- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Shiga toxin producing _Escherichia coli_ (STEC) are noninvasive enteric pathogens that may cause hemorrhagic colitis (HC) and diarrhea-associated hemolytic uremic syndrome (D+ HUS).
We hypothesized that development of D+ HUS is associated with increased serum procalcitonin (PCT) levels. PCT was measured by an immunoluminometric assay in 113 patients. Concentrations of
PCT were different in normal controls, disease control groups (rotavirus enteritis, HC due to non-STEC pathogens, chronic renal failure), and children with uncomplicated O157:H7 HC or D+
HUS. Children with D+ HUS showed higher PCT levels than those with uncomplicated O157:H7 HC, and increased concentrations were noted in cases requiring peritoneal dialysis. Severely
increased concentrations were observed in children with D+ HUS on d 5 or 6 after the onset of enteritis, whereas serial measurements in those with uncomplicated O157:H7 HC remained within
the normal range throughout the first week of illness. PCT was correlated with serum concentrations of lipopolysaccharide (LPS)-binding protein and serum levels of alanine aminotransferase.
Using two separate sets of real-time PCR primers, we were unable to detect elevated PCT mRNA transcripts in nonadherent undifferentiated (monocytic) or differentiated (macrophage-like) THP-1
cells stimulated with purified Shiga toxin-1 and/or LPS. Our data show that serum levels of PCT are associated with the severity of illness in children with D+ HUS. Further studies are
needed to determine the role of PCT in the pathogenesis of thrombotic microangiopathy associated to childhood D+ HUS. SIMILAR CONTENT BEING VIEWED BY OTHERS CORRELATION OF PROCALCITONIN AND
C-REACTIVE PROTEIN LEVELS WITH PATHOGEN DISTRIBUTION AND INFECTION LOCALIZATION IN URINARY TRACT INFECTIONS Article Open access 11 October 2023 EVALUATING THE PROTECTIVE EFFECTS OF AURODOX
IN A MURINE MODEL OF SHIGA TOXIN-PRODUCING _ESCHERICHIA COLI_ Article Open access 01 April 2025 PEDIATRIC SEPSIS DIAGNOSTIC AND PROGNOSTIC BIOMARKERS: PANCREATIC STONE PROTEIN, COPEPTIN, AND
APOLIPOPROTEIN A-V Article Open access 08 February 2023 MAIN D+ HUS may occur after a prodrome of enteritis caused by STEC (1). STEC intimately adhere to intestinal epithelium, but, in
contrast to _Shigella_, they do not invade the submucosae (2). Stxs have been detected on circulating neutrophils (3–6) and within kidneys of children with D+ HUS (7,8). It has been proposed
that after the translocation of Stxs across the intestinal epithelial barrier, the toxins may be disseminated through a neutrophil “piggy-back” mechanism, resulting in the development of
systemic microvascular injury (2). However, many aspects of the pathogenesis of childhood D+ HUS remain unclear. Data from animal models suggest that LPS or proinflammatory cytokines such as
tumor necrosis factor (TNF)-α are cofactors with Stxs in the genesis of thrombotic microangiopathy within renal glomeruli (2). In support of this supposition, Stxs and LPS have been shown
to induce the expression of soluble cytokines by human monocytes _in vitro_ (9), and the depletion of liver and splenic macrophages in mice decreased Stx lethality (10). In response to
infection, the calcitonin I gene is activated leading to the synthesis of calcitonin precursors, including PCT, which is subsequently processed into calcitonin. Increased serum PCT levels
have been noted in baboons or healthy adult volunteers treated with LPS (11,12). The serum half-life of PCT is approximately 24 h, which is longer than the proinflammatory cytokines TNF-α or
IL-6 (12–14). Low concentrations of PCT are found in patients with noninfectious inflammatory processes (<1.0–2.0 μg/L) or viral infections (<0.5–1.0 μg/L). Indeed, interferon-γ
inhibits IL-1β–induced calcitonin mRNA expression (15). Conversely, massive serum PCT elevations have been reported after administration of pan-T cell antibodies (16,17). We hypothesized
that development of D+ HUS is associated with increased serum PCT levels. We compared serum PCT concentrations in children with D+ HUS, those with uncomplicated _E. coli_ O157:H7 HC, disease
control groups, and normal controls. We then stimulated undifferentiated (monocytic) or differentiated (macrophage-like) THP-1 cells with purified Stx-1 and/or LPS to examine PCT mRNA
expression by real-time polymerase PCR. METHODS CLINICAL DATA. From April 1, 1996, to March 1, 2002, children aged <18 y old who presented at Sainte-Justine Hospital with _E. coli_
O157:H7 HC or D+ HUS were eligible for the study. This cohort has been, in part, previously characterized by our group (18). Enteritis was defined as the acute onset of watery diarrhea
(>1 d) with or without abdominal pain. HC was defined as an enteritis followed by grossly visible bloody stools before medical consultation. D+ HUS was defined as a prodrome of enteritis
or HC with 1) thrombocytopenia (<150,000 cells/L); 2) microangiopathic hemolytic anemia (Hb below the third percentile for age- and sex-matched controls) with fragmented red cells on
blood smear; and 3) acute renal failure (serum creatinine values over the 97th percentile for age) (19). Age, sex, date of onset of enteritis, and occurrence of HUS were noted. Results of
stool cultures, complete blood count, and serum ALT levels were recorded. Charts were also reviewed for evidence of nosocomial infections. Among patients with D+ HUS, blood samples were
obtained before starting peritoneal dialysis if needed. The normal control (NC) group included age- and sex-matched patients undergoing elective surgery for inguinal hernia or strabismus. A
disease control group was composed of patients admitted to a general pediatric ward with rotavirus enteritis and negative bacterial stool cultures. Children with HC due to non-STEC pathogens
(_Salmonella_, _n_ = 4; _Yersinia_, _n_ = 1; _Campylobacter_, _n_ = 5; negative culture, _n_ = 5) were also included. The disease control group for renal failure was composed of nine
children on chronic ambulatory peritoneal dialysis with chronic renal failure (CRF) due to reflux nephropathy (_n_ = 4), renal dysplasia (_n_ = 2), glomerulonephritis (_n_ = 2), and
nephrosis (_n_ = 1). In this group, the median serum creatinine was 390 μM (range, 118–1000 μM). They presented no signs of upper respiratory tract infections and had normal temperatures as
well as negative urine and dialysate cultures before obtaining blood samples. LABORATORY DATA. Sorbitol-negative colonies grown on MacConkey-sorbitol agar were subcultured onto blood agar
and screened for _E. coli_ serotype O157 by slide agglutination (Difco, Detroit, MI). Colonies agglutinating with the antiserum were identified as _E. coli_ by standard biochemical
reactions. Identification of pathogen was available within 24–48 h of sample collection. Evidence of rotavirus enteritis was obtained by an ELISA (Pathfinder Rotavirus, Bio-Rad Laboratories,
Redmond, WA). Blood samples were collected, allowed to clot, and centrifuged for 10 min at 4°C and 3000 _g_. Specimens were aliquoted and stored at –80°C until assayed. Serum PCT was
determined by a blind immunoluminometric assay, using two MAb directed against the calcitonin and katacalcin sequences of procalcitonin (BRAHMS PCT-LIA or LUMItest PCT, BRAHMS, Hennigsdorf,
Germany). The variability of the assay was 3–6%. The lower detection limit of the assay was 0.1 μg/L. Values higher than 0.5–1.0 μg/L are usually considered abnormal. ANALYSIS OF PCT MRNA
EXPRESSION BY REAL-TIME PCR The undifferentiated human myelogenous leukemia cell line THP-1 was maintained in RPMI 1640 supplemented with Pen/Strep and 10% fetal bovine serum at 37°C in 5%
CO2. Approximately 5 × 105 cells in 500 μL medium were plated in 12-well cell culture plates and treated with medium alone, purified Stx-1 (400 ng/mL), purified _E. coli_ O111:B4 LPS (200
ng/mL; Sigma Chemical Co., St. Louis, MO) or both for 0, 1, 2, 4, 6, and 8 h. To differentiate cells to the mature macrophage-like state, approximately 1 × 106 cells/mL were plated in
12-well cell culture plates in the presence of 50 ng/mL phorbol 12-myristate 13-acetate for 48 h. Approximately 50% of cells will adhere to the plate. Cells were washed twice with cold,
sterile PBS and fresh RPMI 1640 + fetal bovine serum were added. The medium was replaced daily, until the fourth day, when the experiments were conducted using the same experimental
conditions as for undifferentiated cells. Total RNA from each sample was extracted using the QIAgen QIAshredder and RNeasy Mini Kit (QIAGEN, Valencia, CA) protocols with the added DNase
treatment. RNA was eluted using 50 μL RNase/DNase-free water (Invitrogen, Carlsbad, CA). RNA was reverse transcribed using TaqMan reagents and real-time PCR performed using SYBR Green I
double-stranded DNA binding dye (Applied Biosystems, Foster City, CA). PCT mRNA was measured using primers sets derived from NCBI Sequence Viewer (BC069704): Set 1: Procalcitonin-F
TTCCTGGCTCTCAGCATCTTG Procalcitonin-R CAGACCTGAATGGTGCTGCAT Set 2: Procalcitonin-F2 TCTAAGCGGTGCGGTAATCTG Procalcitonin-R2 CTTGTTGAAGTCCTGCGTGTATG The first set of primers was derived from
the 5′ end of the procalcitonin cDNA, whereas the second set of primers targeted the middle portion of the cDNA. Real-time PCR were done on undifferentiated (monocyte-like) and
differentiated (macrophage-like) THP-1 cells with or without stimulation by Stx1 and/or LPS. Positive controls using primers amplifying GADPH were carried out (20). Reactions were run and
analyzed with the ABI PRISM 7500 sequence detection system (Applied Biosystems) using reaction parameters previously described (9). ETHICS. Written informed consent was obtained from the
parents of all children. The study was approved by the Ethics Committee of Sainte-Justine Hospital. STATISTICS. Descriptive statistics are presented as mean ± SD for data with a normal
distribution; median and range were used otherwise. The _t_ test was used to compare continuous data with a normal distribution and the Mann-Whitney _U_ test was used when the distribution
was abnormal. The Kruskal-Wallis test (α = 0.05) was used to compare age, Δ time between onset of enteritis and blood sample collection, leukocyte count, and serum levels of PCT among the
following independent groups: 1) normal controls; 2) disease controls with rotavirus enteritis; 3) patients with HC caused by non-STEC pathogens; 4) children with uncomplicated _E. coli_
O157:H7 HC; 5) D+ HUS; and 6) disease controls with CRF unrelated to STEC infection. Orthogonal, 2 × 2 comparisons were then performed using the test of Dunn (α = 0.0033). All statistical
tests were two-sided. We performed linear regression analysis between concentrations of PCT, and age, time after the onset of enteritis, leukocyte counts, Hb levels, platelet counts, serum
urea, creatinine, ALT levels, and inflammatory mediators (18,21–24), including LPS-binding protein (25). RESULTS We recruited 113 patients, among whom 154 blood samples were analyzed. Stool
cultures showed evidence of _E. coli_ O157:H7 in 79% (26/33) of children with D+ HUS. As listed in Table 1, the sex distribution was comparable between groups. Children with CRF were older
than any other group (_p_ < 0.0001). Those with rotavirus enteritis were younger than patients with uncomplicated O157:H7 HC (_p_ < 0.002). Children with D+ HUS showed a longer time
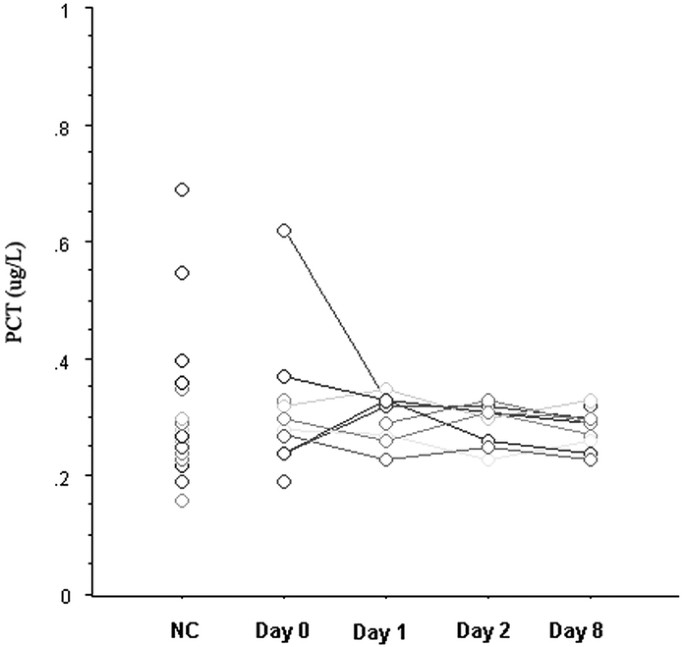
interval between the onset of enteritis and that of blood sampling (_p_ < 0.001), as well as higher white blood counts (_p_ < 0.001). Serial measurements of serum PCT levels among nine
children with uncomplicated _E. coli_ O157:H7 HC are presented in Figure 1, which shows that PCT concentrations remained within the normal range from the onset of enteritis throughout the
first week of the disease. PCT levels among children with STEC infections and control groups are shown in Figure 2. These were significantly different between groups (_p_ < 0.0004).
Children with D+ HUS presented higher PCT concentrations than those with uncomplicated O157:H7 HC (_p_ < 0.002). Serum PCT levels were also increased in children with D+ HUS who required
dialysis: (_n_ = 9) dialyzed, 2.3 (0.2–125) _versus_ (_n_ = 24) nondialyzed, 0.4 (0.2–1.8); _p_ < 0.003. Concentrations of PCT were not correlated to any of the following parameters: age,
Δ time, Hb levels, platelet and total leukocyte counts. PCT was weakly associated to the neutrophil count (_R_2 = 0.1; _p_ < 0.01) and serum creatinine levels (_R_2 = 0.2; _p_ <
0.006). PCT levels are shown as a function of time after the onset of enteritis in Figure 3. Severely increased PCT levels were noted in four patients with HUS on d 5 and 6 of enteritis (8,
13, 59, 125 μg/L). One child had acute pancreatitis and presented with hallucinations (59 μg/L), whereas another presented with convulsions and had particularly severe colitis (125 μg/L).
There was no evidence of nosocomial infections. Children with O157:H7 HC had PCT concentrations within the normal range, except for one measurement (1.9 μg/L) obtained on d 3. As PCT is an
acute phase reactant, its relationship with ALT concentrations was evaluated for the whole study population. Figure 4 shows that most patients with increased serum PCT presented 2- to 5-fold
increased ALT levels. Finally, we noted that PCT was correlated with LPS-binding protein (_n_ = 22, _R_2 = 0.6; _p_ < 0.0001) (25), but not other inflammatory mediators previously
studied by us: (_n_ = 25–50, _R_2 < 0.1, _p_ = NS, for: TNF-α, IL-1β, IL1-receptor antagonist, IFN-γ, IL-6, IL-8, IL-10, selectins-L, -P, -E, intercellular adhesion molecule-1, vascular
cell adhesion molecule-1, granulocyte colony-stimulating factor, growth-related oncogene-α, macrophage inflammatory protein-1β, macrophage chemotactic protein-1, epithelial-cell derived
neutrophil activating peptide-78, transforming growth factor-β1, sFas, and mannan-binding lectin (18,21–24). In light of earlier studies suggesting that freshly isolated human PBMC express
PCT mRNA in response to stimulation with LPS (26), we treated undifferentiated (monocytic) and differentiated (macrophage-like) THP-1 cells with purified Stx1 and/or LPS for 1–8 h. Using two
separate primer sets in real-time PCR analyses, we did not detect PCT mRNA transcripts synthesized in response to the stimulants. DISCUSSION In this study, we found that children with D+
HUS presented with increased serum levels of PCT compared with those with an uncomplicated course of _E. coli_ O157:H7 HC. Higher concentrations were also noted among children with D+ HUS
who required dialysis. Taken together, these results indicate that PCT is associated with severity of illness. We noted very high concentrations among four children with D+ HUS, on the fifth
or sixth day after the onset of enteric symptoms. Conversely, serial measurements of PCT in patients with O157:H7 HC were within the normal range throughout the first week of illness. The
lack of serial measurements before and after the development of D+ HUS constitutes a major limitation of our study as it precludes to calculation of diagnostic predictive values. We
acknowledge that few children with D+ HUS had blood samples drawn during the period of highest risk for the development of D+ HUS, which is around 3–4 d after the onset of enteric symptoms
(27,28). In this regard, a slightly increased PCT level (1.9 μg/L) was noted 3 d after the onset of enteritis in one patient with uncomplicated O157:H7 HC. The magnitude of increase in PCT
concentrations noted among some children with D+ HUS was comparable to that found in two patients with _Salmonella_ enteritis. Although STEC are noninvasive pathogens (2), these PCT
concentrations were similar to values reported among children with septic shock (29,30). Treatment of freshly isolated PBMC with LPS, TNF-α, IL-1β, or IL-6 has been reported to induce PCT at
the mRNA and protein levels (26). PCT has been found within liver, kidneys, aorta, fat, ovaries, bladder, and adrenal glands of baboons treated with endotoxin (11,12). The cellular origin
of PCT within serum of children with D+ HUS is unclear. In this study, we found normal PCT levels among children with CRF on peritoneal dialysis. Slightly increased PCT concentrations have
been noted in adults with chronic renal insufficiency (31), whether or not they required renal replacement therapy (32). In patients with advanced chronic renal disease, PCT may be augmented
due to reduced renal elimination combined with an increased release of PCT by PBMC (32). Conversely, a comparable natural elimination rate of PCT has been noted in patients with normal or
impaired renal function (33). Three studies in baboons or humans suggest that the liver constitutes a major source of PCT within blood (34–36). In this regard, we noted significant
correlations between elevations in serum PCT and levels of LPS-binding protein or ALT. We acknowledge however that LPS-binding protein is also an acute phase reactant protein tht does not
necessarily imply a biologic effect mediated by the lipid A component of endotoxin (25). Using two separate sets of primers, we were unable to detect elevated levels of PCT mRNA from Stx-1
and/or LPS stimulated undifferentiated (monocytic) or differentiated (macrophage-like) THP-1 cells. This is in contrast to previous data by Harrison _et al._ (9,20), who demonstrated under
similar experimental conditions that these toxins, alone or in combination, induced the synthesis of TNF-α, IL-1β, and both C-X-C and CC chemokines. Intracellular PCT has been detected
within peripheral blood monocytes stimulated with _S. aureus_ (37). Oberhoffer _et al._ (26) reported increased expression of PCT mRNA and protein in unfractionated human PBMC stimulated
with LPS, TNF-α, IL-2, or IL-6. The discrepancy with our results may be explained by the fact that our experiments were performed using a lower dose of LPS (0.2 μg/mL _versus_ 10 μg/mL) on a
nonadherent monocytic cell line, or on adherent macrophage-like cells 4 d after differentiation. Indeed, Linscheid _et al._ (15) reported a transient elevation in PCT mRNA expression by
human peripheral blood monocytes immediately after adherence to endothelial cells or plastic surfaces. Furthermore, expression of calcitonin or calcitonin gene-related peptide 1 was noted in
adipose tissue biopsies but not in leukocytes isolated from patients with high serum levels of PCT (15,38). In coculture experiments, adipocyte calcitonin mRNA expression was stimulated by
_E. coli_–activated macrophages in which calcitonin mRNA was undetectable (15). Collectively, these data suggest that macrophage interaction with parenchymal cells stimulate PCT expression
by nonmyeloid cells. In this study, we showed that serum PCT levels are associated with severity of illness in childhood D+ HUS. However, it is unclear how PCT may be used for the diagnosis
or management of children with STEC infections. We speculate that Stx, LPS, or other bacterial virulence factors may have induced cell activation and PCT secretion through complex mechanisms
of cellular cross-talk that could not be reproduced by our experiments utilizing a single cell type. Further studies are needed to determine the role of PCT in the pathogenesis of
thrombotic microangiopathy associated with childhood D+ HUS. ABBREVIATIONS * ALT: alanine aminotransferase * CRF: chronic renal failure * D+ HUS: diarrhea-associated hemolytic uremic
syndrome * HC: hemorrhagic colitis * LPS: lipopolysaccharide * PBMC: peripheral blood mononuclear cell * PCT: procalcitonin * Stxs: Shiga toxins * STEC: Shiga toxin producing _Escherichia
coli_ REFERENCES * Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H 1985 The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing
_Escherichia coli_.. _J Infect Dis_ 151: 775–782 Article CAS Google Scholar * Proulx F, Seidman EG, Karpman D 2001 Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome.
_Pediatr Res_ 50: 163–171 Article CAS Google Scholar * Te Loo DM, van Hinsbergh VW, van den Heuvel LP, Monnens LA 2001 Detection of verocytotoxin bound to circulating polymorphonuclear
leukocytes of patients with hemolytic uremic syndrome. _J Am Soc Nephrol_ 12: 800–806 CAS PubMed Google Scholar * te Loo DM, Monnens LA, van Der Velden TJ, Vermeer MA, Preyers F, Demacker
PN, van Den Heuvel LP, van Hinsbergh VW 2000 Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. _Blood_ 95: 3396–3402 CAS PubMed Google
Scholar * te Loo DM, Heuvelink AE, de Boer E, Nauta J, van der Walle J, Schroder C, van Hinsbergh VW, Chart H, van de Kar NC, van den Heuvel LP 2001 Vero cytotoxin binding to
polymorphonuclear leukocytes among households with children with hemolytic uremic syndrome. _J Infect Dis_ 184: 446–450 Article CAS Google Scholar * Tazzari PL, Ricci F, Carnicelli D,
Caprioli A, Tozzi AE, Rizzoni G, Conte R, Brigotti M 2004 Flow cytometry detection of Shiga toxins in the blood from children with hemolytic uremic syndrome. _Cytometry B Clin Cytom_ 61:
40–44 Article Google Scholar * Uchida H, Kiyokawa N, Horie H, Fujimoto J, Takeda T 1999 The detection of Shiga toxins in the kidney of a patient with hemolytic uremic syndrome. _Pediatr
Res_ 45: 133–137 Article CAS Google Scholar * Chaisri U, Nagata M, Kurazono H, Horie H, Tongtawe P, Hayashi H, Watanabe T, Tapchaisri P, Chongsa-nguan M, Chaicumpa W 2001 Localization of
Shiga toxins of enterohaemorrhagic _Escherichia coli_ in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. _Microb Pathog_ 31: 59–67 Article CAS Google
Scholar * Harrison LM, van Haaften WC, Tesh VL 2004 Regulation of proinflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1.
_Infect Immun_ 72: 2618–2627 Article CAS Google Scholar * Palermo MS, Alves Rosa MF, Van Rooijen N, Isturiz MA 1999 Depletion of liver and splenic macrophages reduces the lethality of
Shiga toxin-2 in a mouse model. _Clin Exp Immunol_ 116: 462–467 Article CAS Google Scholar * Morgenthaler NG, Struck J, Chancerelle Y, Weglohner W, Agay D, Bohuon C, Suarez-Domenech V,
Bergmann A, Muller B 2003 Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. _Horm Metab Res_ 35: 290–295 Article CAS Google Scholar * Dandona P, Nix D, Wilson
MF, Aljada A, Love J, Assicot M, Bohuon C 1994 Procalcitonin increase after endotoxin injection in normal subjects. _J Clin Endocrinol Metab_ 79: 1605–1608 CAS PubMed Google Scholar *
Reinhart K, Karzai W, Meisner M 2000 Procalcitonin as a marker of the systemic inflammatory response to infection. _Intensive Care Med_ 26: 1193–1200 Article CAS Google Scholar *
Brunkhorst FM, Heinz U, Forycki ZF 1998 Kinetics of procalcitonin in iatrogenic sepsis. _Intensive Care Med_ 24: 888–889 Article CAS Google Scholar * Linscheid P, Seboek D, Nylen E,
Langer I, Schlatter M, Becker K, Keller U, Muller B 2003 _In vitro_ and _in vivo_ calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. _Endocrinology_
144: 5578–5584 Article CAS Google Scholar * Sabat R, Hoflich C, Docke WD, Oppert M, Kern F, Windrich B, Rosenberger C, Kaden J, Volk HD, Reinke P 2001 Massive elevation of procalcitonin
plasma levels in the absence of infection in kidney transplant patients treated with pan-T-cell antibodies. _Intensive Care Med_ 27: 987–991 Article CAS Google Scholar * Eberhard OK,
Langefeld I, Kuse ER, Brunkhorst FM, Kliem V, Schlitt HJ, Pichlmayr R, Koch KM, Brunkhorst R 1998 Procalcitonin in the early phase after renal transplantation—will it add to diagnostic
accuracy?. _Clin Transplant_ 12: 206–211 CAS PubMed Google Scholar * Proulx F, Toledano B, Phan V, Clermont MJ, Mariscalco MM, Seidman EG 2002 Circulating granulocyte colony-stimulating
factor, C-X-C, and C-C chemokines in children with _Escherichia coli_ O157:H7 associated hemolytic uremic syndrome. _Pediatr Res_ 52: 928–934 Article CAS Google Scholar * Nicholson J,
Pesce M 2004 Reference ranges for laboratory tests and procedures. In: Behrman R, Kliegman R, Jenson H (eds) _Nelson Textbook of Pediatrics_. Saunders, Philadelphia, pp 2396–2426 Google
Scholar * Harrison LM, van den Hoogen C, van Haaften WC, Tesh VL 2005 Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides.
_Infect Immun_ 73: 403–412 Article CAS Google Scholar * Proulx F, Turgeon JP, Litalien C, Mariscalco MM, Robitaille P, Seidman E 1998 Inflammatory mediators in _Escherichia coli_ O157:H7
hemorrhagic colitis and hemolytic-uremic syndrome. _Pediatr Infect Dis J_ 17: 899–904 Article CAS Google Scholar * Proulx F, Litalien C, Turgeon JP, Mariscalco MM, Seidman E 2000
Circulating levels of transforming growth factor-beta1 and lymphokines among children with hemolytic uremic syndrome. _Am J Kidney Dis_ 35: 29–34 Article CAS Google Scholar * Masri C,
Proulx F, Toledano B, Clermont MJ, Mariscalco MM, Seidman EG, Carcillo J 2000 Soluble Fas and soluble Fas-ligand in children with _Escherichia coli_ O157:H7-associated hemolytic uremic
syndrome. _Am J Kidney Dis_ 36: 687–694 Article CAS Google Scholar * Litalien C, Proulx F, Mariscalco MM, Robitaille P, Turgeon JP, Orrbine E, Rowe PC, McLaine PN, Seidman E 1999
Circulating inflammatory cytokine levels in hemolytic uremic syndrome. _Pediatr Nephrol_ 13: 840–845 Article CAS Google Scholar * Proulx F, Seidman E, Mariscalco MM, Lee K, Caroll S 1999
Increased circulating levels of lipopolysaccharide binding protein in children with _Escherichia coli_ O157:H7 hemorrhagic colitis and hemolytic uremic syndrome. _Clin Diagn Lab Immunol_ 6:
H7 hemorrhagic colitis and hemolytic uremic syndrome. Clin Diagn Lab Immunol 6:773 PubMed Google Scholar * Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, Jager L,
Reinhart K 1999 Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines _in vitro_. _J Lab Clin Med_ 134:
49–55 Article CAS Google Scholar * Buteau C, Proulx F, Chaibou M, Raymond D, Clermont MJ, Mariscalco MM, Lebel MH, Seidman E 2000 Leukocytosis in children with _Escherichia coli_ O157:H7
enteritis developing the hemolytic-uremic syndrome. _Pediatr Infect Dis J_ 19: 642–647 Article CAS Google Scholar * Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI 1997
Predictors of hemolytic uremic syndrome in children during a large outbreak of _Escherichia coli_ O157:H7 infections. _Pediatrics_ 100: E12 Article CAS Google Scholar * Han YY, Doughty
LA, Kofos D, Sasser H, Carcillo JA 2003 Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. _Pediatr Crit Care Med_ 4: 21–25 Article Google
Scholar * Carrol ED, Newland P, Thomson AP, Hart CA 2005 Prognostic value of procalcitonin in children with meningococcal sepsis. _Crit Care Med_ 33: 224–225 Article CAS Google Scholar *
Steinbach G, Bolke E, Grunert A, Storck M, Orth K 2004 Procalcitonin in patients with acute and chronic renal insufficiency. _Wien Klin Wochenschr_ 116: 849–853 Article CAS Google Scholar
* Herget-Rosenthal S, Klein T, Marggraf G, Hirsch T, Jakob HG, Philipp T, Kribben A 2005 Modulation and source of procalcitonin in reduced renal function and renal replacement therapy.
_Scand J Immunol_ 61: 180–186 Article CAS Google Scholar * Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K 2001 The plasma elimination rate and urinary secretion of
procalcitonin in patients with normal and impaired renal function. _Eur J Anaesthesiol_ 18: 79–87 Article CAS Google Scholar * Meisner M, Muller V, Khakpour Z, Toegel E, Redl H 2003
Induction of procalcitonin and proinflammatory cytokines in an anhepatic baboon endotoxin shock model. _Shock_ 19: 187–190 Article CAS Google Scholar * Kretzschmar M, Kruger A,
Schirrmeister W 2001 Procalcitonin following elective partial liver resection—origin from the liver?. _Acta Anaesthesiol Scand_ 45: 1162–1167 Article CAS Google Scholar * Fazakas J,
Gondos T, Varga M, Sarvary E, Horovitz P, Perner F 2003 Analysis of systemic and regional procalcitonin serum levels during liver transplantation. _Transpl Int_ 16: 465–470 Article CAS
Google Scholar * Balog A, Ocsovszki I, Mandi Y 2002 Flow cytometric analysis of procalcitonin expression in human monocytes and granulocytes. _Immunol Lett_ 84: 199–203 Article CAS Google
Scholar * Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B 2004 Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by
macrophage-activated adipocytes. _Crit Care Med_ 32: 1715–1721 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank our research assistants, H. Brassard, A.
Proietti, and R. Trahan, who collected blood specimens. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, Intensive Care Medicine, Sainte-Justine Hospital, University
of Montreal, Montreal, H3T-1C5, Quebec, Canada Hélène Decaluwe & François Proulx * Department of Pediatrics, Leukocyte Biology and Critical Care Medicine, Baylor College of Medicine,
Houston, 77030, Texas Michele M Mariscalco * Department of Pediatrics and Clinical Biology, Hopital Saint-Vincent-de-Paul, Paris, 75014, France Dominique Gendrel & Claude Bohuon *
Department of Medical Microbiology and Immunology, Texas A&M University System Health Science Center, College Station, 77843, >Texas Lisa M Harrison & Vernon L Tesh Authors *
Hélène Decaluwe View author publications You can also search for this author inPubMed Google Scholar * Lisa M Harrison View author publications You can also search for this author inPubMed
Google Scholar * Michele M Mariscalco View author publications You can also search for this author inPubMed Google Scholar * Dominique Gendrel View author publications You can also search
for this author inPubMed Google Scholar * Claude Bohuon View author publications You can also search for this author inPubMed Google Scholar * Vernon L Tesh View author publications You can
also search for this author inPubMed Google Scholar * François Proulx View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to François Proulx. ADDITIONAL INFORMATION This study was presented in part at the 12th International Congress of Immunology and 4th Annual Conference of FOCIS, Montreal, July 20, 2004.
This study was supported by the Fonds de Recherche clinique from Ste-Justine Hospital (F.P.) and U.S. Public Health Service Grant 2RO1 AI34530-10 from the National Institutes of Health
(V.L.T.). RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Decaluwe, H., Harrison, L., Mariscalco, M. _et al._ Procalcitonin in Children with _Escherichia
coli_ O157:H7 Associated Hemolytic Uremic Syndrome. _Pediatr Res_ 59, 579–583 (2006). https://doi.org/10.1203/01.pdr.0000203100.45658.d5 Download citation * Received: 19 July 2005 *
Accepted: 09 November 2005 * Issue Date: April 2006 * DOI: https://doi.org/10.1203/01.pdr.0000203100.45658.d5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative