- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT DNA damage-inducible transcript 3 (DDIT3) is a well-known transcription factor that regulates the expression of apoptosis-related genes for promoting apoptosis during endoplasmic
reticulum stress. Here, we report an unrecognized role of DDIT3 in facilitating necroptosis. DDIT3 directly binds and competitively prevents the p38 MAPK-MK2 interaction and thereby blocking
MK2 activation while stimulating p38 MAPK activation. This blockage of MK2 activation initially prevents RIPK1 phosphorylation at Ser320 (inactivation), subsequently relieving its
suppression of RIPK1 activation. Consequently, p38 MAPK facilitates RIPK1 phosphorylation at Ser166 (activation) through DDIT3 phosphorylation-related mechanisms, leading to necroptosis.
Mechanistically, a 10-amino acid segment (Glu19-Val28) within DDIT3’s N-terminus is identified to account for its pro-necroptotic function. In vivo studies demonstrate that forced expression
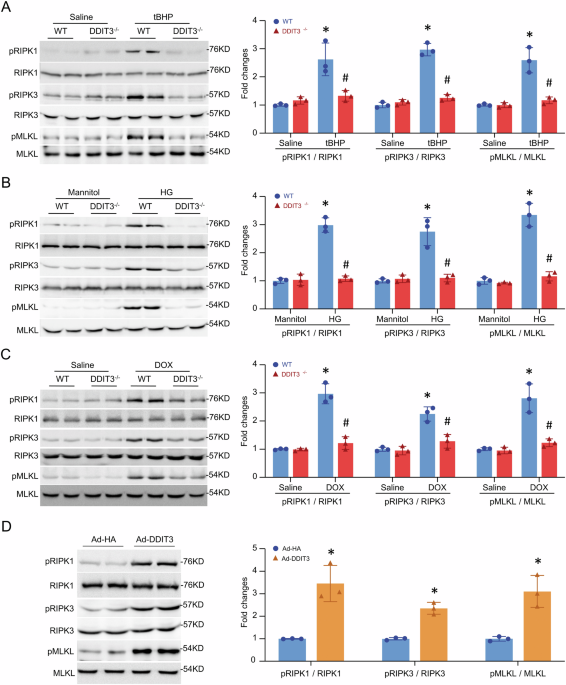
of DDIT3 induces necroptosis, whereas deletion of DDIT3 alleviates necroptosis in mouse hearts under stress. These findings shed light on a novel regulatory mechanism by which DDIT3
promotes RIPK1 activation and subsequent necroptosis. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ANGIOPOIETIN-LIKE PROTEIN 8 DIRECTS DNA DAMAGE RESPONSES TOWARDS APOPTOSIS BY
STABILIZING PARP1-DNA CONDENSATES Article 27 November 2024 P53 INDUCES ARTS TO PROMOTE MITOCHONDRIAL APOPTOSIS Article Open access 24 February 2021 IKK-MEDIATED TRAF6 AND RIPK1 INTERACTION
STIFLES CELL DEATH COMPLEX ASSEMBLY LEADING TO THE SUPPRESSION OF TNF-Α-INDUCED CELL DEATH Article 21 April 2023 DATA AVAILABILITY The datasets generated during and/or analyzed during the
current study are available from the corresponding author on reasonable request. REFERENCES * Guo X, Chen Y, Liu Q. Necroptosis in heart disease: molecular mechanisms and therapeutic
implications. J Mol Cell Cardiol. 2022;169:74–83. Article CAS PubMed PubMed Central Google Scholar * Dondelinger Y, Delanghe T, Rojas-Rivera D, Priem D, Delvaeye T, Bruggeman I, et al.
MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat Cell Biol. 2017;19:1237–47. Article CAS PubMed Google Scholar * Jaco I, Annibaldi A, Lalaoui N, Wilson R, Tenev T,
Laurien L, et al. MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol Cell. 2017;66:698–710.e5. Article CAS PubMed PubMed Central Google Scholar * Linkermann A, Hackl MJ,
Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–804. Article CAS PubMed Google Scholar * Guo Y,
Zhou J, Wang Y, Wu X, Mou Y, Song X. Cell type‐specific molecular mechanisms and implications of necroptosis in inflammatory respiratory diseases. Immunol Rev. 2023;321:imr.13282. *
Chavoshinezhad S, Beirami E, Izadpanah E, Feligioni M, Hassanzadeh K. Molecular mechanism and potential therapeutic targets of necroptosis and ferroptosis in Alzheimer’s disease. Biomed
Pharmacother. 2023;168:115656. Article CAS PubMed Google Scholar * Zhao Y, Main K, Aujla T, Keshavjee S, Liu M. Necroptosis in organ transplantation: mechanisms and potential therapeutic
targets. Cells. 2023;12:2296. Article CAS PubMed PubMed Central Google Scholar * Ye Z, Zhang N, Lei H, Yao H, Fu J, Zhang N, et al. Immunogenic necroptosis in liver diseases:
mechanisms and therapeutic potential. J Mol Med. 2023. https://doi.org/10.1007/s00109-023-02363-y. Article PubMed Google Scholar * Gupta A, Chakole S, Agrawal S, Khekade H, Prasad R,
Lohakare T, et al. Emerging insights into necroptosis: implications for renal health and diseases. Cureus. 2023. https://doi.org/10.7759/cureus.43609. * Ron D, Habener JF. CHOP, a novel
developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev.
1992;6:439–53. Article CAS PubMed Google Scholar * Fornace AJ, Alamo I, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA. 1988;85:8800–4. Article
CAS PubMed PubMed Central Google Scholar * Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. Article CAS PubMed Google
Scholar * Coustry F, Posey KL, Liu P, Alcorn JL, Hecht JT. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am J Pathol. 2012;180:738–48. Article CAS
PubMed PubMed Central Google Scholar * Posey KL, Coustry F, Veerisetty AC, Liu P, Alcorn JL, Hecht JT. Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes
in an inducible transgenic mouse model of pseudoachondroplasia. Am J Pathol. 2012;180:727–37. Article CAS PubMed PubMed Central Google Scholar * Li M, Fu Z, Qi C, Wang Q, Xie H, Li H.
Some macrophages with high expression of CHOP undergo necroptosis in chronic rhinosinusitis. Am J Rhinol Allergy. 2023;37:449–55. Article PubMed Google Scholar * Ma Y, Peng Y, Zhu Q, Gao
A, Chao B, He Q, et al. Novel CHOP activator LGH00168 induces necroptosis in A549 human lung cancer cells via ROS-mediated ER stress and NF-κB inhibition. Acta Pharmacol Sin.
2016;37:1381–90. Article CAS PubMed PubMed Central Google Scholar * Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, et al. Ablation of C/EBP homologous protein attenuates
endoplasmic reticulum–mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–9. Article CAS PubMed Google Scholar * Miyazaki Y, Kaikita K,
Endo M, Horio E, Miura M, Tsujita K, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler
Thromb Vasc Biol. 2011;31:1124–32. Article CAS PubMed Google Scholar * Li J, Zhu H, Shen E, Wan L, Arnold JMO, Peng T. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits
endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–42. Article CAS PubMed PubMed Central Google Scholar *
Ljubkovic M, Gressette M, Bulat C, Cavar M, Bakovic D, Fabijanic D, et al. Disturbed fatty acid oxidation, endoplasmic reticulum stress, and apoptosis in left ventricle of patients with type
2 diabetes. Diabetes. 2019;68:1924–33. Article CAS PubMed Google Scholar * Yang Y, Liu L, Naik I, Braunstein Z, Zhong J, Ren B. Transcription factor C/EBP homologous protein in health
and diseases. Front Immunol. 2017;8:1612. Article PubMed PubMed Central Google Scholar * Rius-Pérez S. p53 at the crossroad between mitochondrial reactive oxygen species and necroptosis.
Free Radic Biol Med. 2023;207:183–93. Article PubMed Google Scholar * Yang T, Wang G, Zhang M, Hu X, Li Q, Yun F, et al. Triggering endogenous Z-RNA sensing for anti-tumor therapy
through ZBP1-dependent necroptosis. Cell Rep. 2023;42:113377. Article CAS PubMed Google Scholar * Cao T, Ni R, Ding W, Ji X, Li L, Liao G, et al. MLKL-mediated necroptosis is a target
for cardiac protection in mouse models of type-1 diabetes. Cardiovasc Diabetol. 2022;21:165. Article CAS PubMed PubMed Central Google Scholar * Zheng D, Zhang Y, Zheng M, Cao T, Wang G,
Zhang L, et al. Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin Sci. 2019;133:1505–21. Article CAS Google Scholar * Maytin
EV, Ubeda M, Lin JC, Habener JF. Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp Cell Res.
2001;267:193–204. Article CAS PubMed Google Scholar * Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science.
1996;272:1347–9. Article CAS PubMed Google Scholar * Hu J-H, Chen T, Zhuang Z-H, Kong L, Yu M-C, Liu Y, et al. Feedback control of MKP-1 expression by p38. Cell Signal. 2007;19:393–400.
Article CAS PubMed Google Scholar * Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar
macrophages to lipopolysaccharide. J Biol Chem. 2005;280:8101–8. Article CAS PubMed Google Scholar * Hu C-D, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel
family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–98. Article CAS PubMed Google Scholar * Abramson J, Adler J, Dunger J, Evans R, Green
T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. Article CAS PubMed PubMed Central Google Scholar * Haar ET,
Prabakhar P, Liu X, Lepre C. Crystal structure of the p38 alpha-MAPKAP kinase 2 heterodimer. J Biol Chem. 2007;282:9733–9. Article PubMed Google Scholar * Gao P, Cao M, Jiang X, Wang X,
Zhang G, Tang X, et al. Cannabinoid receptor 2-centric molecular feedback loop drives necroptosis in diabetic heart injuries. Circulation. 2023;147:158–74. Article CAS PubMed Google
Scholar Download references FUNDING This study was partially supported by a discovery grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2023-04573 to TP),
the Lawson Internal Research Fund (TP), High-level Foreign Experts Project by the Ministry of Science and Technology (G2022014014156L), Research Cooperation and High-level Talent Training
Project of the China Scholarship Council (2022), and Projects of International Cooperation from Jiangsu (BZ2024031, China). AUTHOR INFORMATION Author notes * These authors contributed
equally: Rui Ni, Ting Cao. AUTHORS AND AFFILIATIONS * International Genome Center, Jiangsu University, Zhenjiang, 212013, China Rui Ni, Xiaoyun Ji & Zhaoliang Su * Lawson Health Research
Institute, London Health Sciences Centre, London, ON, N6A 5W9, Canada Rui Ni, Xiaoyun Ji, Zhuxu Zhang & Tianqing Peng * Department of Pathology and Laboratory Medicine, Western
University, London, ON, N6A 5C1, Canada Rui Ni, Xiaoyun Ji, Angel Peng, Zhuxu Zhang, Subrata Chakrabarti & Tianqing Peng * Institute for Cardiovascular Science, Soochow University,
Suzhou, 215123, China Ting Cao * Department of Medicine, Western University, London, ON, N6A 5W9, Canada Zhuxu Zhang & Tianqing Peng * Department of Pharmacology and Systems Physiology,
University of Cincinnati College of Medicine, Cincinnati, OH, 45267, USA Guo-Chang Fan * Department of Physiology and Pharmacology, Western University, London, ON, N6A 5C1, Canada Peter
Stathopulos Authors * Rui Ni View author publications You can also search for this author inPubMed Google Scholar * Ting Cao View author publications You can also search for this author
inPubMed Google Scholar * Xiaoyun Ji View author publications You can also search for this author inPubMed Google Scholar * Angel Peng View author publications You can also search for this
author inPubMed Google Scholar * Zhuxu Zhang View author publications You can also search for this author inPubMed Google Scholar * Guo-Chang Fan View author publications You can also search
for this author inPubMed Google Scholar * Peter Stathopulos View author publications You can also search for this author inPubMed Google Scholar * Subrata Chakrabarti View author
publications You can also search for this author inPubMed Google Scholar * Zhaoliang Su View author publications You can also search for this author inPubMed Google Scholar * Tianqing Peng
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS RN, TC, XJ, and AP conducted the experiments and analyzed the data; RN, TC, GCF, ZZ, SC,
ZS, and TP designed the experiments and wrote the paper; RN, XJ, AP, GCF, ZZ, PS, SC, ZS, and TP discussed the data and revised the paper. PS contributed to protein-protein docking analysis
and structural mechanism. All authors approved the final version to be published. CORRESPONDING AUTHORS Correspondence to Zhaoliang Su or Tianqing Peng. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ETHICAL APPROVAL All mouse experimental procedures were approved by the Animal Use Subcommittee at the University of Western Ontario,
Canada (protocol numbers: 2021-054 and 2020-047), and Soochow University, China (protocol number: 2017-0043). All methods were performed in accordance with the relevant guidelines and
regulations. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY DATA RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement
with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and
applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ni, R., Cao, T., Ji, X. _et al._ DNA damage-inducible transcript 3 positively regulates RIPK1-mediated
necroptosis. _Cell Death Differ_ 32, 306–319 (2025). https://doi.org/10.1038/s41418-024-01385-4 Download citation * Received: 26 December 2023 * Revised: 12 September 2024 * Accepted: 17
September 2024 * Published: 03 October 2024 * Issue Date: February 2025 * DOI: https://doi.org/10.1038/s41418-024-01385-4 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative