- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Resolution of inflammation is an active process mediated by pro-resolution lipid mediators. As resolvin (Rv) D1 is produced in the cornea, pro-resolution mediators could be
effective in regulating inflammatory responses to histamine in allergic conjunctivitis. Two key mediators of resolution are the D-series resolvins RvD1 or aspirin-triggered RvD1 (AT-RvD1).
We used cultured conjunctival goblet cells to determine whether histamine actions can be terminated during allergic responses. We found cross-talk between two types of G protein-coupled
receptors (GPRs), as RvD1 interacts with its receptor GPR32 to block histamine-stimulated H1 receptor increases in intracellular [Ca2+] ([Ca2+]i) preventing H1 receptor-mediated responses.
In human and rat conjunctival goblet cells, RvD1 and AT-RvD1 each block histamine-stimulated secretion by preventing its increase in [Ca2+]i and activation of extracellular regulated–protein
kinase (ERK)1/2. We suggest that D-series resolvins regulate histamine responses in the eye and offer new treatment approaches for allergic conjunctivitis or other histamine-dependent
pathologies. SIMILAR CONTENT BEING VIEWED BY OTHERS SEX-BASED DIFFERENCES IN CONJUNCTIVAL GOBLET CELL RESPONSES TO PRO-INFLAMMATORY AND PRO-RESOLVING MEDIATORS Article Open access 29
September 2022 PROTECTIVE EFFECT OF _TISOCHRYSIS LUTEA_ ON DRY EYE SYNDROME VIA NF-ΚB INHIBITION Article Open access 15 November 2022 OCULAR EFFECTS OF RHO KINASE (ROCK) INHIBITION: A
SYSTEMATIC REVIEW Article 16 September 2024 INTRODUCTION Inflammation has a critical role in many widely occurring diseases, and there now is a general consensus that failed endogenous
resolution mechanisms can lead to uncontrolled and chronic inflammation.1, 2 Uncontrolled inflammation is regarded as a critical component of the pathogenesis of two major diseases of the
ocular surface, dry eye and allergic conjunctivitis, as well as dermatitis in the skin.3, 4 Active resolution of the acute inflammatory response is orchestrated by a novel family of
anti-inflammatory and pro-resolving mediators termed resolvins (Rvs), which are a part of a wider genus of pro-resolving mediators.5, 6 Histamine has a central role in promoting allergic
conjunctivitis7 and is known to directly stimulate conjunctival goblet cell mucin secretion.8 Martin _et al._9 recently demonstrated that RvD1 regulates histamine release by mast cell
degranulation. Herein, we addressed whether RvD1 could regulate the action of histamine on goblet cell secretion and characterized the histamine response by these cells. Recently, we found
that Rvs of the D and E series (RvD1 and RvE1) regulate leukotriene-stimulated conjunctival goblet cell mucin secretion as can occur in the setting of allergic conjunctivitis.10 Here we
report that RvD1 interacts with its receptor G protein-coupled receptor 32 (GPR32) to block histamine-stimulated responses of conjunctival goblet cells that include increase in intracellular
[Ca2+] ([Ca2+]i), activation of extracellular regulated–protein kinase (ERK)1/2, and secretion of high molecular weight glycoproteins, including MUC5AC mucin. RESULTS HISTAMINE-STIMULATED
INCREASE IN [CA2+]I IN CULTURED HUMAN AND RAT CONJUNCTIVAL GOBLET CELLS WAS INHIBITED BY RVS First, we determined whether histamine alters [Ca2+]i using the intracellular Ca2+ probe fura-2
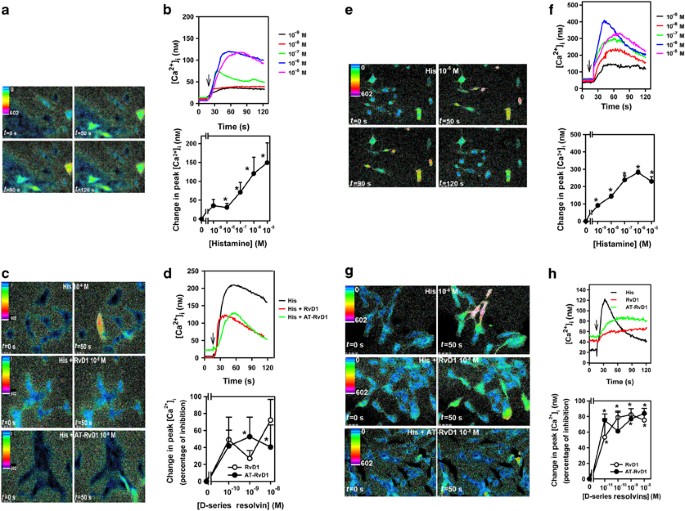
in cultured human conjunctival goblet cells. Histamine increased [Ca2+]i in a concentration-dependent manner, with a maximum increase obtained at 10−5 M (Figure 1a,b). We used RvD1 and its
epimer AT-RvD1, which is produced in the presence of aspirin.5, 11 In goblet cells, exposure to either RvD1 or AT-RvD1 (10−10–10−8 M) blocked histamine-stimulated increases in [Ca2+]i that
were reduced by a maximum of 72.3±24.2 and 52.6±23.%, respectively (Figure 1c,d). In rat goblet cells, histamine also increased [Ca2+]i with maximum levels at 10−6 M, a decreased
concentration of histamine compared with that which was maximum for human cells (Figure 1e,f). At concentrations as low as 10−11 M, both RvD1 and AT-RvD1 significantly blocked the
histamine-stimulated increases in [Ca2+]i (Figure 1g,h). A maximum inhibition of secretion of 82.3±0.3% and 83.9±6.4%. was obtained by RvD1 and AT-RvD1, respectively (Figure 1g,h). RVS BLOCK
HISTAMINE-STIMULATED INCREASE IN [CA2+]I WHEN ADDED BEFORE BUT NOT AFTER HISTAMINE To examine the time-dependency of their effects, RvD1 and AT-RvD1 were each added simultaneously with
histamine or 50 s after histamine at the peak of [Ca2+]i response (Figure 2a–d). When histamine and RvD1 or AT-RvD1 were added simultaneously, the peak [Ca2+]i was significantly decreased
compared with histamine alone (Figure 2a–d). By contrast, when RvD1 or AT-RvD1 were added at the peak of the histamine response, the [Ca2+]i was not altered when the change in [Ca2+]i
(Figure 2a–d) was monitored or when the slope was calculated (data not shown). Both D-series resolvins when added before (Figure 1d,h) or together (Figure 2a–d) with histamine blocked the
increase in [Ca2+]i stimulated by histamine. However, these Rvs could not alter the histamine Ca2+ response if they were added within a minute after histamine had initiated the release of
Ca2+i. RVS AND HISTAMINE RECEPTORS UTILIZE THE SAME INTRACELLULAR CA2+ POOLS GPRs, including histamine receptors, release Ca2+ from intracellular capacitative Ca2+ stores located in the
endoplasmic reticulum.12, 13 Ca2+ release, in turn, activates Ca2+ influx from extracellular stores. The intracellular Ca2+ stores are refilled by Ca2+ATPase pumping Ca2+ back into the
endoplasmic reticulum. As thapsigargin depletes the intracellular Ca2+ store by blocking the Ca2+ATPase, thapsigargin can be used to evaluate use of this store.14 To determine whether Rvs
decrease [Ca2+]i by preventing the release of this store, we measured the effect of Rvs on thapsigargin-induced [Ca2+]i in the presence of extracellular Ca2+. Histamine alone and
thapsagargin alone increased [Ca2+]i by 205.7±48.7 and 146.8±70.1 nM, respectively (Figure 2e). When RvD1 or AT-RvD1 was added before thapsigargin, the thapsigargin-induced increase in
[Ca2+]i was not altered (Figure 2e). When the thapsigargin response was followed by addition of histamine, the histamine response was significantly decreased compared with histamine alone.
When RvD1 or AT-RvD1 were followed by thapsagargin and then histamine, the histamine response was unchanged compared with the histamine response that followed the thapsagargin response.
(Figure 2e). In support of this finding, we demonstrated earlier that after removal of extracellular Ca2+ a small increase in [Ca2+]i remained after histamine 10−5 M stimulation.15, 16 These
results suggest that RvD1 and AT-RvD1 block the histamine-stimulated increase in [Ca2+]i by either directly altering the histamine receptor or by affecting a step before the release of
intracellular Ca2+. HISTAMINE-STIMULATED INCREASE IN ERK 1/2 ACTIVITY IN CULTURED RAT CONJUNCTIVAL GOBLET CELLS WAS INHIBITED BY RVS We next investigated whether histamine affects ERK 1/2
activity. Rat conjunctival goblet cells were stimulated with histamine and ERK1/2 activity measured by western blotting analysis. Histamine increased ERK1/2 activity in a
concentration-dependent manner with a maximum at 10−6 M (Figure 3a). Histamine also activated ERK1/2 in a time-dependent manner with a maximum stimulation at 5 min (Figure 3b). Hence, at
physiological levels histamine activates ERK1/2 along with elevating [Ca2+]i. To determine whether D-series resolvins block histamine stimulation of ERK1/2 activity, we exposed rat goblet
cells to Rvs for 30 min before stimulation with histamine at 10−5 M for 5 min. Both RvD1 and AT-RvD1 significantly inhibited histamine-stimulated ERK1/2 activity, with RvD1 inhibiting by a
maximum of 94.0±7.7 and AT-RvD1 by 81.9±20.9% (Figure 3c). RVS INHIBIT HISTAMINE-STIMULATED MUCIN SECRETION FROM CULTURED RAT AND HUMAN GOBLET CELLS When goblet cell secretion was
investigated, RvD1 or AT-RvD1 exposure completely blocked secretion stimulated by histamine (10−5 M) in human goblet cells and rat goblet cells at two concentrations of histamine (10−5 and
10−6 M, Figure 4a–c). RvD1 and AT-RvD1 blocked histamine-stimulated secretion a maximum of 78.1±9.0% and 90.3±18.6%, respectively, in human goblet cells and 76.9±15.1% and 83.0±9.3%,
respectively, in rat goblet cells. RvD1 and AT-RvD1 blocked histamine-stimulated increase in [Ca2+]i, ERK1/2 activity, and high molecular weight glycoconjugate, including MUC5AC secretion in
both human and rat conjunctival goblet cells. RVS INCREASE MUCIN SECRETION IN CULTURED HUMAN AND RAT CONJUNCTIVAL GOBLET CELLS, [CA2+]I , AND ERK1/2 IN RAT CONJUNCTIVAL GOBLET CELLS RvD1
activates its receptor GPR32,11 which is present in human and rat conjunctival goblet cells,10 and also can activate the lipoxin A4 receptor (ALX/FPR2).17 We tested whether RvD1 and AT-RvD1
themselves alter secretion, [Ca2+]i, and ERK1/2 in conjunctival goblet cells. RvD1 and AT-RvD1 each increased goblet cell secretion in both human (Figure 5a) and rat cells (Figure 5c). A
maximum action was recorded at 10−9 M RvD1 and AT-RvD1 in human cells and at 10−9 M RvD1 and 10−10 M AT-RvD1 in rat cells. The secretory effect of the D-series resolvins was significantly
less than that stimulated by histamine at 10−5 M, which was 2.2±0.2-fold above basal (not shown). RvD1 and AT-RvD1 caused a maximum stimulation at 1 h in both human and rat cells, which was
shorter than histamine, which caused its maximum response at 2 h of stimulation16 (Figure 5b,d). RvD1 and AT-RvD1 increased the peak [Ca2+]i in a concentration-dependent manner (Figure
6a,b), with a maximum increase obtained with 10−9 M RvD1 and 10−8 M AT-RvD1. In the same experiments, histamine increased [Ca2+]i to a higher level elevating it by 220.0±49.0 nM. To
determine the cellular Ca2+ pool used by RvD1 and AT-RvD1, extracellular Ca2+ was omitted before stimulation with the Rvs. In the absence of extracellular Ca2+, RvD1-stimulated increase in
[Ca2+]i was partially decreased, but the increase stimulated by AT-RvD1 was completely blocked (Figure 6c). The role of the intracellular capacitative Ca2+ pool was investigated using
thapsigargin in the absence and presence of extracellular Ca2+. Independent of the presence of extracellular Ca2+, when thapsigargin was added first, the increase in [Ca2+]i by the
subsequent addition of RvD1 was not altered (Figure 6d). When RvD1 was added first, the thapsigargin-induced Ca2+ response was also not altered. Similar results were obtained when AT-RvD1
was used (Figure 6e). Thus, neither RvD1 nor AT-RvD1 appear to use the IP3-sensitive capacitative Ca2+ pool. As RvD1 and AT-RVD1 can also activate the ALX/FPR2 receptor,11 we determined the
role that the ALX receptor has in RvD1- and AT-RvD1-stimulated [Ca2+]i response in rat conjunctival goblet cells. In cells in which the ALX/FPR2 receptor was knocked down with siRNA, the
[Ca2+]i over time in response to RvD1 (Figure 6f), AT-RvD1 (Figure 6g), or as a control lipoxin A4 (data not shown) was abolished. Peak [Ca2+]i response was decreased significantly only with
ALX siRNA (Figure 6h). The [Ca2+]i response was unchanged when cells were incubated with either a scrambled siRNA or the transfection reagent alone (Figure 6f–h). In rat goblet cells, RvD1
and AT-RvD1 each activate the ALX/FPR2 receptor. When ERK1/2 activity was studied, both RvD1 and AT-RvD1 stimulated ERK1/2 activity in a concentration- and time-dependent manner. A maximum
increase in ERK1/2 activity was not reached even at 10−8 M RvD1 or AT-RvD1 (Figure 7a,b). A maximum increase in time was detected at 20 min of Rv stimulation. The Rvs were slower at
activating ERK1/2 than histamine whose maximum stimulation was obtained at 5 min (Figure 7c,d). Both RvD1 and AT-RvD1 themselves caused goblet cell secretion, elevation in [Ca2+]i, and
increase in ERK1/2 activity. H1 HISTAMINE AND DRV1-GPR32 RECEPTORS ARE PRESENT AND CO-LOCALIZE IN CULTURED HUMAN CONJUNCTIVAL GOBLET CELLS As all four histamine receptors (H1–H4) are
activated by histamine in conjunctival goblet cells,8 we focused on the H1 receptor to determine whether RvD1 uses its receptor GPR32 to alter the action of histamine. Using fluorescence
microscopy, we examined the location of the histamine H1 receptors and the DRV1-GPR32 receptor in human goblet cells. Both receptors displayed a punctate pattern of localization (Figure
8a,b). They were detected in the same cells and their immunofluorescence location overlapped (Figure 8c). DRV1-GPR32, USING PROTEIN KINASE C AND GRK 2, BLOCKS THE HISTAMINE-STIMULATED
INCREASE IN [CA2+]I To determine whether RvD1 used its receptor GPR32 to block histamine stimulated [Ca2+]i, we transfected Chinese Hamster Ovary (CHO) cells with DRV1-GPR32 alone, the H1
histamine receptor alone, both receptors, or mock transfection. Cells were stimulated with histamine, RvD1, or RvD1 followed by histamine. When both the H1 and GPR32 receptors were
transfected, histamine increased [Ca2+]i, (Figure 9a) and RvD1 blocked histamine stimulation. RvD1 did not alter [Ca2+]i. When only the H1 receptor was transfected, histamine increased
[Ca2+]i and previous exposure to RvD1 did not alter this response (Figure 9b). When only the GPR32 receptor was transfected, no combination of histamine or RvD1 increased the [Ca2+]i (Figure
9c). In mock-transfected cells, neither histamine nor RvD1 increased [Ca2+]i, and the RvD1 treatment did not alter the histamine response (Figure 9d). Together, these results suggest that
RvD1 uses its receptor DRV1-GPR32 to block the effect of histamine on [Ca2+]i. As a control, cells were also stimulated with RvE1 or RvE1 followed by histamine in CHO cells transfected with
both the H1 receptor and DRV1-GPR32. RvE1 alone had no effect on [Ca2+]i and RvE1 followed by histamine did not have an effect on histamine-stimulated [Ca2+]i (Figure 9e). Results using
transfected CHO cells suggested that RvD1 and AT-RvD1 blocked the histamine-stimulated response at a step before the release of [Ca2+]i, and thus a potential mechanism of action for RvD1 is
to counter-regulate the histamine receptor. Examination of the phosphorylation sites of the H1 receptor using Scan Site (http://scansite.mit.edu/motifscan_id.phtml) and the kinases that
phosphorylate the H1 receptor using Phospho.ELM (http://phospho.elm.eu.org/pELMBlastSearch.html) indicates potential PKC and β-adrenergic receptor kinase 1 (βARK1; also known as GRK2)
phosphorylation sites. CHO cells transfected with the human H1 and GPR32 receptors were stimulated with histamine; RvD1 followed by histamine; or the PKC inhibitor calphostin C (Figure 10a)
or the βARK1inhibitory peptide (Figure 10b) followed either by histamine or RvD1 and then histamine. RvD1 blocked the histamine-induced Ca2+ response. Calphostin C and βARK1 both reversed
the inhibitory effect of RvD1 on the histamine Ca2+i response. Neither calphostin C nor βARK1 alone blocked the histamine receptor response. In rat goblet cells, both the PKC inhibitor
Ro-317549 (Figure 10c) and βARK1 inhibitory peptide (Figure 10d) blocked the RvD1 inhibition of histamine dimaleate, a specific agonist for the H1 histamine receptor. These data indicate
that RvD1 uses PKC and βARK1 to counter-regulate the H1 receptor. RVS INHIBIT HISTAMINE-STIMULATED INCREASE IN [CA2+]I TO THE SAME EXTENT AS H1 RECEPTOR INHIBITOR The inhibitory actions of
both RvD1 and AT-RvD1 on the histamine-induced increase in [Ca2+]i in cultured conjunctival goblet cells was compared with that of the H1 antagonist chlorpheniramine (Figure 11a). Maximally
effective concentrations of the three inhibitors each blocked the histamine-stimulated increase in [Ca2+]i to essentially the same extent (Figure 11b). DISCUSSION Our results demonstrate
that histamine uses its receptors to increase [Ca2+]i, activate ERK1/2, and stimulate secretion. RvD1 blocks the effects of histamine by interacting with its receptor GPR32 to
counter-regulate the histamine receptor to prevent the release of intracellular Ca2+ and the activation of ERK1/2 thus inhibiting secretion. RvD1 must be added before histamine in order to
prevent the release of the intracellular Ca2+ store. AT-RvD1 has similar effects in conjunctival goblet cells as RvD1. Previous studies have demonstrated that RvD1 and AT-RvD1 block
inflammatory processes in a variety of tissues, but the cellular mechanism of this inhibition has not been demonstrated. Here we show that these Rvs prevent the increase in Ca2+ and
activation of ERK1/2 used by histamine and its H1 receptor subtype to induce goblet cell secretion by activating PKC and βARK1 to counter-regulate the histamine receptor. RvD1 is known to
interact with its receptor DRV1-GPR32. Here, we show that there is cross-talk between human GPR32 and the H1 receptor. Use of GPR32 is required for the inhibitory actions of RvD1 on the
signaling pathway activated by another GPR, the H1 receptor activated by histamine. In rat goblet cells, use of the ALX/FPR2 receptor by RvD1 also activates PKC and βARK1 to counter regulate
the H1 receptor. As Ca2+ is a major mechanism by which histamine evokes it’s response, the actions of the D-series resolvins RvD1 and AT-RvD1 on blocking the action of histamine could
prevent allergic or inflammatory responses in any tissue in which histamine receptors are located. These tissues include the lung, skin, immune cells, smooth muscle, blood vessels, glands,
and nerves. The actions of the D-series resolvins on goblet cell functions are complex as a short-term incubation with the Rvs results in significant activation of ERK 1/2. This activity is
short lived, however, as 30 min after addition of the Rvs the ERK 1/2 activity had returned to basal levels. In this study, goblet cells were exposed to D-series resolvins for 30 min before
addition of histamine. Thus Rvs counter-regulate the histamine receptors to prevent activation of ERK 1/2. E-series and D-series resolvins are effective in blocking different types of
inflammatory responses in the ocular surface. The E-series resolvin RvE1 can reverse inflammation in the cornea by blocking corneal hemangiogenesis activated in a suture or pellet implanted
into the mouse cornea.18 RvE1 induces corneal epithelial cell migration and attenuates herpes simplex–induced ocular inflammation, as well as corneal epithelial barrier disruption and goblet
cell loss in a murine model of dry eye.19, 20, 21 RvE1 also stimulates tear production and decreases inflammation in a mouse model of dry eye22 and in humans in phase 1 and 2 clinical trial
reduces the signs and symptoms of dry eye syndrome (ClinicalTrials.gov Identifier NCT00799552). The D-series resolvin RvD1 blocks corneal hemangiogenesis,18 and previously we demonstrated
that RvD1 blocks cholinergic agonist and leukotriene stimulation of conjunctival goblet cell secretion. The present results demonstrate that RvD1 blocks histamine responses and provides a
novel function for Rvs in termination of allergic inflammation. Our findings demonstrate a new use for Rvs as endogenous mediators that could be used to prevent the actions of histamine to
treat allergic responses, including allergic conjunctivitis in both the mild and severe forms. In the eye, nose, and lung, steroids are the mainstay of regulating inflammation, but their use
carries the major side effect of ocular hypertension and glaucoma.23, 24 Rvs are not immunosuppressant, unlike steroids or other anti-inflammatory agents currently used in the eye. RvD1
control of histamine-stimulated responses could represent a novel approach with local anti-inflammatory and pro-resolution mediators to treat allergic conjunctivitis and allergies in other
susceptible tissues such as the lung and skin. METHODS ANIMALS. Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing between 125 and 150 _g_ were anesthetized with CO2 for 1
min, decapitated, and the bulbar and forniceal conjunctival membranes removed from both the eyes. All experiments were approved by the Schepens Eye Research Institute Animal Care and Use
Committee. HUMAN MATERIAL Human conjunctival tissue was obtained from Heartland Lions Eye Bank (Kansas City, MO). Tissue was placed in Optisol media within 6 h of death. CELL CULTURE Goblet
cells from rat and human conjunctiva were grown in organ culture as described previously.25, 26 The tissue plug was removed after nodules of cells were observed. First passage goblet cells
were used in all the experiments. Cultured cells were periodically checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin _Ulex europaeus_
agglutinin (UEA)-1 (detects goblet cell secretory product) to ensure that goblet cells predominated. MEASUREMENT OF [CA2+]I Goblet cells were incubated for 1 h at 37 °C with Krebs–Ringer
bicarbonate buffer with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (KRB-HEPES; 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5
mM glucose (pH 7.45) plus 0.5% bovine serum albumin containing 0.5 μM fura-2/AM (Invitrogen, Grand Island, NY), 8 μM pluronic acid F127, and 250 μM sulfinpyrazone followed by washing in
KRB-HEPES containing sulfinpyrazone. Calcium measurements were made with a ratio imaging system (In Cyt Im2; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm and an
emission wavelength of 505 nm. At least 10 cells were selected in each experimental condition, and experiments were repeated in at least three separate animals. RvD1 or AT-RvD1 (Cayman
Chemical, Ann Arbor, MI) were added 30 min before histamine (Sigma-Aldrich, St. Louis, MO). Calphostin C, Ro-317549, βARK1 inhibitor, and H89 (EMD Millipore, Billerica, MA) were added 15 min
before addition of RvD1. After addition of agonists, data were collected in real time. Data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak
[Ca2+]i was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data are not shown, the plateau [Ca2+]i was affected similarly to the
peak [Ca2+]i. Synthetic Rvs at 10 μg μl−1 (dissolved in ethanol as purchased from the manufacturer) were stored at −80 °C, with minimal exposure to light. Immediately before use, the Rvs
were diluted in KRB-HEPES buffer to the desired concentrations and added to the cells. The cells were then incubated at 37 °C in the dark. Daily working stock dilutions were discarded
following each experiment. Concentrations were confirmed and the Rv structures validated using LC-MS/MS (liquid chromatography-tandem mass spectrometry) and were consistent with reported
characteristics.27 WESTERN BLOTTING Cultured goblet cells were incubated with increasing concentrations of histamine, RvD1, or AT-RvD1. Cells were also incubated with histamine 10−5 M for
0–10 min or RvD1 (10−9 M) or AT-RvD1 (10−9 M) for 0–30 min In additional experiments, cells were preincubated with increasing concentrations of RvD1 or AT-RvD1 for 30 min before addition of
histamine (10−5 M). Cells were lysed in radio-immunoprecipitation assay buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and 1
mM EDTA) in the presence of a protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 2000 _g_ for 30 min at 4 °C. Proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and processed for western blotting. Primary antibodies used were phosphorylated (active) ERK 1/2 or total ERK 2 (Santa Cruz Biotechnology, Santa
Cruz, CA), diluted at 1:1000. Immunoreactive bands were visualized by the enhanced chemiluminescence method. The films were analyzed with Image J software (Rasband, WS, ImageJ, U.S. National
Institute of Health, Bethesda, MD; http://imagej.nih.gov; http://rsbweb.nih.gov/ij/). ERK activation was expressed as fold increase over basal that was set to 1. SECRETION Cultured goblet
cells were serum starved for 2 h before use, preincubated with RvD1 or AT-RvD1 for 30 min, and then stimulated with histamine in serum-free RPMI 1640 supplemented with 0.5% bovine serum
albumin for 0–4 h. Goblet cell secretion was measured using an enzyme-linked lectin assay with the lectin UEA-I. UEA-1 detects high molecular weight glycoconjugates, including mucins
produced by rat goblet cells. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion as described
earlier.10 Glycoconjugate secretion was expressed as fold increase over basal that was set to 1. IMMUNOFLUORESCENCE MICROSCOPY First passage cells were grown on glass cover slips and fixed
in methanol. Both anti-GPR32 (Gene Tex, Irvine, CA) and anti-H1 receptor antibodies (Santa Cruz Biotechnology) were used at 1:100 dilution overnight at 4 °C. Secondary antibodies were
conjugated to either Cy2 or Cy 3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and were used at a dilution of 1:150 for 1.5 h at room temperature. Negative control experiments
included incubation with the isotype control antibody. The cells were viewed by fluorescence microscopy (Eclipse E80i; Nikon, Tokyo, Japan), and micrographs were taken with a digital camera
(Spot; Diagnostic Instruments, Sterling Heights, MI). TRANSFECTION OF CHO CELLS CHO cells (1 × 106 cells) were transfected with mock (pcDNA3), human H1 (NM_000861.2; OriGene, Rockville, MD),
or human GPR32 receptors (O75388;11) using FuGENE transfection reagent (Promega, Madison, WI) following the manufacturer’s instruction. At 48 h post transfection, cells were harvested for
calcium mobilization experiments. KNOCKDOWN OF ALX RECEPTOR Goblet cells were incubated with 100 μM siRNA against a scrambled sequence or ALX receptor for 72 h (ThermoScientific, Waltham,
MA) in Accell delivery media according to the manufacturer’s instructions (ThermoScientific). The siRNA was a pool of four molecules whose sequences were: (1) CCAUCAGGUUCGUUAUUGG (2)
CCUGCAGACAUUGAGAUAA (3) GUUUAAUACUCGUUACGGA and (4) GUACAAACACUUGUGAAA. STATISTICAL ANALYSIS Results were expressed as the fold increase above basal. Results are presented as mean±s.e.m.
Data were analyzed by Student’s _t_-test. _P_<0.05 was considered statistically significant. REFERENCES * Serhan, C.N. _et al_. Resolution of inflammation: state of the art, definitions
and terms. _FASEB J._ 21, 325–332 (2007). Article CAS Google Scholar * Nathan, C. & Ding, A. Nonresolving inflammation. _Cell_ 140, 871–882 (2010). Article CAS Google Scholar *
Stevenson, W., Chauhan, S.K. & Dana, R. Dry eye disease: an immune-mediated ocular surface disorder. _Arch. Ophthalmol._ 130, 90–100 (2012). Article CAS Google Scholar * Guglielmetti,
S., Dart, J.K. & Calder, V. Atopic keratoconjunctivitis and atopic dermatitis. _Curr. Opin. Allergy Clin. Immunol._ 10, 478–485. 410.1097/ACI.1090b1013e32833e32816e32834 (2010). Article
CAS Google Scholar * Serhan, C.N., Chiang, N. & Van Dyke, T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. _Nat. Rev. Immunol._ 8, 349–361
(2008). Article CAS Google Scholar * Serhan, C.N. _et al_. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that
counter proinflammation signals. _J. Exp. Med._ 196, 1025–1037 (2002). Article CAS Google Scholar * Leonardi, A. Allergic Disease of the Conjunctiva and Cornea, in Cornea and External Eye
Disease Reinhard T. & Larkin F., eds. (2010) 97–120 Springer: New York, USA. * Hayashi, D. _et al_. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet
cell secretion. _Invest. Ophthalmol. Vis. Sci._ 53, 2993–3003 (2012). Article Google Scholar * Martin, N. _et al_. Primary human airway epithelial cell-dependent inhibition of human lung
mast cell degranulation. _PLoS One_ 7, e43545 (2012). Article CAS Google Scholar * Dartt, D.A. _et al_. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by
resolvins D1 and E1 to promote resolution of inflammation. _J. Immunol._ 186, 4455–4466 (2011). Article CAS Google Scholar * Krishnamoorthy, S. _et al_. Resolvin D1 binds human phagocytes
with evidence for proresolving receptors. _Proc. Natl. Acad. Sci. USA_ 107, 1660–1665 (2010). Article CAS Google Scholar * Jung, S., Pfeiffer, F. & Deitmer, J.W. Histamine-induced
calcium entry in rat cerebellar astrocytes: evidence for capacitative and non-capacitative mechanisms. _J. Physiol._ 527 (Part 3), 549–561 (2000). Article CAS Google Scholar * Putney,
J.W. New molecular players in capacitative Ca2+ entry. _J. Cell. Sci._ 120, 1959–1965 (2007). Article CAS Google Scholar * Luo, D., Broad, L.M., Bird, G.S. & Putney, J.W. Signaling
pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. _J. Biol. Chem._ 276, 5613–5621 (2001). Article CAS Google Scholar * Li, D., Carozza, R.B., Shatos,
M.A., Hodges, R.R. & Dartt, D.A. Effect of histamine on Ca2+-dependent signaling pathways in rat conjunctival goblet cells. _Invest. Ophthalmol. Vis. Sci._ 53, 6928–6938 (2012). Article
CAS Google Scholar * Hayashi, D. _et al_. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. _Invest. Ophthalmol. Vis. Sci._ 53, 2993–3003
(2012). Article Google Scholar * Bento, A.F., Claudino, R.F., Dutra, R.C., Marcon, R. & Calixto, J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid,
aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. _J. Immunol._ 187, 1957–1969 (2011). Article CAS Google Scholar * Jin, Y. _et al_. Anti-angiogenesis
effect of the novel anti-inflammatory and pro-resolving lipid mediators. _Invest. Ophthalmol. Vis. Sci._ 50, 4743–4752 (2009). Article Google Scholar * Rajasagi, N.K. _et al_. Controlling
herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. _J. Immunol._ 186, 1735–1746 (2011). Article CAS Google Scholar * Zhang, F. _et al_.
Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. _Invest. Ophthalmol. Vis. Sci._ 51, 5601–5609 (2010). Article Google Scholar
* de Paiva, C.S., Schwartz, C.E., Gjorstrup, P. & Pflugfelder, S.C. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine
model of dry eye. _Cornea_ (2012). * Li, N., He, J., Schwartz, C.E., Gjorstrup, P. & Bazan, H.E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse
model. _J. Ocul. Pharmacol. Ther._ 26, 431–439 (2010). Article Google Scholar * Bielory, L. Ocular toxicity of systemic asthma and allergy treatments. _Curr. Allergy Asthma Rep._ 6,
299–305 (2006). Article Google Scholar * Jones, R. & Rhee, D.J. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. _Curr. Opin.
Ophthalmol._ 17, 163–167 (2006). PubMed Google Scholar * Shatos, M.A. _et al_. Isolation and characterization of cultured human conjunctival goblet cells. _Invest. Ophthalmol. Vis. Sci._
44, 2477–2486 (2003). Article Google Scholar * Shatos, M.A. _et al_. Isolation, characterization, and propagation of rat conjunctival goblet cells _in vitro_. _Invest. Ophthalmol. Vis.
Sci._ 42, 1455–1464 (2001). CAS PubMed Google Scholar * Yang, R., Chiang, N., Oh, S.F. & Serhan, C.N. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes.
_Curr. Protoc. Immunol._, Chapter 14, Unit 14, 26 (2011). PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr Wendell Scott for assistance in obtaining human
conjunctiva, Dr Gary Bird and Dr James Putney Jr for their helpful advice with calcium experiments and Fei Gao for performing LC-MS-MS and Cindy Cheng for transfection of CHO cells. This
work was supported by National Institutes of Health NEI RO1-EY019470 to D.A.D. and US National Institutes of Health grants 1P01GM095467 to C.N.S. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Department of Ophthalmology, Massachusetts Eye and Ear, Schepens Eye Research Institute, Harvard Medical School, Boston, Massachusetts, USA D Li, R R Hodges, J Jiao, R B Carozza, M A
Shatos & D A Dartt * Department of Anesthesiology, Center for Experimental Therapeutics and Reperfusion Injury, Perioperative, and Pain Medicine, Harvard Institutes of Medicine, Brigham
and Women’s Hospital, and Harvard Medical School, Boston, Massachusetts, USA N Chiang & C N Serhan Authors * D Li View author publications You can also search for this author inPubMed
Google Scholar * R R Hodges View author publications You can also search for this author inPubMed Google Scholar * J Jiao View author publications You can also search for this author
inPubMed Google Scholar * R B Carozza View author publications You can also search for this author inPubMed Google Scholar * M A Shatos View author publications You can also search for this
author inPubMed Google Scholar * N Chiang View author publications You can also search for this author inPubMed Google Scholar * C N Serhan View author publications You can also search for
this author inPubMed Google Scholar * D A Dartt View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D A Dartt. ETHICS
DECLARATIONS COMPETING INTERESTS C.N.S. is an inventor on patents (resolvins) assigned to BWH and licensed to Resolvyx Pharmaceuticals. C.N.S. is a scientific founder of Resolvyx
Pharmaceuticals and owns equity in the company. C.N.S.’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict
of interest policies. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT
SLIDE FOR FIG. 6 POWERPOINT SLIDE FOR FIG. 7 POWERPOINT SLIDE FOR FIG. 8 POWERPOINT SLIDE FOR FIG. 9 POWERPOINT SLIDE FOR FIG. 10 POWERPOINT SLIDE FOR FIG. 11 RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, D., Hodges, R., Jiao, J. _et al._ Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival
goblet cell secretion. _Mucosal Immunol_ 6, 1119–1130 (2013). https://doi.org/10.1038/mi.2013.7 Download citation * Received: 02 August 2012 * Accepted: 11 January 2013 * Published: 06 March
2013 * Issue Date: November 2013 * DOI: https://doi.org/10.1038/mi.2013.7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative