miRNA processing, was detected in all pulldown experiments performed,
but did not meet our stringent interaction criteria in the let-7 pulldown (Fig. 1e,f, Supplementary Table S1). To determine the overlap between complexes precipitated by miRNA pulldowns and
those previously found to associate with ALG-119, we compared miRNA and ALG-1 co-precipitated factors (Fig. 1e,f, Supplementary Table S1). Eleven (8%) let-7 interactors, ten (18.5%) miR-58
interactors, and five (24%) miR-2 interactors were found to overlap with the ALG-1 co-immunoprecipitated dataset (Fig. 1e, Table 1). Overall, 41 proteins were present in at least two
interaction datasets (Fig. 1e,f, Table 1), potentially representing general miRNA-associated co-factors. In addition, four proteins, HRPK-1, SMG-2, IMPH-1, and SUP-26, were present in 2 out
of 3 pulldowns and the ALG-1 IP (Fig. 1e,f, Table 1). Their homologs were also found to co-immunoprecipitate with human and/or mouse Argonautes17,18,19, suggesting that they may have a
conserved function in miRNA-mediated gene regulation. In fact, we previously confirmed a physical HRPK-1 interaction with ALG-1 and reported _hrpk-1_ to genetically interact with multiple
miRNAs41. Five proteins were commonly captured in all the miRNA pulldowns (Fig. 1e,f, Table 1). Interestingly, one such protein was LET-363, an mTOR homolog42 (Fig. 1e,f, Table 1).
Similarly, we observed overlaps between our miRNA co-precipitates and previously reported miRNA physical interactors43,44 (Supplementary Table S2). Overlaps among our miRNA interaction
datasets and AIN-1 and AIN-2 co-precipitates40,44 were also observed, further emphasizing that our approach captured potential miRISC interactors (Supplementary Table S2). Finally,
candidates identified in genetic screens for miRNA and siRNA pathway genes also intersected with many of our miRNA co-precipitates29,45,46 (Supplementary Table S2). The observed overlaps
among various groups of physical and genetic interactors support the idea that we are detecting real physical interactors of miRNA-centered complexes. Due to a high level of sequence
similarity amongst the _let-7_ miRNA family members, the _let-7_ complementary oligonucleotide precipitates other members of the miRNA family, albeit with reduced efficiency47. To determine
whether distinct populations of proteins might associate with _let-7_ miRNA family members, we performed additional _let-7_ pulldown experiments in _mir-48 mir-241(nDf51); mir-84(n4037)_
mutant animals (Fig. 1g, Supplementary Table S1). 55 (29.4%) proteins were in common among both _let-7_ pulldowns, suggesting that these factors may interact with let-7 itself (Fig. 1h). The
51 (27.3%) proteins that precipitated with the let-7-complementary oligonucleotide from the _mir-48 mir-241(nDf51); mir-84 (n4037)_ animals may similarly represent let-7-interacting
factors, having been enriched in the let-7 pulldown in the absence of miR-48, miR-241, and miR-84 (Fig. 1h). In contrast, 81 (43.3%) proteins were present only in wildtype background
pulldowns (Fig. 1h), suggesting that these factors may normally associate with miR-48, miR-241 and miR-84 (Fig. 1h). While we cannot rule out an association with the remaining let-7 family
miRNAs, miR-793–795, the low relative abundance of these miRNAs19 suggests that miR-793–795 interactors are unlikely to represent significant fractions of the observed co-precipitates.
Finally, some co-precipitates could represent non-specific interactions. RIBONUCLEOPROTEIN COMPLEX COMPONENTS ARE ENRICHED AMONG MIRNA INTERACTORS To understand what biological processes and
functions are represented in the miRNA-precipitated complexes, we performed Gene Ontology (GO) analysis on putative miRNA interactors (Fig. 2a–d, Supplementary Table S3). Factors implicated
in embryonic and larval developmental processes were commonly enriched in all interaction datasets (Fig. 2a–c; ALG-1 interactome analysis is shown in Fig. 2d for comparison19, Supplementary
Table S3). Selective enrichment for splicing associated factors was observed in let-7 and miR-58 PD datasets (Fig. 2a,c, Supplementary Table S3). Components of intracellular
ribonucleoprotein complexes were consistently captured in all datasets (Fig. 2a–d and Ref.19). Enrichment for ribosomal components was observed only in let-7 and ALG-1 datasets (Fig. 2a,b,d
and Ref.19). Unsurprisingly, the RNA/nucleic acid binding term was commonly overrepresented in all the datasets in the molecular function category (Fig. 2a–d and Ref.19). miR-2 interactors
did not show an enrichment for any of the GO terms (Supplementary Table S3), potentially due to the low number of interactors captured in our pulldown and were therefore excluded from
further analysis. As RNA binding proteins (RBPs) were enriched in miRNA co-precipitates (Fig. 2a–d) and RBPs carry distinct domains critical for their RNA binding activity, we examined what
RNA binding domains (RBDs) were present in proteins identified in our pulldowns and ALG-1 IP (Fig. 2e–h, Supplementary Table S3). At least 5 different RBDs were observed among RBPs in all
the datasets. Notably, RBDs such as RNA Recognition Motif, KH, PAZ, and Nucleotide-binding alpha–beta plait domain superfamily were present in RBPs in one or more datasets (Fig. 2e–h,
Supplementary Table S3). Overall, miRNA pulldowns captured factors that may play critical roles during the lifecycle of RNA. To determine whether factors identified through our proteomics
approach form a functional network, we performed network analysis using STRING28 (Fig. 2i–l). STRING predicts candidate protein interactions by utilizing both known and predicted
protein–protein interactions sourced from databases, text mining, experimental, and co-expression data28. Top 40 enriched putative interactors in each dataset, minus the ribosomal proteins,
were chosen for this analysis. Interestingly, we observed that in all the datasets proteins formed functional networks with a significant number of edges (_p_-value < 1.0e−16, as
determined by STRING) (Fig. 2i–l), further supporting the idea that miRNA pulldown-captured proteins form functional complexes that may coordinate with miRNAs to regulate gene expression.
FUNCTIONAL ANALYSIS OF PUTATIVE MIRNA INTERACTORS To identify which putative interactors might functionally coordinate with miRNAs to regulate gene expression, we took advantage of
sensitized genetic backgrounds with reduced miRNA or miRNA family activity. These functional assays with quantifiable phenotypic outputs allow for assessment of a gene’s role in
miRNA-mediated gene repression. Our pulldown experiments targeted miRNAs with varied functions and spatio-temporal expression patterns. Some of the identified interactors of miRNA- or
ALG-1-centered complexes could have broad functional requirements, while others could be specific to a particular tissue or a developmental time. We hypothesized that knockdown of
generally-required factors in multiple sensitized miRNA backgrounds would modulate phenotypes in multiple functional assays. In contrast, spatio-temporal specificity of the putative
interactors may limit their functional relevance to specific miRNAs and may not result in a phenotype in some, or all, of our assays. In addition, the miRNA-centered protein complex analyses
potentially identified interactors that may positively or negatively modulate microRNA activity. Knockdown of these factors in sensitized genetic backgrounds may therefore result in an
enhancement or a suppression of the phenotype associated with reduction of miRNA function. For our functional assessment, we prioritized factors that were highly enriched in our pulldown
and/or ALG-1 IP experiments19 and were captured in multiple datasets. We excluded ribosomal proteins and factors lacking RNAi clones. The 39 candidates assayed ranged from common interactors
of miRNA(s) and ALG-1 (6), common miRNA interactors (6), ALG-1 interactors (16), and specific miRNA interactors [let-7 (10), and miR-58 (1)] (Supplemental Table S4). Among the ALG-1
interactors, we assayed genes encoding for six proteins consistently identified in human and mouse AGO IP (referred to as conserved AGO interactors from hereon). RNAI KNOCKDOWN OF GENES OF
LET-7 AND ALG-1 INTERACTORS ALTERS LET-7(N2853) MUTANT PHENOTYPE let-7 is essential for _C. elegans_ development and promotes transition from the fourth larval stage (L4) to adulthood32.
Loss of _let-7_ function results in vulval bursting and failure of seam cells to differentiate during the L4 to adult transition32. _let-7(n2853)_ is a temperature-sensitive reduction of
function mutation that impairs regulation of let-7 targets, including _lin-41_48. _let-7(n2853)_ mutants have a partially penetrant vulval bursting phenotype at permissive temperature32 (15
°C) (Fig. 3a). To determine whether the identified let-7 and ALG-1 interactors are functionally important for let-7 miRNA activity, we used this well-established genetic background to assay
the effects of gene knockdown on _let-7(n2853)_ bursting phenotype_._ RNAi of six genes enhanced vulval bursting of _let-7(n2853)_ mutant (Fig. 3b,c, Supplementary Table S4). One such gene
(_pab-1)_ encoded a conserved AGO interactor19 (Fig. 3b, Supplementary Table S4) and five genes including _C04E6.11, ech-5_, and _rbd-1,_ which code for let-7 interactors (Fig. 3c,
Supplementary Table S4). RNAi of _cey-1_ suppressed the bursting (Fig. 3c, Supplementary Table S4). Knockdown of _ifg-1_ also mildly suppressed _let-7(n2853)_ vulval bursting from 30 to 6%
(Fig. 3b, Supplementary Table S4), although the suppression did not reach a statistically significant level (Anova p-value = 0.078). RNAi knockdown of these genes in the wild type background
did not result in vulval bursting, suggesting that these genes do not play a central role in gene regulation, only revealing the function in the sensitized _let-7(n2853)_ background (Fig.
3b,c). We cannot, however, rule out the possibility that RNAi knockdown in wild type background may have been ineffective. Overall, these findings support our hypothesis that the identified
let-7 physical interactors play a role in let-7-mediated gene repression. Since dysregulation of _let-7_ target gene _lin-41_ in vulval-uterine system is sufficient to cause vulval
rupturing49, we wanted to determine how depletion of putative physical and genetic _let-7_ interactors affects _lin-41_ expression in the relevant cells. To do this, we used an established
_let-7-lin-41_ reporter system49. We performed RNAi knockdown of genes that enhance (_ech-5_, _rbd-1_, _C04E6.11,_ and _let-363_) and suppress (_cey-1_) _let-7(n2853)_ vulval bursting in the
background of two reporter strains: _pdpy-30::gfp::lin-41 3ʹUTR_ and _pdpy-30::gfp::lin-41 3_ʹ_UTR; let-7(n2853)_49. RNAi depletion of these genes did not alter _pdpy-30::gfp::lin-41
3_ʹ_UTR_ reporter levels in the wildtype background (Fig. 3d,e, Supplementary Table S5), suggesting that these genes do not have a major effect on _lin-41_ levels on their own. However, in
_let-7(n2853)_ background at 15 °C, knockdown of _ech-5_ or _rbd-1_ substantially increased _lin-41_ levels, while _cey-1_ depletion reduced _pdpy-30::gfp::lin-41 3_ʹ_UTR_ reporter levels in
the vulval cells (Fig. 3d,e, Supplementary Table S5). RNAi of _let-363_ led to a mild increase in the reporter levels in _let-7(n2853)_ at 15 °C, although the increase was not statistically
significant (Fig. 3e, Supplementary Table S5). Interestingly, _let-363_ depletion was previously reported to increase levels of let-7 target reporters _hbl-1p::gfp::hbl-1_ in VNC and
_col-10::gfp::lin-41 3′UTR_ in hypodermal cells50. These observations suggest that _ech-5, rbd-1, cey-1_ and perhaps even _let-363_ may be contributing to regulation of vulval bursting by
modulating _let-7_ miRNA activity. RNAI KNOCKDOWN OF GENES OF LET-7 AND ALG-1 INTERACTORS ALTERS MIR-48 MIR-241(NDF51) MUTANT PHENOTYPE We next asked whether miRNA and ALG-1 interactors
might functionally coordinate with other members of the _let-7_ family of miRNAs. _let-7_ family members _mir-48, mir-84_ and _mir-241_ specify developmental timing in _C. elegans_,
regulating seam cell divisions and contributing to L2–L3 larval developmental transition51 (Fig. 4a). Deletion of all three miRNAs (_mir-48 mir-241(nDf51); mir-84(n4037)_) results in
reiteration of L2 stage seam cell divisions, resulting in an increased number of seam cells and delayed terminal cell differentiation in young adults51 (Fig. 4a). Partial deletion (_mir-48
mir-241(nDf51)_) mutants display incompletely penetrant heterochronic phenotype which can be monitored using an adult stage marker, _col-19::gfp,_ expressed in seam and hypodermal cells
(Fig. 4b). RNAi of 11 genes enhanced the abnormal _col-19::gfp_ expression in hypodermal cells (Fig. 4c,d, Supplementary Table S4), with five genes, _pab-1, dlst-1, C43E11.9, snr-4,_ and
_rbd-1_, enhancing the phenotype to > 50% (Fig. 4c,d Supplementary Table S4). This suggests that these potential miRNA interactors are required for regulation of developmental timing
programs. We also examined the effects of gene knockdown on seam cell number. RNAi of three genes, _pqn-70, dlst-1_, and _cey-1_, increased the seam cell number of _mir-48 mir-241(nDf51)_
mutants (Fig. 4f, Supplementary Table S4). RNAi of nine genes suppressed seam cell lineage defect, with knockdown of _let-363_, _rbd-1_, _snr-6,_ and _snr-4,_ restoring the seam cell number
to an average of 13 or lower (Fig. 4e,f, Supplementary Table S4). We should note that while most genes were assayed across a minimum of two independent RNAi experiments, four genes (_snr-6_,
_snr-4_, _let-363,_ and _rnp-7)_ were tested only once, as knockdown of these genes caused lethality, reduced brood size, and slowed growth. Depletion of some genes had varied effects on
hypodermal _col-19::gfp_ expression and seam cell lineage. Knockdown of _C43E11.9, pdi-2,_ and _Y71F9AL.9_ modified _col-19::gfp_ expression but not seam cell number, while knockdown of
_pqn-70_ and _C28H8.3_ modified the seam cell number of _mir-48 mir-241(nDf51)_ animals without affecting hypodermal _col-19::gfp_ expression (Fig. 4d,f). This could perhaps be explained by
distinct roles these genes may play during proliferative seam cell divisions and terminal hypodermal cell fate specification. Since _let-7_ family miRNAs promote L3 cell fates by repressing
_hbl-1_51, we sought to determine whether the genes that affect heterochronic phenotypes in _mir-48 mir-241_(_nDf51_) background do so by regulating _hbl-1_. We used the _hbl-1p::gfp::hbl-1
3′UTR_ fusion construct as a reporter to assess the effects of gene knockdown on levels of HBL-152. Strong _hbl-1_ expression can be seen during embryogenesis with hypodermal
_hbl-1::gfp::hbl-1 3′UTR_ expression decreasing beyond detection at the L3 stage52 (Fig. 4g). RNAi knockdown of _pdi-2, rbd-1,Y71F9AL.9,_ and _cey-1_ resulted in higher percentages of L3
animals expressing the reporter in the wild type background, potentially indicating a miRNA-independent effect (Fig. 4h, Supplementary Table S5). Since depletion of these genes did not
affect _col-19:gfp_ expression or other heterochronic defects in the wild type (Fig. 4d), perhaps the extent of _hbl-1_ derepression was not strong enough to impact developmental timing.
This notion is supported by the observation that _hbl-1_ reporter expression in _mir-48 mir-241_(_nDf51_) background is observed at a higher rate (75%, Fig. 4h). Knockdown of enhancers of
_mir-48 mir-241_(_nDf51_) mutant phenotype derepressed _hbl-1_ reporter expression in the _mir-48 mir-241_(_nDf51_) background (Fig. 4h, Supplementary Table S5). Overall, these findings
suggest that our proteomics approach captured proteins that may co-ordinate with _let-7_ family miRNAs to repress their target, _hbl-1,_ and ultimately coordinate developmental timing_._
DEPLETION OF ALG-1 PHYSICAL INTERACTORS ALTERED LSY-6(OT150) PHENOTYPE We hypothesized that some of the ALG-1 and/or miRNA putative physical interactors could be factors that are generally
required for miRISC activity. To test this, we RNAi depleted them in _lsy-6(ot150)_ background_. lsy-6_ is essential for cell fate determination of chemosensory ASE neurons53. lsy-6
represses an ASER cell fate promoting transcription factor _cog-1_, leading to an ASEL neuronal specific gene expression pattern. Loss of _lsy-6_ leads to dysregulated gene expression of
_cog-1_ and downstream effectors resulting in defective ASEL cell fate which leads to lack of _plim-6::gfp_ reporter. However, the reduction of function mutant _lsy-6(ot150)_ shows partially
penetrant cell fate defective phenotype in approximately 20% of animals53 (Fig. 5a). Knockdown of 8 genes modified _lsy-6(ot150)_ defective phenotype (Fig. 5b,c, Supplementary Table S4)
including previously reported conserved AGO interactors _pab-1,_ and _larp-1_19 (Fig. 5b). Interestingly, we identified two suppressors (_ifg-1,_ and _F28B4.3_) of ASEL cell fate defect
(Fig. 5b,c, Supplementary Table S4). To determine whether these candidate factors can influence ASEL cell fate independent of lsy-6 miRNA, we knocked them down in wild-type worms and
observed no change in _plim-6::gfp_ reporter expression in ASEL cells (Fig. 5b,c)_._ We next determined whether genes that modified _lsy-6_ phenotype upon knockdown were important for lsy-6
mediated target activity using _cog-1_ reporter system54 (Fig. 5d). _cog-1_ is expressed in vulval and uterine cells where lsy-6 is absent (Fig. 5d, top left panel). When _lsy-6_ is
ectopically expressed in these tissues under _cog-1_ promoter, there is reduced expression of _cog-1_ as a result of lsy-6 mediated repression54 (Fig. 5d, top right panel). We performed RNAi
knockdown of top hits from lsy-6 assay in the _cog-1_ reporter strain and observed no difference in _cog-1_ expression (Fig. 5e, Supplementary Table S5), suggesting that these factors do
not regulate _cog-1_ directly in uterine cells. RNAi knockdown of _F33D11.10_ and _psf-1_ (enhancers of _lsy-6(ot150)_ phenotype) restored _cog-1_ expression in the presence of _lsy-6,_
suggesting their requirement for lsy-6 mediated _cog-1_ repression (Fig. 5e). Knockdown of _larp-1_ did not restore _cog-1_ expression to a statistically significant level (Fig. 5e,
Supplementary Table S5). This could be due to RNAi variability among replicates, or possibly because lsy-6 activity was initially assessed in ASE neurons, while _cog-1_ reporter expression
was assessed in uterine tissue. Previously reported tissue-specific composition of miRNA-centered complexes and distinct mechanisms of target suppression44 support this potential explanation
for the observed discrepancy in _larp-1_ effects. _lsy-6(ot150)_ and _cog-1_ reporter assays collectively demonstrate that we identified factors that may directly or indirectly coordinate
with lsy-6, affecting its target _cog-1_ expression. Overall, depletion of miRNA complex interactors did not produce a phenotype in the absence of the sensitized miRNA mutations (Figs. 3b,c,
4c,d, 5b,c). While we cannot rule out inefficient RNAi knockdown as a possible explanation, we hypothesize that the tested factors are not critical for regulation of miRNA target gene
expression, but rather play a modulatory role in miRNA production and/or activity, or influence gene expression downstream of miRNA activity. DISCUSSION To better understand miRNA mediated
gene regulation, we performed miRNA pulldowns to identify components of miRNA-centered complexes. Our proteomics approach captured 211 miRNA-interacting proteins, some of which were
previously reported to precipitate with other miRISC components (Supplementary Table S2). Knockdown of 25 out of 39 genes significantly modulated miRNA mutant phenotypes in one or more
assays, suggesting that our pulldowns captured proteins that coordinate with miRNAs to affect gene regulation (Fig. 6a, Supplementary Table S4). Of the 25 hits, knockdown of five genes
(_pab-1, let-363, rbd-1, cey-1,_ and _lys-8_) and _dcr-1,_ a positive control_,_ consistently modified miRNA phenotypes in two or more assays (Fig. 6a, Supplementary Table S4). Of the 22
candidate genes tested, RNAi of six genes modulated _let-7(n2853)_ vulval bursting phenotype (Fig. 6a,b, Supplementary Table S4). Five of these functional interactors were identified in
let-7 PD experiments, either in let-7 PD alone or in let-7 PD plus additional precipitation experiments (Fig. 6b, Supplementary Table S4), suggesting that let-7 interacting factors indeed
functionally coordinate with let-7 activity. Knockdown of 16 putative interactors did not modify vulval bursting phenotype of _let-7(n2853)_ (Fig. 6b), perhaps due to tissue or time specific
physical interactions of these proteins with let-7 miRNA or ALG-1 complexes. Such spatio-temporal complex compositions could explain the corresponding lack of activity in vulval tissue. We
cannot, however, rule out insufficient RNAi knockdown or non-specific interactions of these proteins with anti-let-7 oligonucleotide. Knockdown of 18/38 candidate genes genetically modified
hypodermal and/or seam cell lineage defects of _mir-48 mir-241(nDf51)_ mutants (Fig. 6c, Supplementary Table S4). 12 of these factors were identified in let-7 pulldowns from wild type and/or
_mir-48 mir-241; mir-84_ mutant backgrounds, suggesting that let-7 PD proteomics captured factors that support let-7 family miRNA activity in developmental timing. As _lsy-6_ miRNA activity
is highly localized and unrelated to miRNAs precipitated in our PD experiments, _lsy-6(ot150)_ mutation provided a convenient genetic background to identify which factors may be broadly
involved miRNA-mediated gene regulation. Of the eight functional hits from the _lsy-6(ot150)_ assay, seven factors were identified as ALG-1 interactors (Fig. 6d, Supplementary Table S4),
consistent with the idea that ALG-1 IP perhaps precipitated proteins with broad specificities. Lack of _lsy-6(ot150)_ phenotype modification by knockdown of let-7 and/or miR-58-associated
proteins suggests that miRNA-centered complexes may be unique to the specific miRNAs, possibly due to distinct spatial or temporal expression patterns. Several genes that modified
_let-7(n2853)_ vulval bursting in our study, _cey-1_, _ifg-1_, _pab-1,_ and _rbd-1_ (Fig. 3b,c, Supplemental Table S4), were previously tested in an RNAi screen for suppressors of
_let-7(n2853)_ vulval bursting, aimed at identifying let-7 target genes55. We observed multiple differences between the results of our RNAi screen, performed at the permissive temperature of
15 °C and the previous work, performed at non-permissive 25 °C55, which eliminates _let-7_ activity. For example, _rbd-1_ knockdown enhanced vulval bursting at 15 °C (Fig. 3c, Supplementary
Table S4), while it suppressed bursting at 25 °C55, suggesting that _rbd-1_ may have both let-7 dependent and independent functions. Knockdown of _cey-1_ suppressed _let-7(n2853)_ vulval
bursting at 15 °C in our study (Fig. 3c, Supplementary Table S4), however, no _let-7(n2853)_ suppression was observed at 25 °C upon _cey-1_ knockdown55. These observations suggest that
_cey-1_ may coordinate with let-7 in target mRNA regulation. Direct comparisons across RNAi studies performed under different conditions can be difficult to interpret and further
explorations will be needed to understand the roles of these genes in _let-7-_mediated regulation of gene expression. How could these putative physical miRNA interactors be coordinating with
miRNAs to regulate gene expression? The factors identified in this study could be acting via multiple mechanisms to affect miRNA mutant phenotypes. Some of the miRNA interactors identified
in this study have wide-ranging roles in regulation of gene expression. Thus, their knockdown could modify the miRNA reduction-of-function phenotypes directly through miRNA regulation and/or
indirectly through regulation of mRNA lifecycle. For example, _pab-1_, a poly(A) binding protein and a homolog of human PABPC156, has well established roles in regulating the stability of
mRNA transcripts by affecting translation initiation and mRNA stabilization and decay. PAB-1 has been previously shown to interact with miRISC19,56 and to aid miRNA-mediated deadenylation56.
The enhancement of miRNA reduction-of-function phenotypes upon _pab-1_ knockdown may therefore be a result of miRNA-dependent and/or independent functions of _pab-1,_ perhaps through loss
of target mRNA deadenylation and subsequent mRNA stabilization. We used the biological and molecular functions predicted by GO term analysis to consider the possible mode of action for the
identified miRNA and miRISC interactors. RNA binding proteins were among the classes of genes enriched in our pulldowns (Fig. 2a–h, Supplementary Table S3). Through functional assays, we
identified nine interactors with predicted and/or experimentally validated RNA binding activity as genetic interactors of miRNA mutants (Supplementary Table S4). Interestingly, knockdown of
genes encoding all nine RNA binding proteins enhanced miRNA mutant phenotypes, consistent with the recent finding that 3’UTR-binding RBPs generally promote miRISC targeting57. Some of these
RBPs could play a role in miRNA processing (Fig. 6e), some RBPs could potentially facilitate miRISC targeting or activity (Fig. 6f), while other RBPs could regulate localization and
stability of miRNAs and/or miRNA targets, ultimately affecting gene regulation. Translation regulators were also captured in miRNA pulldowns and ALG-1 IP (Supplementary Tables S1, S3), with
two of them modifying miRNA phenotypes. Depletion of _ifg-1,_ encoding translation initiation factor 4G (eIF4G)58, suppressed the ASEL cell fate defect of _lsy-6(ot150)_ (Fig. 5b). Given the
potential physical association of IFG-1 with ALG-119, it is possible that IFG-1 and miRNAs share common targets; with loss of IFG-1 reducing translation through loss of initiation, thereby
suppressing target mRNA overexpression in miRNA reduction of function mutants. RNA helicase F33D11.10 co-precipitated in let-7 pulldown (Supplementary Table S1) and was previously identified
as an interactor of ALG-119. Loss of _F33D11.10_ activity enhanced the ASEL cell fate defect of _lsy-6(ot150)_ mutant (Fig. 5c). RNA helicases have been previously implicated in miRNA
processing as well as miRISC activity59 and F33D11.10 may be similarly involved in either facilitating miRNA processing, miRISC activity, or both. A surprising category of interactors
identified in our study was the intermediary metabolic enzymes. RNAi depletion of metabolic enzymes DLST-1, OGDH-1 and ECH-5 modified miRNA mutant phenotypes in our study (Figs. 3c, 4d,f,
Supplementary Table S4). Several reports have suggested that some metabolic enzymes possess RNA binding functions, previously unidentified due to a lack of conventional RNA binding
domains60,61,62. It is possible that the metabolic enzymes identified in our study possess similar dual roles. For instance, _ech-5_ encodes a homolog of human AU RNA binding
methylglutaconyl-CoA hydratase (AUH)63. In humans, AUH plays a dual role as a hydratase and as an RBP, binding AU-rich elements in the 3′ UTR of mRNAs64. Other ARE-binding proteins have been
previously shown to aid in rapid degradation through deadenylation65. In our study, ECH-5 co-precipitated with let-7 miRNAs and _ech-5_ depletion enhanced _let-7(n2853)_ vulval bursting
phenotype (Fig. 3c, Supplementary Table S1). Thus, we might speculate ECH-5 could bridge miRISC complex interaction with deadenylation machinery, with loss of _ech-5_ exacerbating the target
mRNA stabilization in miRNA mutant backgrounds (Fig. 6g). How other metabolic genes such as _dlst-1_ and _ogdh-1_, key players of TCA cycle66,67, influence gene regulation remains unclear.
Thorough investigations into molecular mechanisms by which these factors coordinate with miRNAs in gene regulation will be needed. Interestingly, LET-363, _C. elegans_ mTOR was identified as
an interactor of all three miRNAs in this study (Fig. 1f, Supplementary Table S1). RNAi of _let-363_ enhanced the vulval bursting of _let-7(n2853)_ and suppressed the seam cell lineage
defect of _mir-48 mir-241(nDf51)_ mutant (Figs. 3c, 4f, Supplemental Table S4). While we did not test for functional _let-363_ requirement in our _lsy-6(ot150)_ assay, RNAi of _let-363_ was
previously reported to exacerbate the cell fate specification defect of _lsy-6(ot150)_50_,_ consistent with a _let-363_ role in miRNA-mediated gene regulation. mTOR activation has been
reported to downregulate miRNA biogenesis through Mdm2-mediated DROSHA degradation in mice68. However, the physical association of LET-363 with miRNAs was surprising. If confirmed, these
persistent physical and functional interactions of LET-363 with miRNA-centered complexes should be further explored to establish the mechanistic connection between mTOR and miRNA-mediated
gene regulatory activity. Do miRNAs within the same family associate with same set of protein interactors? _let-7_ family miRNAs are well-studied in _C. elegans_. The four most abundant
members of the _let-7_ family, _let-7, mir-48, mir-84_ and _mir-241,_ are crucial components of the heterochronic pathway, regulating cell fates during larval development32,53. The miRNAs
are thought to function semi-redundantly, with distinct targeting capabilities69. Part of their ability to target unique targets could come from discrete protein interactors adding a layer
of specificity between a _let-7_ family miRNA and its target. Yet not much is known about the protein interacting partners of individual members of this family. By performing pulldowns with
a let-7 specific oligo from wild type and _mir-48 mir-241(nDf51); mir-84(n4037)_ background, we began the task of unraveling which interactors may be specific to let-7 itself or other family
members (Fig. 1h, Supplementary Table S1). For example, ECH-5 and C04E6.11 were identified in let-7 pulldowns from both genetic backgrounds, suggesting that they most likely interact with
let-7 (Supplementary Table S1). RNAi depletion of _ech-5_ or _C04E6.11_ enhanced the _let-7(n2853)_ phenotype but showed no effect on the _mir-48 mir-241(nDf51)_ associated phenotypes (Figs.
3c, 4d). Thus, it is possible that ECH-5 and C04E6.11 specifically interact with, and provide functional support for, let-7 itself. CEY-1 was captured in let-7 pulldowns from both wild type
and _mir-48 mir-241; mir-84_ mutant background, but depletion of _cey-1_ suppressed _let-7(n2853)_ vulval bursting and enhanced _mir-48 mir-241(nDf51)_ mutant phenotypes (Figs. 3c, 4d).
This suggests that _cey-1_ may functionally interact with multiple members of the _let-7_ family, potentially through distinct mechanisms. Previously, miR-241 complementary oligo pulldown
captured CEY-144, although it remains difficult to assess specificity, as miR-241 oligo may capture other members of the family, similar to let-747. Overall, we cannot rule out the
possibility that some of the factors precipitating in miRNA pulldowns are non-specifically interacting with the precipitating oligo, rather than with the miRNA-centered complexes. It is also
possible that let-7 interactions may be altered in the _mir-48 mir-241(nDf51); mir-84(n4037)_ background. Similarly, the severe developmental timing defect of _mir-48 mir-241(nDf51);
mir-84(n4037)_ animals could hinder identification of _bona fide_ interactors of let-7 in that background. However, the high rate of functional relevance of these factors for miRNA-mediated
gene regulation suggests that this approach captures auxiliary factors that may coordinate with miRNAs mediated gene regulation. REFERENCES * Ma, F., Zhang, X. & Yin, K.-J. MicroRNAs in
central nervous system diseases: A prospective role in regulating blood–brain barrier integrity. _Exp. Neurol._ 323, 113094 (2019). Article CAS PubMed PubMed Central Google Scholar *
Ebrahimi, S. O., Reiisi, S. & Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. _J. Cell Physiol._ 235, 8812–8825 (2020). Article CAS PubMed Google
Scholar * Bagga, S. _et al._ Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. _Cell_ 122, 553–563 (2005). Article CAS PubMed Google Scholar * Mayya, V. K. _et
al._ microRNA-mediated translation repression through GYF-1 and IFE-4 in _C. elegans_ development. _Nucleic Acids Res._ 49, gkab162 (2021). Article CAS Google Scholar * Lee, Y. _et al._
The nuclear RNase III Drosha initiates microRNA processing. _Nature_ 425, 415–419 (2003). Article ADS CAS PubMed Google Scholar * Han, J. _et al._ Molecular basis for the recognition of
primary microRNAs by the drosha-DGCR8 complex. _Cell_ 125, 887–901 (2006). Article CAS PubMed Google Scholar * Hutvágner, G. _et al._ A cellular function for the RNA-interference enzyme
dicer in the maturation of the let-7 small temporal RNA. _Science_ 293, 834–838 (2001). Article PubMed Google Scholar * MacRae, I. J., Zhou, K. & Doudna, J. A. Structural
determinants of RNA recognition and cleavage by Dicer. _Nat. Struct. Mol. Biol._ 14, 934–940 (2007). Article CAS PubMed Google Scholar * Kwak, P. B. & Tomari, Y. The N domain of
Argonaute drives duplex unwinding during RISC assembly. _Nat. Struct. Mol. Biol._ 19, 145–151 (2012). Article CAS PubMed Google Scholar * Meijer, H. A., Smith, E. M. & Bushell, M.
Regulation of miRNA strand selection: Follow the leader?. _Biochem. Soc. Trans._ 42, 1135–1140 (2014). Article CAS PubMed Google Scholar * Kim, K. K., Yang, Y., Zhu, J., Adelstein, R. S.
& Kawamoto, S. Rbfox3 controls the biogenesis of a subset of microRNAs. _Nat. Struct. Mol. Biol._ 21, 901–910 (2014). Article CAS PubMed PubMed Central Google Scholar * Guil, S.
& Cáceres, J. F. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. _Nat. Struct. Mol. Biol._ 14, 591–596 (2007). Article CAS PubMed Google
Scholar * Hammell, C. M., Lubin, I., Boag, P. R., Blackwell, T. K. & Ambros, V. nhl-2 Modulates microRNA activity in _Caenorhabditis elegans_. _Cell_ 136, 926–938 (2009). Article CAS
PubMed PubMed Central Google Scholar * Ren, Z., Veksler-Lublinsky, I., Morrissey, D. & Ambros, V. Staufen negatively modulates microRNA activity in _Caenorhabditis elegans_. _G3 Genes
Genomes Genet._ 6, 1227–1237 (2016). CAS Google Scholar * Srikantan, S., Tominaga, K. & Gorospe, M. Functional Interplay between RNA-binding protein HuR and microRNAs. _Curr. Protein
Pept. Sci._ 13, 372–379 (2012). Article CAS PubMed PubMed Central Google Scholar * Kundu, P., Fabian, M. R., Sonenberg, N., Bhattacharyya, S. N. & Filipowicz, W. HuR protein
attenuates miRNA-mediated repression by promoting miRISC dissociation from the target RNA. _Nucleic Acids Res._ 40, 5088–5100 (2012). Article CAS PubMed PubMed Central Google Scholar *
Landthaler, M. _et al._ Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. _RNA_ 14, 2580–2596 (2008). Article CAS PubMed
PubMed Central Google Scholar * Frohn, A. _et al._ Dicer-dependent and -independent Argonaute2 protein interaction networks in mammalian cells*. _Mol. Cell Proteomics_ 11, 1442–1456
(2012). Article CAS PubMed PubMed Central Google Scholar * Zinovyeva, A. Y., Veksler-Lublinsky, I., Vashisht, A. A., Wohlschlegel, J. A. & Ambros, V. R. Caenorhabditis elegans ALG-1
antimorphic mutations uncover functions for Argonaute in microRNA guide strand selection and passenger strand disposal. _Proc. Natl. Acad. Sci._ 112, E5271–E5280 (2015). Article ADS CAS
PubMed PubMed Central Google Scholar * Wu, E. _et al._ A continuum of mRNP complexes in embryonic microRNA-mediated silencing. _Nucleic Acids Res._ 45, 2081–2098 (2017). CAS PubMed
Google Scholar * Iwasaki, S. _et al._ Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. _Mol. Cell_ 39, 292–299 (2010). Article CAS PubMed Google
Scholar * Nishihara, T., Zekri, L., Braun, J. E. & Izaurralde, E. miRISC recruits decapping factors to miRNA targets to enhance their degradation. _Nucleic Acids Res._ 41, 8692–8705
(2013). Article CAS PubMed PubMed Central Google Scholar * Kamath, R. S. & Ahringer, J. Genome-wide RNAi screening in _Caenorhabditis elegans_. _Methods_ 30, 313–321 (2003). Article
CAS PubMed Google Scholar * Li, L. & Zinovyeva, A. Y. Protein extract preparation and Co-immunoprecipitation from _Caenorhabditis elegans_. _J. Vis. Exp._
https://doi.org/10.3791/61243 (2020). Article PubMed Google Scholar * Jannot, G., Vasquez-Rifo, A. & Simard, M. J. Argonaute proteins, methods and protocols. _Methods Mol. Biol._ 725,
233–249 (2011). Article CAS PubMed Google Scholar * Tabb, D. L., McDonald, W. H. & Yates, J. R. DTASelect and contrast: Tools for assembling and comparing protein identifications
from shotgun proteomics. _J. Proteome Res._ 1, 21–26 (2002). Article CAS PubMed PubMed Central Google Scholar * Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and
integrative analysis of large gene lists using DAVID bioinformatics resources. _Nat. Protoc._ 4, 44–57 (2009). Article CAS Google Scholar * Snel, B., Lehmann, G., Bork, P. & Huynen,
M. A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. _Nucleic Acids Res._ 28, 3442–3444 (2000). Article CAS PubMed PubMed Central Google
Scholar * Parry, D. H., Xu, J. & Ruvkun, G. A whole-genome RNAi screen for _C. elegans_ miRNA pathway genes. _Curr. Biol._ 17, 2013–2022 (2007). Article CAS PubMed PubMed Central
Google Scholar * Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A. & Cerón, J. Basic _Caenorhabditis elegans_ methods: Synchronization and observation. _J. Vis. Exp._
https://doi.org/10.3791/4019 (2012). Article PubMed PubMed Central Google Scholar * Pasquinelli, A. E. _et al._ Conservation of the sequence and temporal expression of let-7
heterochronic regulatory RNA. _Nature_ 408, 86–89 (2000). Article ADS CAS PubMed Google Scholar * Reinhart, B. J. _et al._ The 21-nucleotide let-7 RNA regulates developmental timing in
_Caenorhabditis elegans_. _Nature_ 403, 901–906 (2000). Article ADS CAS PubMed Google Scholar * Jan, C. H., Friedman, R. C., Ruby, J. G. & Bartel, D. P. Formation, regulation and
evolution of _Caenorhabditis elegans_ 3′UTRs. _Nature_ 469, 97–101 (2011). Article ADS CAS PubMed Google Scholar * de Lucas, M. P., Sáez, A. G. & Lozano, E. miR-58 family and TGF-β
pathways regulate each other in _Caenorhabditis elegans_. _Nucleic Acids Res._ 43, 9978–9993 (2015). PubMed PubMed Central Google Scholar * Subasic, D. _et al._ Cooperative target mRNA
destabilization and translation inhibition by miR-58 microRNA family in _C. elegans_. _Genome Res._ 25, 1680–1691 (2015). Article CAS PubMed PubMed Central Google Scholar * Marco, A.,
Hooks, K. & Griffiths-Jones, S. Evolution and function of the extended miR-2 microRNA family. _RNA Biol._ 9, 242–248 (2012). Article CAS PubMed PubMed Central Google Scholar *
O’Hern, P. J. _et al._ Decreased microRNA levels lead to deleterious increases in neuronal M2 muscarinic receptors in Spinal Muscular Atrophy models. _Elife_ 6, e20752 (2017). Article
PubMed PubMed Central Google Scholar * Grishok, A. _et al._ Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control _C. elegans_
developmental timing. _Cell_ 106, 23–34 (2001). Article CAS PubMed Google Scholar * Ding, L., Spencer, A., Morita, K. & Han, M. The developmental timing regulator AIN-1 interacts
with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in _C. elegans_. _Mol. Cell_ 19, 437–447 (2005). Article CAS PubMed Google Scholar * Zhang, L. _et al._
Systematic identification of _C. elegans_ miRISC proteins, miRNAs, and mRNA targets by their interactions with GW182 proteins AIN-1 and AIN-2. _Mol. Cell_ 28, 598–613 (2007). Article CAS
PubMed PubMed Central Google Scholar * Li, L., Veksler-Lublinsky, I. & Zinovyeva, A. HRPK-1, a conserved KH-domain protein, modulates microRNA activity during _Caenorhabditis elegans_
development. _PLoS Genet._ 15, e1008067 (2019). Article CAS PubMed PubMed Central Google Scholar * Long, X. _et al._ TOR deficiency in _C. elegans_ causes developmental arrest and
intestinal atrophy by inhibition of mRNA translation. _Curr. Biol._ 12, 1448–1461 (2002). Article CAS PubMed Google Scholar * Wu, E. _et al._ Pervasive and cooperative deadenylation of
3′UTRs by embryonic microRNA families. _Mol. Cell_ 40, 558–570 (2010). Article CAS PubMed PubMed Central Google Scholar * Dallaire, A., Frédérick, P.-M. & Simard, M. J. Somatic and
germline microRNAs form distinct silencing complexes to regulate their target mRNAs differently. _Dev. Cell_ 47, 239-247.e4 (2018). Article CAS PubMed Google Scholar * Kim, J. K. _et
al._ Functional genomic analysis of RNA interference in _C. elegans_. _Science_ 308, 1164–1167 (2005). Article ADS CAS PubMed Google Scholar * Zhou, R. _et al._ Comparative analysis of
argonaute-dependent small RNA pathways in Drosophila. _Mol. Cell_ 32, 592–599 (2008). Article CAS PubMed PubMed Central Google Scholar * Zinovyeva, A. Y., Bouasker, S., Simard, M. J.,
Hammell, C. M. & Ambros, V. Mutations in conserved residues of the _C. elegans_ microRNA argonaute ALG-1 identify separable functions in ALG-1 miRISC loading and target repression. _PLoS
Genet._ 10, e1004286 (2014). Article CAS PubMed PubMed Central Google Scholar * Vella, M. C., Choi, E.-Y., Lin, S.-Y., Reinert, K. & Slack, F. J. The _C. elegans_ microRNA let-7
binds to imperfect let-7 complementary sites from the lin-41 3′UTR. _Gene Dev._ 18, 132–137 (2004). Article CAS PubMed PubMed Central Google Scholar * Ecsedi, M., Rausch, M. &
Großhans, H. The let-7 microRNA directs vulval development through a single target. _Dev. Cell_ 32, 335–344 (2015). Article CAS PubMed Google Scholar * Zhang, P. & Zhang, H.
Autophagy modulates miRNA-mediated gene silencing and selectively degrades AIN-1/GW182 in _C. elegans_. _Embo Rep._ 14, 568–576 (2013). Article CAS PubMed PubMed Central Google Scholar
* Abbott, A. L. _et al._ The let-7 microRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in _Caenorhabditis elegans_. _Dev. Cell_ 9, 403–414
(2005). Article CAS PubMed PubMed Central Google Scholar * Abrahante, J. E. _et al._ The _Caenorhabditis elegans_ hunchback-like gene lin-57/hbl-1 controls developmental time and is
regulated by microRNAs. _Dev. Cell_ 4, 625–637 (2003). Article CAS PubMed Google Scholar * Johnston, R. J. & Hobert, O. A microRNA controlling left/right neuronal asymmetry in
_Caenorhabditis elegans_. _Nature_ 426, 845–849 (2003). Article ADS CAS PubMed Google Scholar * Palmer, R. E., Inoue, T., Sherwood, D. R., Jiang, L. I. & Sternberg, P. W.
_Caenorhabditis elegans_ cog-1 locus encodes GTX/Nkx6.1 homeodomain proteins and regulates multiple aspects of reproductive system development. _Dev. Biol._ 252, 202–213 (2002). Article CAS
PubMed Google Scholar * Rausch, M., Ecsedi, M., Bartake, H., Müllner, A. & Großhans, H. A genetic interactome of the let-7 microRNA in _C. elegans_. _Dev. Biol._ 401, 276–286 (2015).
Article CAS PubMed Google Scholar * Flamand, M. N. _et al._ Poly(A)-binding proteins are required for microRNA-mediated silencing and to promote target deadenylation in _C. elegans_.
_Nucleic Acids Res._ 44, 5924–5935 (2016). Article CAS PubMed PubMed Central Google Scholar * Kim, S. _et al._ The regulatory impact of RNA-binding proteins on microRNA targeting. _Nat.
Commun._ 12, 5057 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Pan, K. Z. _et al._ Inhibition of mRNA translation extends lifespan in _Caenorhabditis elegans_.
_Aging Cell_ 6, 111–119 (2007). Article CAS PubMed Google Scholar * Chu, Y.-D., Chen, H.-K., Huang, T. & Chan, S.-P. A novel function for the DEAD-box RNA helicase DDX-23 in primary
microRNA processing in _Caenorhabditis elegans_. _Dev. Biol._ 409, 459–472 (2016). Article CAS PubMed Google Scholar * Beckmann, B. M. _et al._ The RNA-binding proteomes from yeast to
man harbour conserved enigmRBPs. _Nat. Commun._ 6, 10127 (2015). Article ADS CAS PubMed Google Scholar * Curtis, N. J. & Jeffery, C. J. The expanding world of metabolic enzymes
moonlighting as RNA binding proteins. _Biochem. Soc. Trans._ 49, 1099–1108 (2021). Article CAS PubMed Google Scholar * Rodríguez-Saavedra, C. _et al._ Moonlighting proteins: The case of
the hexokinases. _Front. Mol. Biosci._ 8, 701975 (2021). Article CAS PubMed PubMed Central Google Scholar * Yilmaz, L. S. & Walhout, A. J. M. A _Caenorhabditis elegans_ genome-scale
metabolic network model. _Cell Syst._ 2, 297–311 (2016). Article CAS PubMed PubMed Central Google Scholar * Kurimoto, K. _et al._ AU-rich RNA-binding induces changes in the quaternary
structure of AUH. _Proteins Struct. Funct. Bioinform._ 75, 360–372 (2009). Article CAS Google Scholar * Otsuka, H., Fukao, A., Funakami, Y., Duncan, K. E. & Fujiwara, T. Emerging
evidence of translational control by AU-rich element-binding proteins. _Front. Genet._ 10, 332 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhang, Z. _et al._ Broadly
conserved roles of TMEM131 family proteins in intracellular collagen assembly and secretory cargo trafficking. _Sci. Adv._ 6, eaay7667 (2020). Article ADS CAS PubMed PubMed Central
Google Scholar * Castelein, N., Hoogewijs, D., Vreese, A. D., Braeckman, B. P. & Vanfleteren, J. R. Dietary restriction by growth in axenic medium induces discrete changes in the
transcriptional output of genes involved in energy metabolism in _Caenorhabditis elegans_. _Biotechnol. J._ 3, 803–812 (2008). Article CAS PubMed Google Scholar * Ye, P. _et al._ An
mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. _Mol. Cell._ 57, 708–720 (2015). * Broughton, J. P., Lovci, M. T., Huang, J. L., Yeo, G. W.
& Pasquinelli, A. E. Pairing beyond the seed supports microRNA targeting specificity. _Mol. Cell_ 64, 320–333 (2016). Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS We thank members of the Zinovyeva lab for helpful discussions and technical assistance. We are most grateful to Victor Ambros, in whose lab this work was
initiated. We thank Erik Lundquist and Helge Grosshans for sharing reagents and strains. Some of the strains were provided by the CGC (Caenorhabditis Genetics Center), which is funded by the
National Institutes of Health Office of Research Infrastructure Programs P40-OD010440. AUTHOR INFORMATION Author notes * Ajay A. Vashisht Present address: Genomics Institute of the Novartis
Research Foundation, San Diego, 92121, USA AUTHORS AND AFFILIATIONS * Division of Biology, Kansas State University, Manhattan, 66506, USA Shilpa Hebbar, Ganesh Panzade & Anna Y.
Zinovyeva * Department of Biological Chemistry, David Geffen School of Medicine, University of California, Los Angeles, 90095, USA Ajay A. Vashisht & James A. Wohlschlegel * Department
of Software and Information Systems Engineering, Ben-Gurion University of the Negev, 8410501, Beer-Sheva, Israel Isana Veksler-Lublinsky Authors * Shilpa Hebbar View author publications You
can also search for this author inPubMed Google Scholar * Ganesh Panzade View author publications You can also search for this author inPubMed Google Scholar * Ajay A. Vashisht View author
publications You can also search for this author inPubMed Google Scholar * James A. Wohlschlegel View author publications You can also search for this author inPubMed Google Scholar * Isana
Veksler-Lublinsky View author publications You can also search for this author inPubMed Google Scholar * Anna Y. Zinovyeva View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS S.H.: Study concept and design, performing the experiments, analysis and interpretation of data, prepared figures, manuscript writing. G.P.P.: Data
analysis. I.V.-L.: Data analysis and editing manuscript. J.A.W. and A.A.V.: Proteomics experiments and data analysis. A.Y.Z.: Study concept and design, performing the experiments, editing
manuscript, technical and material support, study supervision. CORRESPONDING AUTHOR Correspondence to Anna Y. Zinovyeva. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE S5. SUPPLEMENTARY TABLE S1. SUPPLEMENTARY TABLE S2. SUPPLEMENTARY TABLE S3. SUPPLEMENTARY TABLE S4. RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hebbar, S., Panzade, G.,
Vashisht, A.A. _et al._ Functional identification of microRNA-centered complexes in _C. elegans_. _Sci Rep_ 12, 7133 (2022). https://doi.org/10.1038/s41598-022-10771-2 Download citation *
Received: 17 December 2021 * Accepted: 08 April 2022 * Published: 03 May 2022 * DOI: https://doi.org/10.1038/s41598-022-10771-2 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative








