- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND/OBJECTIVES As an essential development in the new century, surface ablation procedures have attracted increasing attention. There exists a concern regarding the risk of
infectious keratitis. Hence, we aimed to investigate the rate and predisposing factors of infectious keratitis after photorefractive keratectomy (PRK). SUBJECTS/METHODS This retrospective
study was designed in two phases. First, the rate of post-PRK keratitis of Farabi Eye Hospital was investigated. In other words, the targeted population was the patients who developed
keratitis after performing procedure at Farabi Eye Hospital. In the second phase, all the patients with the diagnosis of post-PRK keratitis were studied regardless of the centre where
surgery was performed. Patients with the diagnosis of infectious keratitis between 2014 and 2020 were enrolled and following information was analyzed: demographics, presentation time after
surgery, perioperative medications, culture results, risk factors, medical treatment, complications, and final visual acuity. RESULTS The total number of PRK procedures in our centre was
24,986 (13,253 patients), in which 6 eyes of 5 patients developed keratitis. Beside these 5 patients, 24 referred patients (24 eyes) from the other centres were enrolled. Finally, a total
number of 29 patients (30 eyes) were included. Our analysis revealed that manipulation of contact lens, dry eye, and blepharitis were the essential predisposing factors for keratitis
development. CONCLUSION The overall post-PRK keratitis occurrence rate of our study was 0.02%. Our observation highlighted the importance of preoperative examination and treatment of the
lids and dry eye disease. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS CORNEAL FOREIGN BODIES: ARE ANTISEPTICS AND
ANTIBIOTICS EQUALLY EFFECTIVE? Article 13 January 2023 CLINICAL COMPARATIVE ANALYSIS OF CULTURE-PROVEN BACTERIAL KERATITIS ACCORDING TO PRIOR TOPICAL STEROID USE: A RETROSPECTIVE STUDY IN A
TERTIARY REFERRAL CENTER OF SOUTH KOREA Article Open access 02 September 2023 PREDISPOSING FACTORS, MICROBIOLOGICAL FEATURES AND OUTCOMES OF PATIENTS WITH CLINICAL PRESUMED CONCOMITANT
MICROBIAL AND HERPES SIMPLEX KERATITIS Article 19 February 2021 INTRODUCTION As an essential development in the new century, surface ablation procedures have attracted increasing attention
[1,2,3]. Given their submicron precision, these techniques are commonly applied for corneal surgeries including photorefractive keratectomy (PRK), epithelial laser in situ keratomileusis
(epi-LASIK) and laser-assisted subepithelial keratectomy (LASEK) [1]. PRK is the preferable choice in condition of higher risk of flap dislocation, thin cornea and a predisposition to trauma
[4,5,6]. However, risk of infectious keratitis development after PRK is still a concern [2]. Breaking down the barrier function of the corneal epithelium and using bandage contact lens and
topical corticosteroids have been identified as the pivotal predisposing factors for infectious keratitis after PRK surgery [1, 7, 8]. Moreover, history of blepharitis, corneal surgery,
contamination during surgery, dry eye, lack of perioperative antibiotics, and herpes simplex virus (HSV) infection have been indicated as risk factors for infectious keratitis progression
[1, 7, 9]. Across the incidence rate of post-PRK infectious keratitis, previous studies have been reported a rate of 0.02–0.2% [2, 7, 10]. Differences in standardization methods,
intraoperative practices and sterilization procedures reveal this variation [11]. So far, few studies have been addressed the rate of post-PRK keratitis [1, 10]. In this study, we aimed to
investigate one of the largest series of post-PRK infectious keratitis up to date and share our experience regarding prevention and management of infectious keratitis. MATERIALS AND METHODS
This research was undertaken in accordance with Declaration of Helsinki and confirmed by Farabi Eye Hospital Institutional Review Board. Informed written consent was obtained from all
subjects. This retrospective case-series study comprised 24,986 eyes from 13,253 patients, who underwent PRK surgery consecutively at Farabi Eye Hospital, Tehran, Iran, between January 2014
and January 2020. The current study was designed in two phases. In the first phase, the occurrence rate of post-PRK keratitis of Farabi Eye Hospital was investigated; in other words, the
targeted population was the patients who developed keratitis after performing the procedure at Farabi Eye Hospital. In the second phase, all the patients with the diagnosis of post-PRK
keratitis were studied regardless of the centre where their surgery was performed. Using the medical records system of Farabi Eye Hospital, data were obtained. Patients with the diagnosis of
infectious keratitis within 6 months after PRK surgery were identified by an electronic search of medical records using the keywords PRK/surface ablation and infectious or PRK/surface
ablation and keratitis/corneal ulcer. Overall, the following information was obtained for review and analysis: age, gender, involved eye, presentation time after surgery, perioperative
medications, preoperative preparation, culture results, duration of follow-up, risk factors, medical treatment, complications, uncorrected visual acuity (UCVA) and best-corrected visual
acuity (BCVA). In this study, infectious keratitis was diagnosed based on symptoms, slit-lamp findings, microbiology results or their combination. Clinical diagnostic criteria encompassed
corneal infiltration compatible with infectious keratitis excluding other causes of non-infectious keratitis. In all the patients suspected for infectious aetiology, cultures were obtained.
Sabouraud, chocolate and blood agar plates were applied as primary culture panels. Besides, vancomycin and amikacin were prescribed empirically until the availability of definitive culture
results. To ascertain whether patients were suitable candidates for corneal refractive surgery, all of them underwent a complete ophthalmologic examination. The surgical suite met the
criteria for ophthalmologic laser procedures. Instruments were autoclaved before PRK surgery with Statim 2000 or 5000 with reservoir (SciCan). In the field of preoperative preparation, the
patients were instructed to perform lid hygiene during the three days before the surgery. Preoperatively, patient’s eyes were cleaned and prepped with povidone–iodine. The eyelashes were
draped and 2 drops of proparacaine 0.5% were given in less than 10 min before the surgery. Also, topical fluoroquinolones were given to reduce the chance of infection. During the surgery,
the epithelium was debrided with exposure to 20% alcohol for 20 seconds or mechanically using a hockey knife based on the surgeon’s preference. Laser ablation was carried out in the right
eye first and then, in the left eye using a Technolas 217C or 217- Z-100 excimer laser (Bausch & Lomb) or the Mel 80 excimer laser (Carl Zeiss Meditec AG). Topical mitomycin-C (0.02%)
was used on the ablated surface immediately after laser ablation based on surgeon’s preference to reduce the incidence of primary or recurrent haze. A bandage soft contact lens was fitted
after surgery and the patients underwent on a topical combination of tobramycin 3 mg/mL–dexamethasone 1 mg/mL (Tobradex) four times a day until the contact lens was removed and diclofenac
sodium 0.1% four times per day for 2 days together with preservative-free artificial tears. Also, based on availability of different drugs in Iran and surgeon’s preference, chloramphenicol,
ciprofloxacin, levofloxacin, and ofloxacin were the other wide spectrum used antibiotics in postoperative treatment. Once the contact lens was removed, the patient received a tapering
regimen of fluorometholone 0.1% and preservative-free artificial tears for 1.5 months. All the patients were examined 24 h, 7 days, and 1 and 3 months after surgery unless complications
required more frequent visits. The primary endpoint of this study was the incidence of infectious keratitis within 6 months after the surgery and the secondary endpoints were culture
results, response to treatment, and visual outcome. Descriptive statistics was accomplished to ascertain the incidence of infectious keratitis and to describe the study population. Outcomes
reported in the clinical records were compiled in an Excel spread sheet (Microsoft Corp.). Data were analyzed using SPSS 21.0 software. RESULTS The total number of PRK procedures in our
centre at the mentioned 6-year time period was 24,986 (13,253 patients), in which 6 eyes of 5 patients developed keratitis. Therefore, the overall keratitis occurrence rate of our centre was
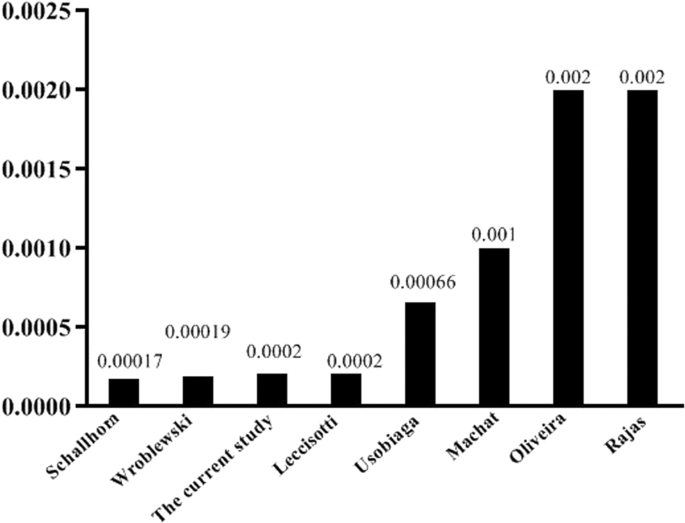
estimated as 0.02%. Figure 1 illustrates the comparison of keratitis incidence after photorefractive keratectomy between the published studies. Moreover, 29 patients (30 eyes) with the
diagnosis of post-PRK keratitis underwent treatment in our centre (24 referred patients and 5 patients of our centre). The mean age of the patients was 34.62 ± 10.6 years old and females
accounted for 55.2% of the patients (16/29). Across the involved eye of all the patients, 21 infection (70%) was in the left eye and 9 (30%) involved the right (Table 1). It should be
mentioned that infection was bilateral in one patient. The mean follow-up time was 6.2 ± 5.64 months (range 1–24). With respect to admission time after surgery, 22 patients presented between
1 and 7 days (early onset) (mean 4.14 days; range 1–7) and 7 patients presented after 7 days (late onset) (mean 37.43 days; range 8–120). The mean time of symptom presentation time from
surgery was 12.17 ± 24.35 days (within–1 120). In the field of clinical symptoms, decreased vision was the most prevalent symptom (in 27 patients (93%)). Afterwards, photophobia was present
in 21 patients (72.4%), eye pain in 19 patients (65.5%), discomfort in 18 patients (62.1%), eye discharge in 17 patients (58.6%), and excess tears in 8 patients (27.6%). In the initial
evaluation at the emergency department, ciliary injection and corneal infiltration were diagnosed in all the patients. In addition, corneal epithelial defect, hypopyon, corneal satellite
infiltration, and endophthalmitis were detected in 17 (58.6%), 12 (41.4%), 3 (10.3%) and 1 (3.4%) of the patients, respectively. Cultures were obtained from all the cases that were suspected
for infectious keratitis. Of all the cultures, 22 eyes had positive results and 8 eyes had culture-negative keratitis. Among culture-positive eyes, the most commonly identified pathogen was
_staphylococcus aureus_ (16 eyes, 72.8%). Fungi, the second most commonly microorganism, was diagnosed in 2 (9.2%) eyes. Both of the cases developed filamentous fungal keratitis in our
study with positive culture for _Aspergillus_. In addition, _enterobacter, acanthamoeba_, _methicillin-resistant staphylococcus aureus_ (_MRSA_) and _Staphylococcus epidermidis_ each
accounted for 1 case (4.5%) (Table 2). After accurately investigating the plausible predisposing factors, the following were determined. First, as the most prevalent factor, contact lens
manipulation (history of lens exchange or hand touch by the patient) was significant in 6 patients (21%). Second, dry eye and blepharitis each accounted for four of the patients (14%). In
three patients (10%), a history of ocular trauma and lens fall out were found. Moreover, two patients (7%) had a previous history of diabetes mellitus. Two patients (7%) were healthcare
workers; one was an intensive care unit staff, in whom a _S. epidermidis_ ulcer progressed, and the second was a medical doctor, in whom _MRSA_ ulcerations were developed. Additionally,
history of HSV keratitis and history of corneal transplantation were blamed to be responsible for 2 (7%) and 1 (3%) keratitis development. Figure 2 represents the overall pattern of
predisposing factors. Empirical treatments with intensive regimen with topical vancomycin (50 mg/dL) and amikacin (15 mg/dL) were started in all the patients. In the cases involving _S.
aureus_, a broad-spectrum combination consisted of fortified vancomycin with the aminoglycoside or a fluoroquinolone was the most common treatment. Oral doxycycline was added in 9 cases.
With regard to the fungal inherent of the culture results, antifungal regimen (natamycin or voriconazole) was started in two cases. In addition, case 28 received polyhexamethylene biguanide
due to the identified pathogen (_acanthamoeba_) (Table 3). Final visual acuity ranged from 20/20 to 20/70. The final BCVA was 20/20 or better in 17 patients (58.6%), 20/40 or better (but
worse than 20/20) in 10 cases (34.5%), and worse than 20/40 in two cases (6.9%) (Table 3). All the patients responded to the medical therapy, except two cases. Patient 19 developed
endophthalmitis, necessitating urgent surgery. Furthermore, patient 10 underwent penetrating keratoplasty (PKP) 24 months after the initiation of the infection. DISCUSSION Surface ablation
procedures have attracted increasing attention during recent decades [1]. As a devastating complication of laser refractive surgeries, infectious keratitis can result in remarkable loss of
BCVA in eyes with great visual potential [1]. There are published works regarding post-PRK keratitis in the literature. The rate of infectious keratitis after PRK and the related risk
factors were investigated in our study. All the diagnoses were made by a cornea specialist who was expert and experienced in the field of keratitis. The other differential diagnoses include
non-infectious keratitis or a form of immunologic predisposition, peripheral ulcerative keratitis (PUK) and staphylococcal marginal hypersensitivity. In all the differential diagnoses,
corticosteroid is one of the major treatment columns and the sole use of antibiotics is not useful whereas use of corticosteroids in infectious keratitis can lead to catastrophic outcome.
Hence, appropriate clinical response to antibiotics is a differentiating factor to exclude the aforementioned diagnoses. In the current study, we found 6 eyes in 24,986 procedures, with the
occurrence rate of 0.02%. It should be borne in mind that estimating the accurate incidence highly relies on the completeness of follow-up of all the patients. In this regard, most of our
patients attended all the scheduled visits. A 24-h phone number is available for the patients who underwent every type of interventions in our centre. The patients are advised to make a
phone call in condition of experiencing any symptoms or problems. Also, we ask for the reason of not presenting through a phone call if a scheduled follow-up visit is missed or delayed.
Therefore, we believe that the reported rate is reasonably accurate. Comparing the current study with previous efforts, our observed occurrence rate for post-PRK infectious keratitis (0.02%)
fell within the reported range in the literature [12, 13]. Our observed rate was similar to the report by Leccisotti et al. [12] who found 2 cases of infectious keratitis (0.02%) among
10,452 PRK procedures. In addition, our results were nearly consistent with those of the study by Schallhorn et al. [7] and Wroblewski et al. [10] (rates of 0.017% and 0.019%, respectively).
On the other hand, our proposed rate was almost tenfold lower than the 0.2% estimated by Rojas et al. [2] and Oliveira et al. [13] Justifying the mentioned rates, the exact reason for
higher rates in these studies was unclear. However, the most notable difference between studies with higher and lower rates was that those with lower occurrence of infectious keratitis
mostly performed later (2006–2020) [2, 13]. The later time led to the strict adherence to techniques that had been recommended by the previous studies as well as published guidelines, from
which the previous studies would not have benefited. In the field of the involved eye, we found more infectious keratitis in the left eye. To explain the potential contributor, the
preoperative process of ocular prepping in PRK was commonly performed simultaneously in both eyes, with the right side frequently performed first. Given the passing time and probability of
not meeting the sterility process, the infection occurred more commonly in the second eye [1]. This phenomenon could partially explain the higher observed infectious keratitis in the left
eye, although due to the inherent biases of the current study, more longitudinal studies were warranted to accurately respond to this question. Comparing PRK and LASIK, there has been a
legitimate debate regarding the post-operation keratitis [1, 2]. Rojas et al. [2] found 5.7 times higher keratitis incidence rate in those who underwent surface ablation than those who
performed LASIK procedure. In this respect, breaking down of the eye barrier due to corneal epithelial defect, using a bandage contact lens and topical corticosteroids for controlling wound
healing has been identified as the most essential culprit of increased risk of infectious keratitis in the patients who performed PRK surgery [14, 15]. In contrast, in a recent meta-analysis
study, it was revealed that the keratitis incidence was 4.5 times higher in LASIK than PRK [1]. Creation of the corneal flap and higher rates of performing LASIK surgery were considered as
the potential explanation for the observed rate [1, 16]. Altogether, it seems that infectious keratitis serves as an essential complication after both PRK and LASIK surgeries, necessitating
more precise care to prevent its consequences. Generally, infectious keratitis after surface ablation procedures could be classified as either early onset (occurring within one week after
surgery) or late onset (occurring after one week after the surgery) [17]. We found that the percentage of early infectious (75.8%) was higher than the late-onset subtype. In line with this
concept, Rojas et al. [2] found that 71.79% of involved eyes had early-onset keratitis. Moreover, 90% of PRK cases in the study by Schallhorn et al. [7] were presented within two weeks of
the surgery. Regarding the observed results, it seems imperative to implement aggressive treatment strategy for observed infiltration in the first postoperative week after PRK. Hence, we
recommend performing smear, culture and aggressive treatment in those present with any infiltrations that is central or paracentral, larger than 2 mm, and associated with significant pain or
AC reaction, or fails to respond rapidly to the standard therapy. It should be mentioned case #1 and case #4 of our study who had a long elapsed time after surgery (120 days and 70 days,
respectively) was complicated with persistent epithelial defect. So, it is not irrational to contribute their keratitis to PRK. In our study, the most common microorganisms responsible for
infectious keratitis were _S. aureus_, fungi, _enterobacter_, _acanthamoeba_, _MRSA_ and _S. epidermidis_. No patient with mycobacterial infection was detected. Our results were in
accordance with the previous two largest series of post-PRK keratitis, indicating that Gram-positive microorganisms were responsible in almost all cases [18, 19]. Therefore, it seems that
Gram-positive organisms posed the greatest risk of infectious keratitis after PRK surgery and, due to the remarkable ocular morbidity associated with infectious keratitis, we strongly
recommend the use of prophylactic antibiotics after PRK. Overall, it was demonstrated that the common bacterial pathogens in the early onset type were _staphylococcus_, _streptococcus_ and
Gram-negative species. On the other hand, opportunistic microorganisms including fungi, _nocardia_ and atypical _mycobacteria_ were the important pathogens in the late-onset subtype [20]. In
line with this concept, we observed two cases with fungi infectious, both presenting after one week from operation. Similarly, Garg et al. [21] found fungi as the most contributors to
post-Lasik keratitis. Ascertaining the plausible contributor to infection, several sources including periocular flora, surgical instruments, surgeon’s hands and environmental factors have
been identified [22]. Accordingly, Feizi et al. [22] found that the rate of corneal interface contamination was 24.5%. In addition, _S. epidermidis_ was recognized as one of the most
commonly retrieved organisms during intraocular surgeries. It is a normal inhabitant of the eyelids, eyelashes and conjunctiva, and it is believed that the microorganisms that cause
postoperative complications originate from the eyelids and conjunctiva [23,24,25,26]. Therefore, regular follow-up of the patients who underwent PRK surgeries could be an efficient solution
to timely diagnosis and treatment of those with post-PRK infectious keratitis. Appraising the potential risk factors for post-PRK keratitis, several factors such as dry eye, blepharitis and
lens manipulation have been identified before [20]. We found that manipulation of contact lens, dry eye, and blepharitis were the most essential predisposing factors in our study. These
observations highlighted the importance of preoperative examination and treatment of the lids and dry eye disease [27]. Indeed, eyelid hygiene could decrease the bacterial load on corneal
surface, which in turn could reduce the risk of infectious keratitis [28]. Regarding our protocol, we started hygiene procedure not more than 3 days before PRK due to the point that longer
time could alter the pattern of ocular flora. We also found that history of diabetes mellitus and HSV keratitis could facilitate the keratitis development. Despite the previous reports
regarding good visual outcomes in those with HSV keratitis, our both patients who had a history of HSV keratitis developed some degrees of visual loss because of the scarring [29, 30]. Our
findings were in accordance with a previous study indicating development of scarring and visual loss in all the cases with HSV keratitis. American Academy of Ophthalmology has been
introduced history of HSK as a relative contraindication for corneal refractive surgery [31]. Nagy et al. reported an incidence of 0.14% (19 of 13,200) for post-PRK HSV keratitis, in which
it was remarkably higher than rate of 0.03% for general population [32, 33]. Moshirfar et al. recently published a narrative review on corneal refractive surgery in patients with a history
of HSK [34]. In this review, three animal studies, two prospective case series, one case report, and one retrospective study regarding post-PRK herpetic ocular disease were reviewed
suggesting excimer laser PRK as a trigger of HSK. To solve this issue prophylaxis with anti-viral agents is strongly recommended. The recommended regimen is oral acyclovir 400 mg twice daily
or valacyclovir 500 mg once daily for two weeks prior to surgery, which is continued postoperatively until cessation of topical steroids. Surgery is not recommended for patients with
multiple recurrences. The other point is that surgery should be postponed until at least one year from the last episode of recurrence. Patients with no corneal haze, nummular keratopathy, or
neovascularization are suitable candidates for corneal refractive surgery [34]. It is of note a comprehensive external examination of the eyelids, corneal sensation, and slit-lamp
examination should be performed in these patients. It seems patients with exposure to a healthcare environment should be considered at additional risk for developing MRSA keratitis following
refractive surgery. Previously, Solomon et al. [27] reported a series of MRSA keratitis following refractive surgery. In their study, nine out of 12 patients were either healthcare workers
(hospital lab technician, medical resident, obstetrician-gynaecologist, nurse, emergency room physician) or exposed to a healthcare setting (history of hospitalization before surgery, two
volunteers at nursing homes, and husband of a healthcare worker). Except one case (the medical resident who underwent PRK), the rest of the cases underwent LASIK. To prevent MRSA keratitis
in such cases, prophylactic treatment of blepharitis, considering fourth-generation fluoroquinolone or bacitracin for preoperative prophylaxis, monocular treatment in the patients with known
MRSA carriage, and avoiding contact lens manipulation in PRK patients are recommended. Drawing from previous guidelines and studies, treatment of post-PRK infectious keratitis with
aggressive antibiotic agents in addition to removing the soft contact lens has been suggested [35]. In our study, we started empirical treatment with vancomycin and amikacin in all the
patients. Besides, we administrated a broad-spectrum combination consisting of fortified vancomycin with an aminoglycoside or a fluoroquinolone according to the ASCRS guideline
recommendations [28]. We found that all the patients responded to medical therapy except two cases. Our prescribed medication is nearly consistent with the study by Rojas et al. [2] and a
complete response in all the cases were achieved. Visual acuity results in the current study were reasonably satisfactory and fell within the range reported by previous studies. We found
that the final BCVA was 20/20 or better in 58.6% of patients, 20/40 or better (worse than 20/20) in 34.5% and worse than 20/40 in 6.9% of patients. Consistent with this notion, Rojas et al.
[2] reported that 58.97% of the cases had corrected distance visual acuity (CDVA) of 20/20 or better, 20/40 or better in 33.3% and worse than 20/40 in 7.69%. In addition, Wroblewski et al.
[10] reported the final CDVA of 20/30, 20/25, 20/16, 20/20, and 20/20 in five patients with infectious keratitis. Besides, Donnenfeld et al. [18] reported the final visual acuity between
20/20 and 20/100 among post-PRK cases with infectious keratitis. In this regard, CDVA was 20/20 in 5 cases, 20/40 or better in 11 cases and worse than 20/40 in 2 cases, with 1 patient
awaiting PKP. Finally, in the study by Oliveira et al. [13], the final CDVA was 20/20 or better in 7 of 9 cases of culture-proven infectious keratitis after PRK and 20/40 or better in the
remaining 2 cases. We would like to emphasize the strengths of this study. First, to the best of our knowledge, this is one of the largest studies regarding evaluating the incidence of
post-PRK keratitis. In addition, all the patients earned regular follow-up after infectious keratitis diagnosis, making the reported rates and consequences more accurate. On the other hand,
the present study was subject to a number of potential limitations. First, due to the descriptive inherent of the present study, we could not assess accurately the casual association between
predisposing factors and keratitis. In addition, we had several negative cultures, which could be due to technical reasons such as alteration during transport, or previous treatment with
antibiotics. Also, we are not certain about the candidacy of patients who underwent PRK at other facilities, which can be one of the weaknesses of our study. CONCLUSION In conclusion,
infectious keratitis is a devastating complication of laser refractive surgeries, which could result in remarkable loss of visual acuity in eyes with great visual potential. The overall
post-PRK keratitis occurrence rate of our study was 0.02%. We found that Gram-positive cocci were the most prevalent microorganism in the eyes with keratitis infection. Besides, our analysis
revealed that manipulation of contact lens, dry eye, and blepharitis were the most essential predisposing factors for keratitis development. All the patients were prescribed suitable
antibiotics regimen and the final visual acuity results in the current study were reasonably satisfactory. SUMMARY WHAT WAS KNOWN BEFORE * There exists a critical concern regarding the risk
of infectious keratitis development after PRK. * As a devastating complication of laser refractive surgeries, infectious keratitis can result in remarkable loss of vision. WHAT THIS STUDY
ADDS * Our study revealed that manipulation of contact lens, dry eye, and blepharitis were the most essential predisposing factors for keratitis development. * The overall post-PRK keratitis
occurrence rate of our study was 0.02%. DATA AVAILABILITY The data is available from the corresponding author on reasonable request. REFERENCES * Afsharpaiman S, Zare M, Yasemi M,
Jamialahmadi T, Sahebkar A. The prevalence of infectious keratitis after keratorefractive surgery: a systematic review and meta-analysis study. J Ophthalmol. 2020;2020:6329321.
https://doi.org/10.1155/2020/6329321. Article PubMed PubMed Central Google Scholar * de Rojas V, Llovet F, Martínez M, Cobo-Soriano R, Ortega-Usobiaga J, Beltrán J, et al. Infectious
keratitis in 18,651 laser surface ablation procedures. J Cataract Refract Surg. 2011;37:1822–31. https://doi.org/10.1016/j.jcrs.2011.04.037. Article PubMed Google Scholar * Bower KS,
Weichel ED, Kim TJ. Overview of refractive surgery. Am Fam Physician. 2001;64:1183–90. CAS PubMed Google Scholar * Tomás-Juan J, Murueta-Goyena Larrañaga A, Hanneken L. Corneal
regeneration after photorefractive keratectomy: a review. J Optom. 2015;8:149–69. https://doi.org/10.1016/j.optom.2014.09.001. Article PubMed Google Scholar * O’Keefe M, Kirwan C. Laser
epithelial keratomileusis in 2010 - a review. Clin Exp Ophthalmol. 2010;38:183–91. https://doi.org/10.1111/j.1442-9071.2010.02198.x. Article PubMed Google Scholar * Bower KS, Woreta F.
Update on contraindications for laser-assisted in situ keratomileusis and photorefractive keratectomy. Curr Opin Ophthalmol. 2014;25:251–7. https://doi.org/10.1097/icu.0000000000000055.
Article PubMed Google Scholar * Schallhorn JM, Schallhorn SC, Hettinger K, Hannan S. Infectious keratitis after laser vision correction: Incidence and risk factors. J Cataract Refract
Surg. 2017;43:473–9. https://doi.org/10.1016/j.jcrs.2017.01.017. Article PubMed Google Scholar * Heidemann DG, Clune M, Dunn SP, Chow CY. Infectious keratitis after photorefractive
keratectomy in a comanaged setting. J Cataract Refract Surg. 2000;26:140–1. https://doi.org/10.1016/s0886-3350(99)00336-3. Article CAS PubMed Google Scholar * Faramarzi A, Feizi S,
Javadi MA, Kanavi MR, Yazdizadeh F, Moein HR. Bilateral nocardia keratitis after photorefractive keratectomy. J Ophthalmic Vis Res. 2012;7:162–6. PubMed PubMed Central Google Scholar *
Wroblewski KJ, Pasternak JF, Bower KS, Schallhorn SC, Hubickey WJ, Harrison CE, et al. Infectious keratitis after photorefractive keratectomy in the United States army and navy.
Ophthalmology. 2006;113:520–5. https://doi.org/10.1016/j.ophtha.2005.09.038. Article PubMed Google Scholar * Moshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and
noninfectious keratitis after laser in situ keratomileusis Occurrence, management, and visual outcomes. J Cataract Refract Surg. 2007;33:474–83. https://doi.org/10.1016/j.jcrs.2006.11.005.
Article PubMed Google Scholar * Leccisotti A, Bartolomei A, Greco G, Manetti C. Incidence of bacterial keratitis after photorefractive keratectomy. J Refract Surg. 2005;21:96. Article
PubMed Google Scholar * de Oliveira GC, Solari HP, Ciola FB, Lima AL, Campos MS. Corneal infiltrates after excimer laser photorefractive keratectomy and LASIK. J Refract Surg.
2006;22:159–65. Article PubMed Google Scholar * Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, et al. Incidence of contact-lens-associated microbial keratitis and
its related morbidity. Lancet. 1999;354:181–5. https://doi.org/10.1016/s0140-6736(98)09385-4. Article CAS PubMed Google Scholar * Dart JK, Radford CF, Minassian D, Verma S, Stapleton F.
Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–54. https://doi.org/10.1016/j.ophtha.2008.05.003. 1654.e1641-1643
Article CAS PubMed Google Scholar * Santhiago MR, Kara-Junior N, Waring GOT. Microkeratome versus femtosecond flaps: accuracy and complications. Curr Opin Ophthalmol. 2014;25:270–4.
https://doi.org/10.1097/icu.0000000000000070. Article PubMed Google Scholar * Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published
literature. Surv Ophthalmol. 2004;49:269–80. https://doi.org/10.1016/j.survophthal.2004.02.007. Article PubMed Google Scholar * Donnenfeld ED, O’Brien TP, Solomon R, Perry HD, Speaker
MG, Wittpenn J. Infectious keratitis after photorefractive keratectomy. Ophthalmology. 2003;110:743–7. https://doi.org/10.1016/s0161-6420(02)01936-x Article PubMed Google Scholar * Leal
F, Hofling-lima AL, de Freitas D, Campos M. AAnálise laboratorial das ceratites infecciosas secundáriasà cirurgia refrativa.
https://www.scielo.br/j/abo/a/ZdZdtrNXQHxbWTrm8s8xkfq/?lang=pt&format=pdf. * Cheng H-C. Infectious keratitis after excimer laser corneal surgery. Taiwan J Ophthalmol. 2014;4:101. Article
Google Scholar * Garg P, Chaurasia S, Vaddavalli PK, Muralidhar R, Mittal V. Microbial keratitis after LASIK. J Refract Surg. 2010;26:209–16. https://doi.org/10.3928/1081597x-20100224-07.
Article PubMed Google Scholar * Feizi S, Jadidi K, Naderi M, Shahverdi S. Corneal interface contamination during laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:1734–7.
https://doi.org/10.1016/j.jcrs.2007.05.037. Article PubMed Google Scholar * Detorakis ET, Siganos DS, Houlakis VM, Kozobolis VP, Pallikaris IG. Microbiological examination of bandage soft
contact lenses used in laser refractive surgery. J Refract Surg. 1998;14:631–5. Article CAS PubMed Google Scholar * Seal D, Reischl U, Behr A, Ferrer C, Alió J, Koerner RJ, et al.
Laboratory diagnosis of endophthalmitis: comparison of microbiology and molecular methods in the European Society of Cataract & Refractive Surgeons multicenter study and susceptibility
testing. J Cataract Refract Surg. 2008;34:1439–50. https://doi.org/10.1016/j.jcrs.2008.05.043. Article PubMed Google Scholar * Endophthalmitis Vitrectomy Study Group. Results of the
Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis
Vitrectomy Study Group. Arch Ophthalmol. 1995;113:1479–96. Article Google Scholar * Assia EI, Jubran RZ, Solberg Y, Keller N. The role of intraocular lenses in anterior chamber
contamination during cataract surgery. Graefe’s Arch Clin Exp Ophthalmol. 1998;236:721–4. https://doi.org/10.1007/s004170050148. Article CAS Google Scholar * Solomon R, Donnenfeld ED,
Perry HD, Rubinfeld RS, Ehrenhaus M, Wittpenn JR, et al. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am J Ophthalmol. 2007;143:629–34.
Article PubMed Google Scholar * Solomon R, Donnenfeld ED, Holland EJ, Yoo SH, Daya S, Güell JL, et al. Microbial keratitis trends following refractive surgery: results of the ASCRS
infectious keratitis survey and comparisons with prior ASCRS surveys of infectious keratitis following keratorefractive procedures. J Cataract Refract Surg. 2011;37:1343–50.
https://doi.org/10.1016/j.jcrs.2011.05.006. Article PubMed Google Scholar * Kabra A, Lalitha P, Mahadevan K, Prajna NV, Srinivasan M. Herpes simplex keratitis and visual impairment: a
case series. Indian J Ophthalmol. 2006;54:23–27. https://doi.org/10.4103/0301-4738.21610. Article PubMed Google Scholar * Jarade EF, Tabbara KF. Laser in situ keratomileusis in eyes with
inactive herpetic keratitis. Am J Ophthalmol. 2001;132:779–80. https://doi.org/10.1016/s0002-9394(01)01092-3. Article CAS PubMed Google Scholar * Chuck RS, Jacobs DS, Lee JK, Afshari NA,
Vitale S, Shen TT, et al. Refractive errors & refractive surgery preferred practice pattern®. Ophthalmology. 2018;125:P1–P104. Article PubMed Google Scholar * Nagy ZZ, Keleman E,
Kovács A. Herpes simplex keratitis after photorefractive keratectomy. J Cataract Refractive Surg. 2003;29:222–3. Article Google Scholar * Labetoulle M, Auquier P, Conrad H, Crochard A,
Daniloski M, Bouée S, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–95. e881. Article CAS PubMed Google Scholar * Moshirfar M, Milner DC,
Baker PA, McCabe SE, Ronquillo YC, Hoopes PC. Corneal refractive surgery in patients with a history of herpes simplex keratitis: a narrative review. Clin Ophthalmol. 2020;14:3891. Article
PubMed PubMed Central Google Scholar * de Rojas Silva MV, Díez-Feijóo E, Javaloy J, Sánchez-Salorio M. Prophylactic perioperative antiviral therapy for LASIK in patients with inactive
herpetic keratitis. J Refract Surg. 2006;22:404–6. Article PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Eye Research Center, Farabi Eye
Hospital, Tehran University of Medical Sciences, Tehran, Iran Mohammad Soleimani, Mohammad Keykhaei, Seyed Ali Tabatabaei, Hossein Farrokhpour & Kasra Cheraqpour * Imam Hossein Medical
Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran Mansoor Shahriari * School of Medicine, Iran University of Medical Sciences, Tehran, Iran Bahareh Ramezani Authors *
Mohammad Soleimani View author publications You can also search for this author inPubMed Google Scholar * Mohammad Keykhaei View author publications You can also search for this author
inPubMed Google Scholar * Seyed Ali Tabatabaei View author publications You can also search for this author inPubMed Google Scholar * Mansoor Shahriari View author publications You can also
search for this author inPubMed Google Scholar * Hossein Farrokhpour View author publications You can also search for this author inPubMed Google Scholar * Bahareh Ramezani View author
publications You can also search for this author inPubMed Google Scholar * Kasra Cheraqpour View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
MS1 and KC conceptualized the work. MK, MS1, and HF collected the data. MS1, SAT, MS2, and BR analyzed the data. MK and KC wrote the manuscript. MS2, HF, BR, and KC performed critical
revision on the manuscript. All the authors read and approved the final version of manuscript. CORRESPONDING AUTHOR Correspondence to Kasra Cheraqpour. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Soleimani, M., Keykhaei, M., Tabatabaei, S.A. _et al._ Post photorefractive
keratectomy (PRK) infectious keratitis; six-year experience of a tertiary eye hospital. _Eye_ 37, 631–637 (2023). https://doi.org/10.1038/s41433-022-02009-2 Download citation * Received: 23
July 2021 * Revised: 07 February 2022 * Accepted: 22 February 2022 * Published: 10 March 2022 * Issue Date: March 2023 * DOI: https://doi.org/10.1038/s41433-022-02009-2 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative








