- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND/OBJECTIVES To examine the clinical characteristics, risk factors and outcomes of contact lens-related bacterial keratitis (CLBK) in a large UK tertiary referral centre.
SUBJECTS/METHODS A retrospective analysis of all patients who presented to the Queen’s Medical Centre, Nottingham, UK, with suspected CLBK between October 2015 to September 2022 (a 7-year
period) was performed. Relevant data on demographic factors, CL wear behaviour, causes, clinical characteristics, and outcomes were analysed. RESULTS We included 138 patients with CLBK; the
mean age was 42.0 ± 17.8 years and 74 (53.6%) patients were male. Most CLBK were related to soft CL wear (94.5%), particularly monthly disposable (42.5%) and daily disposable (24.4%) CLs.
Poor CL wear behaviour/hygiene was documented in 57.1% cases. Among the 64 (46.4%) microbiological-positive cases (n = 73 organisms), _Pseudomonas aeruginosa_ (36, 49.3%) and _Staphylococcus
spp_. (16, 21.9%) were most commonly identified. Six (4.3%) cases were polymicrobial. Most (97.0%) patients were successfully treated with topical antibiotics alone, with 80.6% achieving
good final corrected-distance-visual-acuity (CDVA) of ≥ 0.30 logMAR. Poor visual outcome (final CDVA < 0.30 logMAR) was significantly associated with presenting CDVA < 0.6 logMAR (_p_
= 0.002) and central ulcer (_p_ = 0.004). Poor corneal healing (complete healing of > 30 days from initial presentation) was significantly associated with age > 50 years (_p_ = 0.028),
female gender (_p_ = 0.020), and infiltrate size >3 mm (_p_ = 0.031). CONCLUSIONS Poor CL wear behaviour/hygiene is commonly observed in CLBK, highlighting the importance of improved
counselling and awareness regarding CL use and hygiene. When presented early and managed appropriately, most patients are able to achieve good clinical outcomes with medical treatment alone.
SIMILAR CONTENT BEING VIEWED BY OTHERS CORNEAL FOREIGN BODIES: ARE ANTISEPTICS AND ANTIBIOTICS EQUALLY EFFECTIVE? Article 13 January 2023 PREDISPOSING FACTORS, MICROBIOLOGICAL FEATURES AND
OUTCOMES OF PATIENTS WITH CLINICAL PRESUMED CONCOMITANT MICROBIAL AND HERPES SIMPLEX KERATITIS Article 19 February 2021 INFECTIOUS KERATITIS: AN UPDATE ON EPIDEMIOLOGY, CAUSATIVE
MICROORGANISMS, RISK FACTORS, AND ANTIMICROBIAL RESISTANCE Article Open access 07 January 2021 INTRODUCTION Infectious keratitis (IK) represents the leading cause of corneal blindness
worldwide [1, 2]. Global incidence of IK has been estimated at approximately 2.5 to 799 cases per 100,000 population/year, with a considerably higher incidence in the low- and middle-income
countries (LMICs). It is a painful and potentially sight-threatening ocular disease that often requires intensive treatment and/or hospitalisation, posing significant impact on patients’
quality of life, healthcare systems, and economy [3]. IK can be caused by a wide range of pathogens, including bacteria, fungi, protozoa, and viruses. Bacterial keratitis (BK) has been
consistently shown to be the most common cause of microbial keratitis (i.e., bacterial, fungal, and protozoan keratitis) in developed countries, including the UK, accounting for > 90% of
all cases of microbial keratitis in some studies [4, 5]. The innate defence mechanisms at the ocular surface ensure that IK rarely occurs without any predisposing factor [2, 6, 7]. Among
all, contact lens (CL) wear is recognised as one of the main risk factors for BK [7,8,9,10]. Some studies have highlighted ~10-80 times increased risk of developing IK in CL wearers compared
to healthy non-CL wearers [11, 12]. This has a significant implication on global health as the number of contact lens wearers has been estimated at 140 million worldwide and is likely to
increase in the coming decades [13]. CL-related IK tends to affect younger patients and working adults more commonly due to their higher social and occupational needs for CL [8, 14]. This
could have a considerably negative effect on work productivity and economy as a result of lost working days. CL-related IK is most commonly caused by _Pseudomonas aeruginosa_, likely
attributed to its ability to survive in contact lens solution and ocular environment and to form biofilm on CL [8, 9, 15]. Several risk factors associated with CL-related IK have been
highlighted; these included CL material and design, overnight wear, showering/swimming with CL use, and poor hygiene, amongst others [16,17,18]. Despite the prevalent number of CL wearers
and the potential impact of CL-related IK, there was no study in the past decade that had specifically evaluated the clinical characteristics and outcomes of CLBK in the UK. In view of the
gap in the literature, this study aimed to provide an up-to-date analysis of CL-related BK (CLBK) in a major tertiary ophthalmic referral centre in the UK. MATERIALS AND METHODS This was a
retrospective study which captured all patients who had presented to Queen’s Medical Centre, Nottingham, UK between October 2015 and September 2022 (a 7-year period) with suspected CLBK
bacterial keratitis and underwent corneal sampling. The study was approved as a clinical audit by the Clinical Governance team at the Nottingham University Hospitals NHS Trust (Ref: 19–265C)
and was conducted in accordance with the tenets of Declaration of Helsinki. CASE IDENTIFICATION, INCLUSION AND EXCLUSION Potential eligible patients were first identified through the local
microbiology database [8, 19]. Both microbiological-positive and microbiological-negative presumed CLBK cases were included. Microbiological-positive cases were defined by the presence of
clinical characteristics of BK (e.g., corneal epithelial defect, infiltrate, and anterior chamber activity) with microbiological confirmation of bacterial organism(s) from corneal sampling.
Microbiological-negative cases were defined by the presence of clinical characteristics of BK in the absence of positive microbiological result and was treated with intensive topical
antibiotic treatment alone (but no other antimicrobial treatment). Exclusion criteria included cases that were not related to CL wear, non-bacterial related or mixed infections (i.e. mixed
bacterial, fungal and/or Acanthamoeba infections). DATA COLLECTION Relevant data regarding demographic factors, risk factors, presenting features, corrected-distance-visual-acuity (CDVA),
causative organisms, treatment, and outcomes were collected from local electronic health record systems using a standardised excel proforma. Details on CL wear included the type of CL worn
(e.g. daily/monthly disposable soft CL, extended wear soft CL, rigid gas permeable (RGP) CL, therapeutic bandage CL, and cosmetic CL), overnight wear, contact with water (e.g. showering or
swimming with CL wear), and frequency/duration of wear. Extended wear was referred to the CL type that were designed to be worn continuously, including overnight, whereas overnight wear was
referred to the behavioural use of CL overnight (irrespective of whether the CL was designed as extended wear or not). The size of epithelial defect and infiltrate was defined by the maximal
linear dimension of the ulcer and was divided into 3 categories: (1) small ( < 3.0 mm); (2) moderate (3.1–6 mm); and (3) large ( > 6.0 mm) [8]. The location of ulcer was defined as:
(1) peripheral: ulcer located fully within 3 mm of the limbus; (2) paracentral: any part of the ulcer involving the paracentral cornea ( > 3 mm from the limbus) but not affecting the
visual axis; and (3) central: any part of the ulcer affecting the visual axis. MICROBIOLOGICAL INVESTIGATIONS All patients who presented with suspected CLBK with any of the following
clinical characteristics, including (1) ulcer of >1 mm diameter, (2) central location, (3) presence of anterior chamber activity and/or hypopyon, and/or (4) atypical clinical
presentation, underwent corneal sampling as per the departmental guideline [8]. Corneal samples were sent for microscopic examination, microbiological culture and susceptibility testing,
and/or 16S/18S polymerase chain reaction (PCR; only introduced since June 2021) [20]. CL and CL solution were not routinely sent for culture, and therefore the results were not included in
the analysis. Cultures were inoculated on chocolate, blood, fastidious anaerobic, and Sabouraud dextrose agars to isolate bacterial and fungal organisms. For suspected _Acanthamoeba_
keratitis, corneal sampling was performed for either culture (using non-nutrient agar with _Escherichia coli_ overlay) or PCR [21]. However, as stated, fungal and _Acanthamoeba_ positive
cases were excluded from this study. CLINICAL MANAGEMENT All patients were started on intensive topical antibiotics with either fluoroquinolone monotherapy (levofloxacin 0.5% or moxifloxacin
0.5%) or dual therapy using fortified cefuroxime 5% plus fortified aminoglycoside (e.g., amikacin 2.5% or gentamicin 1.5%) or levofloxacin 0.5%. Topical antibiotics were given every hourly
for first 48 h then slowly tapered off over a few weeks-months, depending on the treatment response and clinical progress. Adjustments to treatment were made (if necessary) depending on the
microbiological results and clinical response to treatment. Hospitalisation was indicated in moderate/severe or potentially sight-threatening cases, presence of considerable corneal melt or
threatened/actual corneal perforation, or patients who might not manage or comply with treatment at home [8]. Systemic antibiotic was administered if there was risk or evidence of
sclerokeratitis or intraocular involvement such as endophthalmitis (which would also require intravitreal antibiotics). STATISTICAL ANALYSIS Statistical analysis was performed using SPSS
software version 28.0 (IBM Corp; Armonk, NY, USA). For descriptive and analytic purposes, cases were divided into microbiological-positive and microbiological-negative cases. Chi square test
or Fisher’s Exact test was used to analyse the difference between categorical variables whereas Student’s T-test or Mann-Whitney U-test was used to compare the means between two groups
where appropriate. CDVA was recorded and analysed in logMAR unit. CDVA of counting fingers, hand movement, perception of light, and no perception of light were converted to 1.9, 2.3, 2.8,
and 3.0 logMAR, respectively [22]. Multivariable logistic regression analysis was conducted to examine for any prognostic factors for poor visual outcome (defined as a final CDVA < 0.30
logMAR) and poor corneal healing (defined as > 30 days for complete corneal healing). Odds ratios (OR) were calculated and presented with a 95% confidence interval (CI). _P_-value of <
0.05 was considered statistically significant. RESULTS A total of 138 patients (_n_ = 138 eyes) were included. Patients’ mean age was 42.0 ± 17.8 years, with 120 (87.0%) being of working
age (i.e. 16–64 years old) and 74 (53.6%) being male (Table 1). No bilateral cases were identified. The mean follow-up duration was 2.9 ± 5.3 months. Of all cases, 64 (46.4%) were
microbiological-positive. CLINICAL FEATURES, CAUSATIVE ORGANISMS AND ANTIMICROBIAL SUSCEPTIBILITY RESULTS The baseline clinical characteristics are presented in Table 1. The mean duration of
symptoms prior to presentation was 3.3 ± 5.8 days. The majority of the patients presented with a CDVA of ≥0.30 logMAR (i.e., 6/12 or better Snellen vision; 59, 42.8%) and an ulcer with
small epithelial defect (106, 76.8%), small infiltrate (116, 84.1%), paracentral location (74, 53.6%), and absence of hypopyon (104, 75.4%). When comparing microbiological-positive and
microbiological-negative cases, microbiological-positive cases had a worse presenting CDVA, larger epithelial defect and infiltrate, and higher proportion of hypopyon (all _p_ < 0.05;
Table 1). Of the 64 microbiological-positive cases, 63 cases were culture-positive (including one case of smear-positive case) and one case was culture-negative but PCR-positive (which
identified _P. aeruginosa_ and _Abiotrophia defective_). Of all 73 bacteria isolated, _P. aeruginosa_ (36, 49.3%) and _Staphylococcus spp_. (16, 21.9%) were the most common bacteria (Table
2). There were six (4.3%) cases of polymicrobial infection (i.e. infection caused by more than one bacterial species) identified. Gram-positive bacteria demonstrated excellent susceptibility
to vancomycin (100%) and aminoglycosides (94.1%–100%), and moderate-to-good susceptibility to penicillin (84.6%) and fluoroquinolones (50%–77.8%). Gram-negative bacteria exhibited excellent
susceptibility to aminoglycosides (95.2%–100%) and moderate-to-good susceptibility to fluoroquinolones (75%) and cefuroxime (60.0%; Supplementary Table 1). DETAILS OF CONTACT LENS WEAR AND
RISK FACTORS Details of CL wear, including the types of CL and behavioural risk factors, are detailed in Table 3. Based on the available information, the most common type of CL was monthly
disposable refractive CL (56, 40.6%) followed by daily disposable refractive correcting lenses (30, 21.7%). A small number of cases were related to the use of RGP CL (7, 5.5%),
therapeutic/bandage CL (3, 2.4%), and cosmetic CL (1, 0.9%). None of the cases was related to orthokeratology RGP CL. There was a lack of information on the types of CL material as these
data were not routinely recorded in medical case notes. Of the 119 patients with details of CL wear, 68 (57.1%) patients were noted to have at least one behavioural risk factor, including
overnight wear (43, 36.1%), contact with water (59, 49.6%) and long duration of CL wear ( > 16 hours/day; 26/95, 27.4%). In addition to CL wear, 41 (29.7%) cases had at least one
additional risk factor, including ocular surface disease (32, 23.2%), systemic immunosuppression/diabetes (10, 7.2%), trauma (9, 6.5%), previous corneal graft (5, 3.6%), and concomitant use
of topical steroids (5, 3.6_%)_. CLINICAL MANAGEMENT AND OUTCOMES Full follow-up details were available for 134 (97.1%) patients. The majority (130, 97.0%) of patients were successfully
treated with intensive topical antibiotics alone, including levofloxacin / moxifloxacin monotherapy (65, 48.5%) and dual therapy (69, 51.5%), consisting of cefuroxime in combination with
amikacin, gentamicin, or levofloxacin. Topical steroids were used in 55 (41.0%) cases, with a mean start time of 11.1 ± 11.6 days after the initial presentation. Topical steroids were used
in both microbiological-positive (31, 50%) and microbiological-negative (24, 33.3%) cases for a mean duration of 23.6 ± 20.3 days, after excluding cases that required long-term topical
steroid use (e.g. eyes with previous keratoplasty) from the analysis. Hospitalisation for intensive treatment was warranted in 52 (37.7%) patients, with microbiological-positive cases having
a greater need and longer duration of hospitalisation (p < 0.01) (Table 1). Three (2.2%) patients underwent optical penetrating keratoplasty for treating post-infection corneal scar and
one (0.7%) patient required amniotic membrane grafting for managing persistent corneal epithelial defect. Recurrence of infection and corneal melt were noted in 11 (8.2%) and 6 (4.5%) cases,
respectively, but were successfully managed with topical antibiotics. No significant adverse event, such as corneal perforation or uncontrollable infection requiring therapeutic
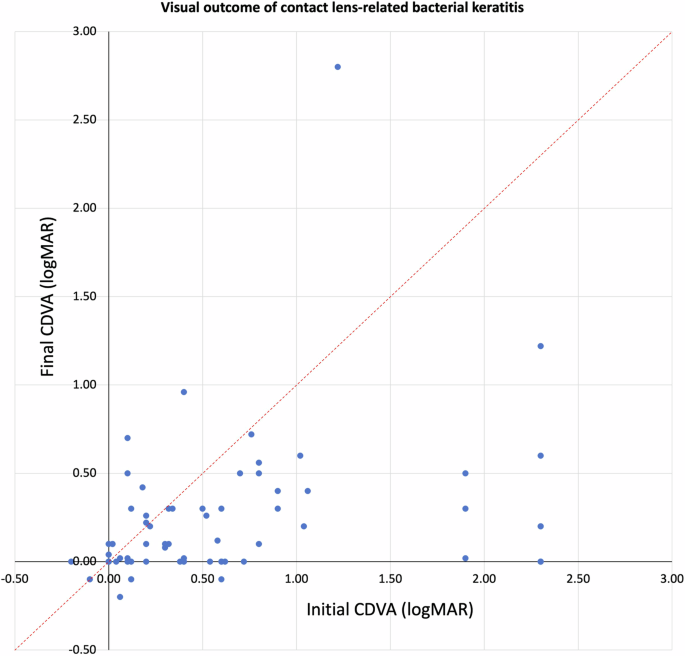
keratoplasty or anophthalmic surgery, was noted. Of the 134 patients, the mean CDVA improved significantly from 0.70 ± 0.76 logMAR at presentation to 0.21 ± 0.40 logMAR at final follow-up
(_p_ < 0.001), with 114 (85.1%) patients achieving an equal or better final CDVA compared to the initial CDVA (Fig. 1). The proportion of patients with CDVA of ≥ 0.30 logMAR increased
from 42.5% to 80.6% (_p_ < 0.001). The mean complete corneal healing time was 21.4 ± 15.2 days, with 31 (23.1%) cases having a delayed corneal healing (i.e. > 30 days). PROGNOSTIC
FACTORS Multivariable logistic regression analysis demonstrated that poor visual outcome was significantly influenced by presenting CDVA < 0.6 logMAR [OR 5.63 (95% CI, 1.85–17.17); _p_ =
0.002] and centrally located ulcer [OR 4.53 (95% CI, 1.62–12.71); _p_ = 0.004] (Table 4). Poor corneal healing time was significantly associated with age >50 years [OR 2.85 (95% CI,
1.12–7.25); _p_ = 0.028], female gender [OR 3.08 (95% CI, 1.19–7.94); _p_ = 0.020], and infiltrate >3 mm diameter [OR 3.53 (95% CI, 1.12–11.09); _p_ = 0.031]. Other factors such as eye
laterality, presence of hypopyon and positive microbiological results did not significantly affect the visual outcome or corneal healing time (all _p_ > 0.05). DISCUSSION CL wear
represents one of the most common risk factors for IK in developed countries, accounting for 30–65% of all IK cases [1, 7, 8]. The incidence of CL-related IK was estimated at 10–130 per
100,000 people-year, highlighting its potential burden on global health and economy [1, 11, 23]. To the best of our knowledge, this study represents the largest UK study that had
specifically examined the clinical characteristics, risk factors, causes, and outcomes of CLBK over the past decade. DEMOGRAPHIC AND RISK FACTORS Majority (87%) of our patients were of
working adult age, which was similarly observed in other studies (usually ~25–55 years old) [1, 8, 16, 24, 25]. Some studies have identified an increase in incidence of CL-related IK in
younger patients ( ~ 20–30 years old), potentially related to poorer CL hygiene and use of cosmetic CLs [17, 26]. A range of modifiable risk factors have previously reported, including
extended wear, overnight wear, contact with water, poor CL storage case hygiene/care, and poor hand hygiene. [16,17,18, 23, 24] In our study, we noted poor CL wear behaviours/hygiene such as
overnight wear, extended wear and contact with water in > 50% of our patients, and this figure may be potentially underestimated due to possible under-recording in clinical notes.
Furthermore, the type of CL serves as another significant influencing factor for CL-related IK [12, 18, 23]. Extended wear soft CL has been shown to have the highest risk of CL-related IK,
followed by daily soft CL (non-extended wear) and rigid gas-permeable CL [15, 23, 24, 27, 28]. Our study showed that monthly disposable CL was the most common type (42.5%) of CL whereas
extended wear CL was the least common choice (7.1%). In view of the low number of CLBK cases associated with extended overnight wear CL (despite its inherent higher risk of causing
infection), one may infer a reducing trend in the use of extended overnight wear CL in this UK population. While all these risk factors increase the risk of CLBK, we did not observe any
significant influence of these risk factors on microbiological positivity. CLINICAL FEATURES AND CAUSATIVE ORGANISMS Majority of our cases were of mild severity (84% with < 3 mm
infiltrate), which might be attributed to the early presentation to the hospital (the mean duration of symptoms before presentation was 3.3 days). This has significant clinical implications
as visual outcome of IK, including CLBK, is highly dependent on timely diagnosis and treatment [8, 29]. The relatively mild severity of infection may also be attributed to the low proportion
( < 10%) of extended wear CL in our cohort. Extended wear CL can increase the retention time and growth of the organisms on the ocular surface, resulting in more severe infection, as
opposed to daily replacement of CL [24]. We also observed that microbiological positivity was correlated with the severity of infection, which was similarly observed in previous studies [8,
30]. In contrast, a US study conducted more than a decade ago reported that a considerably higher proportion of CLBK patients presented with more severe infection, with an ulcer > 4 mm in
size (46%) and hypopyon (36%) [31]. The disparity in severity may be related to the difference in the proportion of cases affected by _P. aeruginosa_ (63% in the US study), ocular
co-morbidities, and/or time interval between onset of symptoms and presentation to the hospital. _P. aeruginosa_ (49.3%) and _Staphylococcus spp_. (21.9%) were shown to be the most common
organisms in our study, paralleling the findings of many other studies [9, 16, 24, 28, 31]. However, a study conducted in South India identified _Serratia spp_. as the most common organism
for CLBK, whereas a Japanese study reported _Staphylococcus epidermidis_ as the most common causative organism [9, 25]. Such heterogeneity highlights the geographical and temporal variations
of the disease and the importance of up-to-date regional analysis of the causative organisms (as it may serve as a useful guide for treatment, particularly when the microbiological result
is negative). CLINICAL AND VISUAL OUTCOMES The majority (97.0%) of our patients were successfully managed with topical antibiotics alone (without systemic antibiotic or surgical
intervention), with 80.6% achieving a final CDVA of ≥ 0.30 logMAR. Enzor et al. [32] reported a slightly higher rate of surgical intervention (14%) for their patients with CLBK. However,
they only included cases affected by _P. aeruginosa_, which is notoriously known to cause severe corneal infection, stromalysis and visual loss [9, 28, 32]. Nonetheless, CLBK has been shown
to have a better visual outcome than non-CLBK [32]. This may be attributed to a younger age, less ocular/systemic comorbidities and earlier presentation of the disease in patients with CLBK
(as CL wearers may be more aware/concern of any new ocular symptoms that can interfere with their CL wear). Indeed, when compared to our previous BK study (which included 65% non-CLBK and
35% CLBK) [8], the mean duration of symptoms prior to presentation was 5.3 days in the previous study (as opposed to 3.3 days in this study), suggesting that CL wearers are more likely to
seek earlier medical attention. In addition, 35% of the patients in the previous study had more severe infection at presentation than this study (35% vs. 16% with ulcers of >3 mm
infiltrate). We also observed that poor visual outcome was significantly associated with poorer presenting CDVA and central ulcer. These findings were similarly observed in other CLBK
studies conducted in France and the US [10, 32], highlighting the importance of prompt diagnosis and treatment in CLBK and IK in general [16]. Furthermore, we found that corneal healing was
negatively correlated with increased age and larger infiltrate size. Increased cell senescence in ageing cornea may affect the epithelial adhesion molecules function, phagocytic ability of
reactive polymorphonuclear cells, and the innate and/or adaptive immune responses at the ocular surface, resulting in slower eradication of infection and delayed corneal healing [33]. In
addition, older patients generally have multiple ocular/systemic co-morbidities (e.g. ocular surface diseases, diabetes, previous corneal surgeries, etc.), which can contribute to poor wound
healing. Interestingly, female gender was associated with poorer corneal healing, which contrasted the findings observed by Das et al. [25] and Konda et al. [27]. While sex-specific
difference in corneal healing has been shown in mice studies (with female mice corneas having a slower healing rate due to the effect of oestradiol) [34], this observation has not been
supported by other preclinical or human corneal studies [35]. Our finding may be confounded by other variables (e.g., difference in types and severity of co-morbidities) and requires further
elucidation. While we found that microbiological positivity was associated with more severe infection, it did not influence the visual outcome or the corneal healing time, similarly
observed in other studies [31, 36]. One plausible explanation is the ability to initiate/administer the optimal antibiotic therapy based on the positive microbiological and
susceptibility/resistance results, thereby achieving a similarly good outcome as microbiological-negative cases. In addition, a significantly higher proportion of patients with
microbiological-positive CLBK were hospitalised for intensive treatment and monitoring (for a longer duration), which might have contributed to the good clinical recovery and outcomes.
Reassuringly, only a few patients required surgical interventions, including amniotic membrane grafting, which has been shown to expedite corneal healing in bacterial keratitis [37]. STUDY
LIMITATIONS One of our study limitations is that we only included cases that had undergone corneal sampling; hence some of the very mild CLBK cases (i.e. small ( < 1 mm),
non-sight-threatening ulcer) might not have been captured by this study. However, these very mild cases usually have less impact on the patients and are responsive to topical antibiotics; if
not, the affected patients would re-present to our unit for further management (which would include corneal sampling and be included in our study). Another limitation is the inclusion of
microbiological-negative CLBK cases. However, all cases were only included after careful analysis of patients’ case notes to confirm eligibility. In addition, microbiological-negative cases
represent >50% of all IK cases in the real-world setting in many regions, including the UK [5, 19]. Therefore, a good understanding of the clinical characteristics, management and
outcomes of microbiological-negative cases are invaluable for clinicians and patients. Implementation of molecular diagnostics and next-generation sequencing may potentially ameliorate the
low culture yield in the future [38,39,40]. As this study was not a case-control study, we could not ascertain the relative/absolute risk difference of CLBK among each CL type. It would also
be interesting to analyse the impact of CL material on the clinical characteristics of CLBK as it has been shown to influence the severity of infection [18], though these data were not
routinely collected in daily clinical practice. Nonetheless, we observed that 57% of our patients reported poor CL hygiene, and this figure might be underestimated as these risk factors
might be under-recorded in medical notes. In conclusion, CLBK represents as a significant ocular condition that can have a negative impact on patient’s vision, quality of life, healthcare
resources, and work productivity (as affected patients are usually of working age). Timely presentation, diagnosis and treatment is key to achieving a good outcome. Poor CL behavioural risk
factors are commonly observed among our patients, highlighting the need for improved education and awareness of CL care/hygiene. SUMMARY WHAT WAS KNOWN BEFORE * Contact lens (CL) wear is a
major risk factor for bacterial keratitis. * Poor CL wear behaviour/hygiene increases the risk of CL-related bacterial keratitis (CLBK). * There is no study in the UK that had specifically
analysed the risk factors, causes and outcomes of CLBK in the past decade. WHAT THIS STUDY ADDS * This study provides one of the most comprehensive and up-to-date analyses of CLBK in the UK
in the past decade. * With prompt diagnosis and treatment, most patients with CLBK can be successfully treated with intensive topical antibiotics alone and achieve a good outcome. *
Microbiological positivity is significantly associated with the initial severity of CLBK but has no significant influence on the final visual outcome or corneal healing time. DATA
AVAILABILITY The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. REFERENCES * Ting DSJ, Ho CS, Deshmukh
R, Said DG, Dua HS. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye (Lond) 2021;35:1084–101. Article PubMed
Google Scholar * Stapleton F. The epidemiology of infectious keratitis. Ocul Surf. 2023;28:351–63. Article PubMed Google Scholar * Moussa G, Hodson J, Gooch N, Virdee J, Penaloza C,
Kigozi J, et al. Calculating the economic burden of presumed microbial keratitis admissions at a tertiary referral centre in the UK. Eye (Lond) 2021;35:2146–54. Article PubMed Google
Scholar * Ting DSJ, Ho CS, Cairns J, Gopal BP, Elsahn A, Al-Aqaba M, et al. Seasonal patterns of incidence, demographic factors and microbiological profiles of infectious keratitis: the
Nottingham Infectious Keratitis Study. Eye (Lond) 2021;35:2543–9. Article PubMed Google Scholar * Tan SZ, Walkden A, Au L, Fullwood C, Hamilton A, Qamruddin A, et al. Twelve-year analysis
of microbial keratitis trends at a UK tertiary hospital. Eye (Lond) 2017;31:1229–36. Article CAS PubMed Google Scholar * Ting DSJ, Galal M, Kulkarni B, Elalfy MS, Lake D, Hamada S, et
al. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011-2020: A 10-Year Study. J Fungi (Basel). 2021;7:966. * Khoo P, Cabrera-Aguas MP, Nguyen V, Lahra MM,
Watson SL. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefes Arch Clin Exp Ophthalmol. 2020;258:1745–55. Article PubMed Google
Scholar * Ting DSJ, Cairns J, Gopal BP, Ho CS, Krstic L, Elsahn A, et al. Risk Factors, Clinical Outcomes, and Prognostic Factors of Bacterial Keratitis: The Nottingham Infectious Keratitis
Study. Front Med. (Lausanne) 2021;8:715118. Article PubMed Google Scholar * Hatami H, Ghaffari Jolfayi A, Ebrahimi A, Golmohammadi S, Zangiabadian M, Nasiri MJ Contact Lens Associated
Bacterial Keratitis: Common Organisms, Antibiotic Therapy, and Global Resistance Trends: A Systematic Review. Front Ophthalmol. 1: https://doi.org/10.3389/fopht.2021.759271 (2021). *
Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol 2003;87:834–8. Article
CAS PubMed PubMed Central Google Scholar * Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, et al. Epidemiology of ulcerative keratitis in Northern California. Arch
Ophthalmol. 2010;128:1022–8. Article PubMed Google Scholar * Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet 1991;338:650–3.
Article CAS PubMed Google Scholar * Nichols JJ, Willcox MD, Bron AJ, Belmonte C, Ciolino JB, Craig JP, et al. The TFOS International Workshop on Contact Lens Discomfort: executive
summary. Invest Ophthalmol Vis Sci. 2013;54:Tfos7–tfos13. Article PubMed PubMed Central Google Scholar * Cope JR, Collier SA, Nethercut H, Jones JM, Yates K, Yoder JS. Risk Behaviors for
Contact Lens-Related Eye Infections Among Adults and Adolescents - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:841–5. Article PubMed PubMed Central Google Scholar *
Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26:185–93. Article CAS
PubMed Google Scholar * Lim CH, Carnt NA, Farook M, Lam J, Tan DT, Mehta JS, et al. Risk factors for contact lens-related microbial keratitis in Singapore. Eye (Lond) 2016;30:447–55.
Article CAS PubMed Google Scholar * Stellwagen A, MacGregor C, Kung R, Konstantopoulos A, Hossain P. Personal hygiene risk factors for contact lens-related microbial keratitis. BMJ Open
Ophthalmol. 2020;5:e000476. Article PubMed PubMed Central Google Scholar * Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB. Incidence of keratitis of varying severity among
contact lens wearers. Br J Ophthalmol. 2005;89:430–6. Article CAS PubMed PubMed Central Google Scholar * Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba M, Boswell T, et al. 12-year
analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol 2021;105:328–33.
Article PubMed Google Scholar * Hammoudeh Y, Suresh L, Ong ZZ, Lister MM, Mohammed I, Thomas DJI, et al. Microbiological culture versus 16S/18S rRNA gene PCR-Sanger sequencing for
infectious keratitis: A three-arm, diagnostic cross-sectional study. Front Med. 2024;11:1393832. Article Google Scholar * Wong TL, Ong ZZ, Marelli L, Pennacchi A, Lister M, Said DG, et al.
False positive microbiological results in Acanthamoeba keratitis: the importance of clinico-microbiological correlation. Eye (Lond). 2023;37:3699–701. * Ong ZZ, Wong TL, Suresh L, Hammoudeh
Y, Lister M, Said DG, et al. A 7-year review of clinical characteristics, predisposing factors and outcomes of post-keratoplasty infectious keratitis: the Nottingham infectious keratitis
study. Front Cell Infect Microbiol. 2023;13:1250599. Article PubMed PubMed Central Google Scholar * Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, Brian G, et al. The incidence
of contact lens-related microbial keratitis in Australia. Ophthalmology 2008;115:1655–62. Article PubMed Google Scholar * Stapleton F, Naduvilath T, Keay L, Radford C, Dart J, Edwards K,
et al. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One 2017;12:e0181343. Article PubMed PubMed Central Google Scholar * Das S,
Sheorey H, Taylor HR, Vajpayee RB. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch Ophthalmol. 2007;125:1182–5. Article
PubMed Google Scholar * Sauer A, Bourcier T. Microbial keratitis as a foreseeable complication of cosmetic contact lenses: a prospective study. Acta Ophthalmol. 2011;89:e439–42. Article
PubMed Google Scholar * Konda N, Garg P, Sharma S, Willcox MDP. Risk Factors for Contact Lens-Related Microbial Keratitis and Associated Vision Loss in a South Indian Population. Eye
Contact Lens 2021;47:118–26. Article PubMed Google Scholar * Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, et al. Incidence of contact-lens-associated microbial
keratitis and its related morbidity. Lancet 1999;354:181–5. Article CAS PubMed Google Scholar * Prajna NV, Krishnan T, Mascarenhas J, Srinivasan M, Oldenburg CE, Toutain-Kidd CM, et al.
Predictors of outcome in fungal keratitis. Eye (Lond.) 2012;26:1226–31. Article PubMed Google Scholar * Ung L, Wang Y, Vangel M, Davies EC, Gardiner M, Bispo PJM, et al. Validation of a
Comprehensive Clinical Algorithm for the Assessment and Treatment of Microbial Keratitis. Am J Ophthalmol. 2020;214:97–109. Article PubMed Google Scholar * Yildiz EH, Airiani S,
Hammersmith KM, Rapuano CJ, Laibson PR, Virdi AS, et al. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea 2012;31:1097–102. Article PubMed Google Scholar
* Enzor R, Bowers EMR, Perzia B, Perera C, Palazzolo L, Mammen A, et al. Comparison of Clinical Features and Treatment Outcomes of Pseudomonas aeruginosa Keratitis in Contact Lens and
Non-Contact Lens Wearers. Am J Ophthalmol. 2021;227:1–11. Article PubMed Google Scholar * Galletti JG, de Paiva CS. The ocular surface immune system through the eyes of aging. Ocul Surf.
2021;20:139–62. Article PubMed PubMed Central Google Scholar * Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective
lipid mediator circuits in the cornea. Faseb J 2012;26:1506–16. Article CAS PubMed PubMed Central Google Scholar * McKay TB, Priyadarsini S, Karamichos D. Sex Hormones, Growth Hormone,
and the Cornea. Cells. 2022;11:224. * Otri AM, Fares U, Al-Aqaba MA, Miri A, Faraj LA, Said DG, et al. Profile of sight-threatening infectious keratitis: a prospective study. Acta
Ophthalmol. 2013;91:643–51. Article PubMed Google Scholar * Ting DSJ, Henein C, Said DG, Dua HS. Amniotic membrane transplantation for infectious keratitis: a systematic review and
meta-analysis. Sci Rep. 2021;11:13007. Article CAS PubMed PubMed Central Google Scholar * Somerville TF, Corless CE, Sueke H, Neal T, Kaye SB. 16S Ribosomal RNA PCR Versus Conventional
Diagnostic Culture in the Investigation of Suspected Bacterial Keratitis. Transl Vis Sci Technol. 2020;9:2. Article PubMed PubMed Central Google Scholar * Low L, Fuentes-Utrilla P,
Hodson J, O’Neil JD, Rossiter AE, Begum G, et al. Evaluation of full-length nanopore 16S sequencing for detection of pathogens in microbial keratitis. PeerJ. 2021;9:e10778. Article PubMed
PubMed Central Google Scholar * Ting DSJ, Chodosh J, Mehta JS. Achieving diagnostic excellence for infectious keratitis: A future roadmap. Front Microbiol. 2022;13:1020198. Article PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS Funding/Support: DSJT acknowledges support from the Birmingham Health Partners (BHP) Clinician Scientist Fellowship,
the Medical Research Council / Fight for Sight Clinical Research Fellowship (MR/T001674/1), and the FFS / John Lee, Royal College of Ophthalmologists Primer Fellowship (24CO4). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Academic Ophthalmology, School of Medicine, University of Nottingham, Nottingham, UK Lakshmi Suresh, Yasmeen Hammoudeh, Jessica Cairns, Dalia G. Said,
Harminder S. Dua & Darren S. J. Ting * Department of Ophthalmology, Western Eye Hospital, London, UK Charlotte S. Ho * New Cross Eye Hospital, Wolverhampton, UK Zun Zheng Ong *
Department of Ophthalmology, Luton Hospital, Luton, UK Bhavesh P. Gopal * Department of Ophthalmology, Queen’s Medical Centre, Nottingham, UK Lazar Krstic, Dalia G. Said & Harminder S.
Dua * Department of Ophthalmology, King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation, Sutton-in-Ashfield, UK Ahmad Elsahn * Department of Microbiology, Queen’s Medical Centre,
Nottingham, UK Michelle M. Lister * Academic Unit of Ophthalmology, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK Darren S. J. Ting * Birmingham and Midland
Eye Centre, Birmingham, UK Darren S. J. Ting Authors * Lakshmi Suresh View author publications You can also search for this author inPubMed Google Scholar * Yasmeen Hammoudeh View author
publications You can also search for this author inPubMed Google Scholar * Charlotte S. Ho View author publications You can also search for this author inPubMed Google Scholar * Zun Zheng
Ong View author publications You can also search for this author inPubMed Google Scholar * Jessica Cairns View author publications You can also search for this author inPubMed Google Scholar
* Bhavesh P. Gopal View author publications You can also search for this author inPubMed Google Scholar * Lazar Krstic View author publications You can also search for this author inPubMed
Google Scholar * Ahmad Elsahn View author publications You can also search for this author inPubMed Google Scholar * Michelle M. Lister View author publications You can also search for this
author inPubMed Google Scholar * Dalia G. Said View author publications You can also search for this author inPubMed Google Scholar * Harminder S. Dua View author publications You can also
search for this author inPubMed Google Scholar * Darren S. J. Ting View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Study conceptualisation
and design: DSJT; Data collection: LS, YH, CSH, ZZO, JC, BPG, LK, AE, MML; Data analysis: LS, DSJT; Data interpretation: LS, DGS, HSD, DSJT; Drafting of initial manuscript: LS, DSJT;
Critical revision and approval of final manuscript: All authors; Study supervision: DSJT CORRESPONDING AUTHOR Correspondence to Darren S. J. Ting. ETHICS DECLARATIONS COMPETING INTERESTS DGS
serves as a member of the Eye editorial board. All other authors have competing interests to declare. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Suresh, L., Hammoudeh, Y., Ho, C.S. _et al._ Clinical features, risk
factors and outcomes of contact lens-related bacterial keratitis in Nottingham, UK: a 7-year study. _Eye_ 38, 3459–3466 (2024). https://doi.org/10.1038/s41433-024-03323-7 Download citation
* Received: 20 December 2023 * Revised: 07 August 2024 * Accepted: 03 September 2024 * Published: 12 September 2024 * Issue Date: December 2024 * DOI:
https://doi.org/10.1038/s41433-024-03323-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative