- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT An unusual stylar dimorphism occurs in _Narcissus_, a plant genus of insect-pollinated Mediterranean geophytes. To determine the characteristics of the sexual polymorphism, we
investigated floral variation in 46 populations of _N. assoanus_ (section Jonquillae) and 21 populations of _N. dubius_ (section Tazettae) in SW France. Flowers possess two stamen levels in
each morph that occupy slightly different positions within the floral tube. In long-styled plants (L-morph), the stigma is located within or slightly above the upper-level stamens, whereas
in short-styled plants (S-morph) the stigma is placed well below the lower-level stamens. The stigma-height dimorphism is distinct from heterostyly because the reciprocity of stigma and
anther positions in the two style morphs is only weakly developed and there are no differences between the style morphs in pollen size or production. In both species, mean stigma–anther
separation is much greater in the S-morph than the L-morph. In _N. assoanus_, population style-morph ratios vary from isoplethy (1L:1S) to L-biased, whereas in _N. dubius_ they are usually
strongly L-biased or occasionally contain only the L-morph. Populations fixed for the S-morph, or with S-biased morph ratios, were not observed. In _N. assoanus_, style-morph ratios were
associated with population size: large continuous populations always exhibited 1:1 morph ratios, whereas smaller, fragmented populations were often L-biased. This pattern was not evident in
_N. dubius_. We argue that biased style-morph ratios largely result from morph-specific differences in assortative mating. SIMILAR CONTENT BEING VIEWED BY OTHERS CONVERGENT EVOLUTIONARY
PATTERNS OF HETEROSTYLY ACROSS ANGIOSPERMS SUPPORT THE POLLINATION-PRECISION HYPOTHESIS Article Open access 09 February 2024 WITHIN-INDIVIDUAL PHENOTYPIC PLASTICITY IN FLOWERS FOSTERS
POLLINATION NICHE SHIFT Article Open access 11 August 2020 DIMORPHIC FLOWERS MODIFY THE VISITATION ORDER OF POLLINATORS FROM MALE TO FEMALE FLOWERS Article Open access 19 June 2020
INTRODUCTION Sexual polymorphisms involving discrete variation in the length or position of the style are reported from many unrelated flowering plant families (Webb & Lloyd, 1986;
Barrett et al., 2000a,b). The most common stylar polymorphism is heterostyly which Darwin (1877) first examined in detail and has been the focus of a great deal of subsequent research
(reviewed in Ganders, 1979; Barrett, 1992; Richards, 1997). Heterostylous populations have two (distyly) or three (tristyly) floral morphs which differ reciprocally in the placement of
stigmas and anthers (Lloyd & Webb, 1992a). Another, less well known, stylar polymorphism is characterized by discrete variation in the position of the stigma but little or no reciprocal
positioning in anther placement between the two style morphs. Populations exhibiting stigma-height dimorphism comprise two floral morphs, one in which the stigma is at the same level as the
stamens or protrudes beyond them (long-styled or L-morph) and the other in which the stigma is located below the stamens (short-styled or S-morph). Some authors recognize stigma-height
dimorphism as distinct from heterostyly (Charlesworth & Charlesworth, 1979; Ganders, 1979; Jernstedt, 1982; O’Brien & Calder, 1989; Barrett & Richards, 1990; Lloyd & Webb,
1992a; Arroyo & Dafni, 1995; Barrett et al., 1996), whereas others view the polymorphism as part of the variation encompassed within heterostyly (Philipp & Schou, 1981; Dulberger,
1992; Richards, 1997 and pers. comm.). Unfortunately, there have been few detailed studies of species with stigma-height dimorphism to assess the nature of floral variation and the
evolutionary and functional relationships between this polymorphism and heterostyly. This is unfortunate because stigma-height dimorphism plays an important role in some models of the
evolution of distyly (Lloyd & Webb, 1992b). Stigma-height dimorphism occurs commonly in _Narcissus_ (Amaryllidaceae), a genus of approximately 40 species of insect-pollinated geophytes
largely native to the Mediterranean basin. Dulberger (1964 and unpubl. data) reported stigma-height dimorphism in _N. tazetta_ populations from Israel (and see Arroyo & Dafni, 1995).
Using controlled crosses she demonstrated that self-incompatibility was not of the heteromorphic type, although the inheritance of style length conformed to the single-locus two-allele
control common in distylous species, with the allele for short styles dominant. Stigma-height dimorphism has subsequently been documented in several additional species from three sections of
the genus (Apodanthae, Jonquillae, Tazettae) by Barrett et al. (1996). Despite the widespread distribution of stigma-height dimorphism in _Narcissus_, heterostyly only occurs in distylous
_N. albimarginatus_ of section Apodanthae (J. Arroyo & S. C. H. Barrett, 2000) and tristylous _N. triandrus_ of section Ganymedes (Barrett et al., 1997; Sage et al., 1999), and the
evolutionary relationships between stigma-height dimorphism, distyly and tristyly are unclear. In their model of the evolution of heterostyly, Lloyd & Webb (1992b) suggested that
stigma-height dimorphism is rare in flowering plants because it may be difficult to maintain (and see Charlesworth & Charlesworth, 1979). According to this view, selection rapidly
favours the evolution of discrete anther-height variation in populations with stigma-height dimorphism and hence the polymorphism represents a transient stage in the evolution of heterostyly
(and see O’Brien & Calder, 1989). However, in _Narcissus_ the contrasting frequencies and phylogenetic distributions of the two polymorphisms are not in accord with this model and
further studies are clearly warranted to explain the evolutionary stability of stigma-height dimorphism in the genus. In large distylous populations with frequent sexual recruitment,
equilibrium morph ratios are generally isoplethic (1L:1S) and result from disassortative mating governed by the heteromorphic incompatibility system typical of most heterostylous plants
(reviewed in Ganders, 1979). However, in _N. tazetta_ (Dulberger, 1964) and _N. triandrus_ (Barrett et al., 1997) both inter- and intramorph pollinations are fully compatible and morph
ratios are governed by the influence of floral morphology on the relative fitness of the morphs as female and male parents (Barrett et al., 1996). Intramorph mating in _Narcissus_ spp.
provides opportunities for variation in levels of assortative mating in the style morphs resulting in populations with biased morph ratios or those containing only a single morph. Unlike
heterostylous species with conventional heteromorphic incompatibility, a wide range of style-morph ratios is therefore predicted in natural populations of _Narcissus_ spp. We examine these
issues in populations in _N. assoanus_ and _N. dubius_, two species from different sections of the genus which occur in SW France. Preliminary observations suggested that both species
exhibit stigma-height dimorphism but that the patterns of sex-organ variation differ in association with several features of their floral biology that might be expected to influence
pollination and mating. Here we report the findings of a comparative study in which the goal was to provide insights into the evolution and maintenance of stigma-height dimorphism. In this
paper we ask the following questions: (i) what are the patterns of sex-organ variation in natural populations of _N. assoanus_ and _N. dubius_ and do the two species exhibit a true
dimorphism for stigma height? (ii) are there morph-specific differences in pollen size and production in either _Narcissus_ spp. (a feature commonly associated with distyly)? (iii) what
style-morph ratios characterize populations of _N. assoanus_ and _N. dubius_ and what factors might explain any differences observed both within and between species? In a companion paper
(Baker et al., 2000), we examine fitness components of the style morphs in an effort to understand the selective mechanisms maintaining the polymorphism and the contrasting style-morph
ratios in the two species reported in this paper. MATERIALS AND METHODS NATURAL HISTORY OF _NARCISSUS ASSOANUS_ AND _N. DUBIUS_ _Narcissus assoanus_ (section Jonquillae) is a diminutive
species, approximately 10–15 cm in height, that is widespread in S Spain and SW France. In SW France, it typically occurs in meadows and stony pastures on limestone from sea level to 700 m
altitude. Plants produce a single inflorescence with one to three deep-yellow flowers with prominent coronas and long floral tubes. In SW France the vast majority of plants produce a single
flower. Flowering time depends on altitude and usually occurs from late February to April. _Narcissus dubius_ (section Tazettae) is larger (20–25 cm), with inflorescences of one to seven
white flowers also with prominent coronas and long floral tubes. In SW France flowering begins in mid-February and ends in late March. The species has a more restricted distribution,
occurring primarily along the east coast of S Spain and in SW France at less than 300 m altitude and in some areas is found in sympatry with _N. assoanus. Narcissus dubius_ occurs almost
exclusively in stony, limestone garrigues and on or around cliff faces. _Narcissus assoanus_ is pollinated by butterflies, hawkmoths and solitary bees, whereas _N. dubius_ is pollinated by
hawkmoths, flies and solitary bees. PATTERNS OF SEX-ORGAN VARIATION To establish whether _N. assoanus_ and _N. dubius_ exhibit a true dimorphism for stigma height, we sampled populations of
both species for floral measurements at peak flowering (late February–March for _N. dubius_; March–April for _N. assoanus_). All sampling was undertaken in the Languedoc-Roussillon region of
SW France in an area bounded by the Rhône River to the east and the city of Perpignan near the France–Spain border to the west. In each population, flowers were sampled randomly from
throughout the entire population with care taken to not sample more than one inflorescence from dense clumps which may have arisen via bulb fragmentation (particularly in _N. dubius_).
Floral measurements were made on a single mature flower from at least 45 randomly selected individuals from 15 _N. assoanus_ and 10 _N. dubius_ populations. These populations were chosen
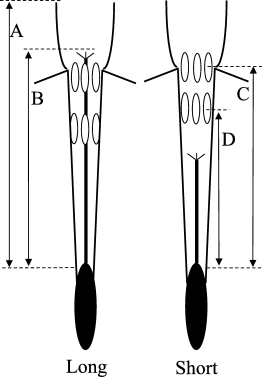
from throughout the range of style-morph ratios revealed from our survey (see below). The following floral traits were measured to 0.01 mm using digital callipers: flower length, stigma
height, and the distance to the top and bottom of the upper- and lower-level stamens. For each stamen level, the distance to the middle of the anthers was calculated by taking the mean of
the distance to the top and bottom of the anthers within a level. All measurements were made from the top of the ovary (Fig. 1). To account for the influence of flower size on patterns of
sex-organ variation we adjusted the measurements of stigma height and upper- and lower-stamen height for flower length. We first performed an analysis of covariance (ANCOVA) using JMP
statistical software (SAS, 1994), in which population and style morph were treated as main effects and flower length was treated as a covariant. Secondly, two- and three-way interaction
terms were eliminated from the model using backward elimination if they contributed less than 5% of the variation in organ height (cf. Sokal & Rohlf, 1995). Finally, we adjusted organ
position as follows: _adjusted organ position_=_organ position_ − _b_ (_flower length_ − _mean flower length_), where _b_ represents the slope of the line of organ position plotted against
flower length. This process accounts for variation in organ position caused by variation in flower size among individuals but does not significantly alter the value of mean organ position.
Differences in mean adjusted organ position between the morphs for populations of each species were then assessed using ANOVA with population and style morph as main effects. POLLEN
CHARACTERISTICS All three anthers from a given stamen level were collected prior to anthesis and placed in Eppendorf sample tubes. Anthers were allowed to dry and fully dehisce before the
tube was filled with 70% ethanol. Pollen size and production were quantified using an electronic Elzone 282 particle counter with a 190 μm aperture. Each sample was vortexed for 15 s to
dislodge pollen grains from anthers and 0.5 mL was removed from the tube and added to a vial containing 24.5 mL of saline solution (0.5% NaCl). The particle counter counted the number of
particles in 0.25 mL samples, assigned them to logarithmic size classes, and calculated the mean geometric size of the pollen grains. For each pollen sample, mean estimates of pollen size
and number are based on four 0.25 mL subsamples. We counted pollen samples from 12 plants of each style morph in one _N. assoanus_ population and 12 and 10 plants of the L- and S-morph,
respectively, from a _N. dubius_ population. Pollen size and number were analysed separately in each population using a two-way ANOVA with style morph and stamen level as main effects.
SURVEYS OF STYLE-MORPH RATIOS Floral measurements established that populations of both _N. assoanus_ and _N. dubius_ were dimorphic for stigma height. Plants could be classified as either L-
or S-styled based on the placement of the stigma with respect to the two stamen levels. We conducted an extensive survey to determine the relative frequencies of the two style morphs in
populations of each species. During spring in years 1996–98 we sampled 46 and 21 populations of _N. assoanus_ and _N. dubius_, respectively, for style-morph ratios. Pooled goodness-of-fit
_G_-tests were calculated to determine whether pooled morph ratios differed significantly from 1L:1S. We also calculated _G_heterogeneity statistics to test for heterogeneous morph ratios
among populations. For each population, two or three independent estimates of population size were made and the average value was taken. Data on style-morph ratios and individual sizes for
populations in the survey are available upon request from A.M.B. RESULTS VARIATION IN SEX-ORGAN POSITION IN NATURAL POPULATIONS Populations of _N. assoanus_ and _N. dubius_ both exhibit a
stigma-height dimorphism despite considerable variation in the relative positions of stigmas and anthers among individuals within each population (Fig. 2). With the exception of monomorphic
populations of _N. dubius_ fixed for the L-morph, there is a clear discontinuity in style length between individuals of the L- and S-morph. The data presented in Fig. 2 were not adjusted to
account for flower size and therefore some of the variation undoubtedly results from developmental or environmental influences. However, despite these sources of variability, a fundamental
dimorphism in stigma height is evident. A two-way ANOVA on the data obtained from all populations with style morph and population as main effects indicated that mean stigma height (mm) was
significantly different between the style morphs in both species (_N. assoanus_: L-morph=17.43, SE=0.082; S-morph=8.94, SE=0.079; _F_1,692= 3956.66, _P_ < 0.001; _N. dubius_:
L-morph=15.61, SE=0.095; S-morph=9.49, SE=0.215; _F_1,314= 295.07, _P_ < 0.001). When sex-organ position is adjusted to account for differences in flower size, the ANOVA results were not
qualitatively different (Table 1). In all analyses of variation in sex-organ position, there were no significant differences in the results obtained between the adjusted or raw data.
Consequently, all subsequent _F_-values we present are those using data that were adjusted to account for flower size. Although mean stigma height was strongly differentiated between the
style morphs in both species, the corresponding positions of the two stamen levels differed much less, although consistent differences were evident when data were examined across
populations. In a two-way ANOVA with morph and population as main effects, mean positions of upper- and lower-level stamens (mm) were significantly lower in the floral tube of the L-morph
compared to the S-morph in both species (Table 1). In both species, mean stamen position differs significantly among populations and the interaction between population and style morph was
significant in _N. dubius_ but not in _N. assoanus_ (Table 2). The two stamen levels in the L-morph were more strongly differentiated from one another than the corresponding stamen levels of
the S-morph, especially in _N. assoanus_. This can be seen by inspection of Fig. 1 which is drawn to scale using mean values obtained from _N. assoanus_ populations. In both species,
flowers of the L-morph typically have stigmas positioned within or slightly above the upper-level stamens. In contrast, stigmas are located well below the lower-level stamens in the S-morph
(Fig. 2). These contrasts in the relative position of stigmas and anthers result in striking differences in the degree of herkogamy exhibited by the style morphs (Fig. 3). Over the 15
sampled populations of _N. assoanus_, mean stigma–anther separation in the S-morph was 5.4 times greater than in the L-morph. The same pattern was also observed in _N. dubius_, where the
degree of stigma–anther separation between the style morphs was even greater. Mean stigma–anther separation in _N. dubius_ was 33 times greater in the S-morph than in the L-morph averaged
over the seven dimorphic populations sampled. In _N. dubius_ most flowers of the L-morph have their stigmas positioned within the upper-level stamens (Fig. 2). VARIATION IN POLLEN SIZE AND
NUMBER We found no evidence for morph-specific or stamen-level differences in pollen size in either of the species under study (_N. assoanus_: L-morph=21.86 μm, SE= 0.081; S-morph=21.70 μm,
SE=0.126; _F_3,44=0.7225, _P_=0.5440; _N. dubius_: L-morph=25.89 μm, SE=0.100; S-morph=25.33 μm, SE=0.475; _F_3,40=0.5230, _P_= 0.6690). Also, there were no significant differences between
style morphs or stamen levels in the number of pollen grains produced in either species (_N. assoanus_: L-morph=64 912 pollen grains per flower, SE=2260; S-morph=64 875, SE=3743;
_F_3,44=0.3926, _P_=0.7589; _N. dubius_: L-morph=59 113, SE=2733; S-morph= 50 355, SE=3609; _F_3,40=1.3810, _P_=0.2625). STYLE-MORPH RATIOS IN NATURAL POPULATIONS All 46 populations of _N.
assoanus_ sampled were dimorphic for style length. Style-morph ratios varied from 1L:1S (_N_=25 populations) to L-biased (_N_=21 populations). There was no relation between style-morph
ratios (frequency of S-morph) and the degree of S-level organ reciprocity (mean difference in height of lower anthers of L-morph and stigmas of S-morph) among the 15 populations for which
detailed floral measurements were undertaken (_r_=0.20, _P_=0.476). No populations with a statistically significant excess of the S-morph were observed. A significant excess of the L-morph
was evident when the morph-ratio data were pooled across all populations (L-morph=0.62, S-morph=0.38; _G_pooled=204.56, d.f.=1, _P_ < 0.001) and there was significant heterogeneity among
population morph ratios (_G_het=44603.18, d.f.=45, _P_ < 0.001). Of the 21 populations of _N. dubius_ sampled for morph ratios, 15 were dimorphic and the remaining six were monomorphic,
containing only the L-morph. Dimorphic populations were strongly L-biased and, like _N. assoanus_, there was also a significant excess of the L-morph when data were pooled across populations
(L-morph= 0.93, S-morph=0.07; _G_pooled=1208.23, d.f.=1, _P_ < 0.001). Significant heterogeneity among population morph ratios was also evident (_G_het=52.45, d.f.=20, _P_ < 0.001).
In _N. assoanus_, the observed morph-ratio variation among populations was nonrandomly distributed over the geographical region sampled (Fig. 4). On the other hand, in _N. dubius_, with the
exception of a small concentration of four monomorphic populations located along the Gardon river valley north of Nîmes, there was no obvious geographical pattern to morph-ratio variation
(Figure 5). Populations of _N. assoanus_ closest to the coast occurring in the garrigue landscape surrounding Montpellier were smaller and more isolated from one another. All exhibited
strongly L-biased morph ratios. In contrast, populations further inland and away from Montpellier were more likely to have isoplethic morph ratios. This geographical pattern was associated
with habitat fragmentation and population size. Inland populations tended to be much larger than those closer to Montpellier where suitable habitats for the species are more restricted in
area. In particular, populations in the NW portion of the region sampled on ‘Le Causse de Blandas’ and ‘Le Causse du Larzac’ limestone plateaux were very large in size and always exhibited
isoplethic morph ratios. Figure 6(a) illustrates the relation between population size and morph ratio in _N. assoanus_. Whereas smaller populations display variable morph ratios, very large
populations were always isoplethic. In _N. dubius_ there was no association between population size and morph ratio (Figure 6b). However, populations of this species never attain the size of
the larger _N. assoanus_ populations. DISCUSSION The major findings of this study are that populations of _N. assoanus_ and _N. dubius_ possess a sexual polymorphism involving discrete
variation in style length. Minor differences in the positions of the two stamen levels within a flower also occur. Population surveys revealed different patterns of variation in style-morph
ratios in the two species, although L-biased morph ratios commonly occur in both. Here, we begin by reviewing what is known about stigma-height dimorphism in plants and address the question
of whether the two _Narcissus_ species should be considered heterostylous. We then discuss functional aspects of the dimorphism and consider the selective mechanisms that may account for the
contrasting patterns of morph-ratio variation revealed in our surveys. STIGMA-HEIGHT DIMORPHISM AND ITS RELATIONSHIP TO HETEROSTYLY Reports of stigma-height dimorphism are infrequent in
other genera of flowering plants. They include: _Anchusa_ spp. (Dulberger, 1970; Philipp & Schou, 1981) and _Lithodora_ spp. (S.C.H. Barrett, J.D. Thompson & D. Manicacci, unpubl.
data) in the Boraginaceae; _Linum grandiflorum_ (Darwin, 1877; Dulberger, 1992) in the Linaceae; _Chlorogalum angustifolium_ (Jernstedt, 1982; Barrett et al., 2000b) in the Liliaceae;
_Epacris impressa_ (O’Brien & Calder, 1989) in the Epacridaceae; _Kalmiopsis leachiana_ (Barrett et al., 2000a) in the Ericaceae; and _Anigozanthos humilis_ (S.D. Hopper, pers. comm.) in
the Haemodoraceae. In common with dimorphic _Narcissus_ spp., populations of these taxa are generally characterized by a bimodal distribution of style length but little or no
differentiation in stamen position in the two style morphs. Several genera with stigma-height dimorphism occur in families in which heterostyly is common (e.g. Boraginaceae and Linaceae),
raising the question of whether the polymorphism represents a transitional stage in the evolution of distyly (and see Lloyd & Webb, 1992a,b). However, in other cases (e.g. Liliaceae and
Ericaceae) heterostyly is absent from the families and the evolution of stigma-height dimorphism appears to involve isolated events of unknown adaptive significance. Heterostyly has been
defined as ‘a genetically determined polymorphism in which the morphs differ in the sequence of heights at which the anthers and stigmas are presented within their flowers’ (Lloyd &
Webb, 1992a, p. 152). The morphological features of _N. assoanus_ and _N_. _dubius_ flowers match this description well. However, Lloyd & Webb (1992a) elaborated this definition further
to emphasize that the position of stigmas and anthers in the floral morphs must differ in a reciprocal manner. Indeed this reciprocal herkogamy (Richards, 1986, p. 262) was considered by
Lloyd & Webb (1992a) to be a defining feature of heterostyly and on this basis they distinguished distyly from stigma-height dimorphism using _Narcissus_ as an example of the latter
(also see Ganders, 1979, p. 608). We agree with this perspective, which differs from the view held by Dulberger (1992) and Richards (1997 and pers. comm.) who advocate a broader view of
heterostyly which includes species without reciprocal herkogamy. Our more restricted definition of heterostyly excludes species that lack a clear reciprocal correspondence between stigmas
and anthers. Additionally, although pollen size and production commonly differ between the style morphs in heterostylous species, there is no evidence of morph-specific differences in pollen
characters of _N. assoanus_ or _N. dubius_. Based on these findings, we prefer to classify _N. assoanus_ and _N. dubius_ as nonheterostylous. As discussed further in Baker et al. (2000),
other features of the reproductive systems of _N. assoanus_ and _N. dubius_ are atypical for heterostylous plants. We believe these distinguishing features merit the recognition of
stigma-height dimorphism as a plant sexual polymorphism distinct from yet functionally similar to heterostyly. FUNCTIONAL CONSEQUENCES OF SEX-ORGAN DEPLOYMENT Casual observations of the two
style morphs in _N. assoanus_ and _N. dubius_ indicated that stamen levels were located at similar positions within the floral tube. However, our detailed measurements revealed subtle
differences between the style morphs in both species. Are these differences of functional significance for pollination and mating? This seems unlikely in the case of the upper-level stamens,
where there is less than 0.5 mm difference between the morphs in their position in both species. In fact, within most populations there was no significant difference between the height of
the upper-level stamens in the two morphs (nine of 15 populations for _N. assoanus_; four of seven for _N. dubius_ following _t_-tests, results not shown). It is difficult to imagine that
this small difference in mean height of upper-level stamens has significant morph-specific influences on pollen dispersal, given the large amount of variation among individual plants in
their position (Fig. 2). The difference between style morphs in the position of lower-level stamens was much greater than for upper-level stamens and hence may have functional significance.
This difference was evident in both species but was more pronounced in _N. assoanus_ [mean difference (mm): 1.7 in _N. assoanus_ and 1.0 in _N. dubius_]. Lower-level stamens in the L-morph
were always positioned significantly lower in the floral tube relative to the corresponding stamen level of the S-morph. Interestingly, this difference was a consistent feature of all
populations we sampled in both species, suggesting that the positioning may influence pollen dispersal. It is possible that the lower-level stamens are under disruptive selection in the two
style morphs. Selection to reduce the height of these stamens in the L-morph may increase male fertility because of more proficient pollen dispersal to stigmas of the S-morph. In contrast,
selection to increase the height of lower-level stamens in the S-morph may reduce self-pollination and self-interference and also increase the effectiveness of pollen dispersal to stigmas of
the L-morph. Because upper-level stamens in both morphs probably transfer most of their pollen to stigmas of the L-morph, selection would be more likely to maintain them at a similar
position corresponding to the height of stigmas in the L-morph. The most striking morphological difference between the style morphs of both species involves the degree of herkogamy that they
exhibit. Stigmas and anthers are in close proximity in flowers of the L-morph, whereas there is considerable spatial separation in the S-morph. There is substantial evidence that the degree
of herkogamy has important functional consequences for mating patterns, especially selfing rates (see Belaoussoff & Shore, 1995 and references therein). Plants with weakly developed
herkogamy usually experience more self-pollination than those with well separated sex organs. Indeed, studies of self-pollen deposition in caged individuals of _N. assoanus_ demonstrated
that flowers of the L-morph often have upwards of 200 self pollen grains deposited on stigmas through autonomous self-pollination, whereas flowers of the S-morph always have fewer than 15
(A.M. Baker, J.D. Thompson & S.C.H. Barrett, unpubl. data). Similar results were also obtained in _N. tazetta_ by Arroyo & Dafni (1995), a species that exhibits the same
morph-specific differences in herkogamy. What are the likely functional consequences of different rates of autonomous self-pollination in the style morphs? As yet there is no clear answer to
this question. Experimental studies have demonstrated that _N. assoanus_ is moderately self-sterile, whereas _N. dubius_ is highly self-compatible. These differences between the two species
are reflected in contrasting selfing rates in natural populations (Baker et al., 2000). Yet comparative data on the mating systems and female fertility of the style morphs of both species
failed to detect significant morph-specific influences on maternal selfing rates or seed-set (Baker et al., 2000). This suggests that although the quantity and composition (self vs.
outcrossed) of pollen deposited on stigmas of the style morphs are likely to be different, postpollination mechanisms may act to filter heterogeneous pollen loads resulting in the similar
maternal fitness components that we observed. FACTORS INFLUENCING MORPH-RATIO VARIATION Our surveys of _N. assoanus_ and _N. dubius_ in SW France revealed a wide range of style-morph ratios
ranging from isoplethy to populations fixed for the L-morph. This finding is consistent with the results of earlier studies of _N. tazetta_ (Arroyo & Dafni, 1995) in Israel and _N.
papyraceus_ in S Spain (Barrett et al., 1996). In these surveys, similar patterns of variation were reported, with many populations exhibiting biased morph ratios and monomorphic populations
fixed only for the L-morph. A survey of nine populations of five dimorphic _Narcissus_ species, including two populations of _N. assoanus_ from S Spain, also revealed L-biased morph ratios
in all populations (Barrett et al., 1996). Collectively, these results raise the question of why _Narcissus_ populations often exhibit L-biased morph ratios? Barrett et al. (1996) and Baker
et al. (2000) developed pollination and mating models, respectively, that can explain biased style-morph ratios in _Narcissus_ populations with a stigma-height dimorphism. The models
indicate that L-biased morph ratios will occur if there are higher levels of assortative mating in this morph compared to the S-morph. Morph-specific differences in sex-organ deployment in
_N. assoanus_ and _N. dubius_ suggest that this is likely to occur. Although upper-level anthers of the L-morph are well positioned to transfer pollen to stigmas of other plants of this
morph, the high degree of herkogamy in the S-morph reduces the likelihood of proficient assortative pollen transfer. Most pollen transferred to S-stigmas probably originates from the
lower-level anthers of the L-morph, which are positioned below the corresponding anther level in the S-morph. Morph-specific differences in the ratio of assortative to disassortative mating
resulting from asymmetric pollen transfer within and between the style-morphs can explain the L-biased morph ratios observed in _Narcissus_ spp. (Baker et al., 2000). Why are monomorphic
populations of _Narcissus_ spp. almost always fixed for the L-morph? If offspring from plants of the S-morph are largely the products of disassortative mating they will mostly be
heterozygous (_Ss_) at the locus controlling style length, assuming that inheritance is of the single-locus control demonstrated in _N. tazetta_ (Dulberger, 1964) and found in most
heterostylous species (Ganders, 1979). Heterozygosity at the _S_-locus is also promoted in populations with L-biased morph ratios. This is because plants of the S-morph are more likely to
mate with individuals of the L-morph because of the high abundance of the latter. Hence homozygous short-styled plants (_SS_) should occur rather infrequently in most populations of
_Narcissus_ spp. because of low levels of assortative mating in this morph and L-biased morph ratios. Interestingly in her studies on the inheritance of style-length in _N. tazetta_,
Dulberger (1964) found no homozygous short-styled plants despite the absence of intrinsic barriers to intramorph mating. Elsewhere, a similar finding was also reported by Schou & Philipp
(1984) in their genetic studies of _Anchusa officinalis_, a species that exhibits stigma-height dimorphism, intramorph compatibility and strongly L-biased morph ratios. The apparent rarity
of homozygous plants of the S-morph restricts opportunities for S-monomorphy. This is because heterozygous plants of the S-morph that found new populations will always segregate both morphs,
thus guaranteeing establishment of stylar dimorphism. In contrast, L-morph (_ss_) founders will only produce long-styled offspring, giving rise to populations monomorphic for this morph.
Thus according to this hypothesis the contrasting occurrence of L- vs. S-morph monomorphism arises because of morph-specific differences in mating and the dominance relationships at the
_S_-locus governing stylar dimorphism. However, two alternative hypotheses could also explain the absence of S-monomorphy in _Narcissus_ spp. Strong selection against homozygous (_SS_)
plants because of inbreeding depression could prevent the establishment of monomorphic populations composed of the S-morph. In this regard, Richards (1997, p. 284) has argued that
S-morph-linked recessive lethality is ‘a pervasive feature of heterostylous plants’ (and see Richards, 1998), but unfortunately the experimental evidence for this phenomenon is only mixed at
best (Shore & Barrett, 1985; Eckert & Barrett, 1993). Nevertheless, it would be worth investigating this possibility through controlled crosses of short-styled plants. Finally, it
is possible that plants of the S-morph are poor at establishing populations because their concealed stigmas are only accessible to long-tongued pollinators which may be less inclined to
visit isolated plants. According to this hypothesis, fertility selection favours the L-morph as a founding morph because its sex organs are more accessible to short-tongued generalist
pollinators that might be more likely to visit small, newly established populations. We have not been able to detect morph-specific differences in female fertility in established populations
of _N. assoanus_ or _N. dubius_, but it would certainly be worth comparing patterns of seed-set in experimental monomorphic populations composed of the L- vs. the S-morph to test this
hypothesis. Populations with equal frequencies of the two style morphs were commonly observed in _N. assoanus_, indicating that stigma-height dimorphism can result in equivalent levels of
disassortative mating. This finding is significant because it is an assumption for Lloyd & Webb’s (1992a,b) model for the evolution of distyly from stigma-height dimorphism. The floral
morphologies of isoplethic populations were similar to those exhibiting biased morph ratios and there was no evidence of a greater reciprocal correspondence in anther and stigma positions
that might favour symmetrical disassortative mating. Isoplethic populations tended to occur in the less disturbed landscapes and particularly on the upland limestone plateaux north-west of
Montpellier where populations were all very large. It is possible that the frequency and composition of pollinators visiting isoplethic vs. nonisoplethic populations differ in ways that
influence pollen transfer and mating patterns. Observations of pollinator visits to _N. assoanus_ indicate that the primary visitors are butterflies (_Gonepteryx cleopatra_), solitary bees
(_Anthophora_ spp.) and day-flying hawkmoths (_Macroglossum stellatarum_) but as yet we have not been able to determine whether visitation patterns differ between populations with
contrasting style-morph ratios. Elsewhere it has been argued that differences in the types of pollinators visiting populations of _N. tazetta_ (Arroyo & Dafni, 1995), _N. papyraceus_ and
_N. triandrus_ (Barrett et al., 1996) may explain the striking patterns of morph-ratio variation that these species also exhibit. To distinguish how different pollinators might influence
pollination and mating, measurements of pollen transfer and male fertility are required. Isoplethic style-morph ratios were not observed in _N. dubius_ and the S-morph was generally either
rare or absent from populations. Strongly biased morph ratios in this species probably reflect the mating consequences of weak disassortative mating compared with _N. assoanus_. In common
with many plant species that flower in very early spring (Schemske et al., 1978), _N. dubius_ is highly self-compatible. Not unexpectedly, populations of this species exhibit higher selfing
rates than the self-sterile _N. assoanus_ (Baker et al., 2000). Higher selfing in _N. dubius_ is also probably influenced by opportunities for geitonogamy because of the multiflowered
inflorescences of this species. It is possible that weak disassortative mating may be destabilizing the stigma-height dimorphism in _N. dubius_ resulting in the gradual elimination of the
S-morph from many populations. Selection against the S-morph may also be occurring in hill populations of _N. tazetta_ (Arroyo & Dafni, 1995) and _N. papyraceus_ (Barrett et al., 1996),
two other members of section Tazettae. In conclusion, stigma-height dimorphism represents a curious and perplexing floral design. It seems probable that the polymorphism functions to promote
more proficient pollen transfer among plants in a manner similar to heterostyly. However, how disassortative pollen transfer is achieved, at least in some populations, without well
developed sex-organ reciprocity is unclear. The wide range of style-morph ratios that characterize populations of _Narcissus_ spp. suggests that the patterns of pollen transfer within and
between style morphs vary considerably. Detailed studies of the pollination biology of populations with contrasting style-morph ratios should provide insights into how this occurs.
REFERENCES * Arroyo, J. and Dafni, A. (1995). Variation in habitat, season, flower traits, and pollinators in dimorphic _Narcissus tazetta_ L. (Amaryllidaceae) in Israel. _New Phytol_ 129:
135–145. Article Google Scholar * Arroyo, J. and Barrett, S. C. H. (2000). Discovery of distyly in _Narcissus_ (Amaryllidaceae). _American Journal of Botany_ in press. Article CAS Google
Scholar * Baker, A. M., Thompson, J. D. and Barrett, S. C. H. (2000). The evolution and maintenance of stigma-height dimorphism in _Narcissus_ II. Fitness comparisons between style morphs.
_Heredity_ 84: in press. Article Google Scholar * Barrett, S. C. H. (ed.) (1992). _Evolution and Function of Heterostyly_. Springer, Berlin. Book Google Scholar * Barrett, S. C. H. and
Richards, J. H. (1990). Heterostyly in tropical plants. _Mem N Y Bot Gard_, 55: 35–61. Google Scholar * Barrett, S. C. H., Lloyd, D. G. and Arroyo, J. (1996). Stylar polymorphisms and the
evolution of heterostyly in _Narcissus_ (Amaryllidaceae). In: Lloyd, D. G. and Barrett, S. C. H. (eds) _Floral Biology. Studies on Floral Evolution in Animal-Pollinated Plants_ pp. 339–376.
Chapman & Hall, New York. Google Scholar * Barrett, S. C. H., Cole, W. W., Arroyo, J., Cruzan, M. B. and Lloyd, D. G. (1997). Sexual polymorphisms in _Narcissus triandrus_
(Amaryllidaceae): is this species tristylous? _Heredity_ 78: 135–145. Article Google Scholar * Barrett, S. C. H., Jesson, L. K. and Baker, A. M. (2000a). The evolution and function of
stylar polymorphisms in flowering plants. _Annals of Botany_ in press. * Barrett, S. C. H., Baker, A. M. and Jesson, L. K. (2000b). Mating strategies in monocotyledons. In: Wilson, K. L. and
Morrison, D. (eds) _Systematics and Evolution of Monocots_ pp. 56–267. CSIRO Publishing, Australia. Google Scholar * Belaoussoff, S. and Shore, J. S. (1995). Floral correlates and fitness
consequences of mating-system variation in _Turnera ulmifolia_. _Evolution_. 49: 545–556. Article Google Scholar * Charlesworth, D. and Charlesworth, B. (1979). A model for the evolution
of distyly. _Am Nat_ 114: 467–498. Article Google Scholar * Darwin, C. (1877). _The Different Forms of Flowers on Plants of the Same Species_ Reprinted in 1986. University of Chicago
Press, Chicago, IL. * Dulberger, R. (1964). Floral dimorphism and self-incompatibility in _Narcissus tazetta_ L. _Evolution_. 18: 361–363. Article Google Scholar * Dulberger, R. (1970).
Floral dimorphism in _Anchusa hybrida_ Ten. _Isr J Bot_ 19: 37–41. Google Scholar * Dulberger, R. (1992). Floral polymorphisms and their functional significance in the heterostylous
syndrome. In: Barrett, S. C. H. (ed.) _Evolution and Function of Heterostyly_ pp. 41–84. Springer, Berlin. Chapter Google Scholar * Eckert, C. G. and Barrett, S. C. H. (1993). The
inheritance of tristyly in _Decodon verticillatus_ (Lythraceae). _Heredity_. 71: 473–480. Article Google Scholar * Ganders, F. R. (1979). The biology of heterostyly. _N Z J Bot_, 17:
607–635. Article Google Scholar * Jernstedt, J. A. (1982). Floral variation in _Chlorogalum angustifolium_ (Liliaceae). _Madroño_ 29: 87–94. Google Scholar * Lloyd, D. G. and Webb, C. J.
(1992a). The evolution of heterostyly. In: Barrett, S. C. H. (ed.) _Evolution and Function of Heterostyly_ pp. 151–178. Springer, Berlin. Chapter Google Scholar * Lloyd, D. G. and Webb, C.
J. (1992b). The selection of heterostyly. In: Barrett, S. C. H. (ed.) _Evolution and Function of Heterostyly_ pp. 179–207. Springer, Berlin. Chapter Google Scholar * O’brien, S. P. and
Calder, D. M. (1989). The breeding biology of _Epacris impressa_: Is this species heterostylous? _Aust J Bot_, 37: 43–54. Article Google Scholar * Philipp, M. and Schou, O. (1981). An
unusual heteromorphic incompatibility system: Distyly, self-incompatibility, pollen load, and fecundity in _Anchusa officinalis_ (Boraginaceae). _New Phytol_ 89: 693–703. Article Google
Scholar * Richards, A. J. (1986). _Plant Breeding Systems_. 1st edn. Allen & Unwin, London. Google Scholar * Richards, A. J. (1997). _Plant Breeding Systems_. 2nd edn. Chapman &
Hall, London. Book Google Scholar * Richards, A. J. (1998). Lethal linkage and its role in the evolution of plant breeding systems. In: Owens, S. J. and Rudall, P. J. (eds) _Reproductive
Biology in Systematics, Conservation and Economic Botany_ pp. 71–83. Royal Botanic Gardens, Kew. * Sage, T. L., Strumas, F., Cole, W. W. and Barrett, S. C. H. (1999). Differential ovule
development following self- and cross-pollination in _Narcissus triandrus_ (Amaryllidaceae). _Am J Bot_, 86: 855–870. Article CAS Google Scholar * SAS (1994). JMP Users Guide version
3.0.2. SAS Institute, Cary, NC. * Schemske, D. W., Willson, M. F., Melampy, M. N., Miller, L. J., Vernier, L., Schemske, K. M. and Best, L. B. (1978). Flowering ecology of some spring
woodland herbs. _Ecology_ 59: 351–366. Article Google Scholar * Schou, O. and Philipp, M. (1984). An unusual heteromorphic incompatibility system 3. On the genetic control of distyly and
self-incompatibility in _Anchusa officinalis_ L. (Boraginaceae). _Theor Appl Genet_, 68: 139–144. Article CAS Google Scholar * Shore, J. S. and Barrett, S. C. H. (1985). The genetics of
distyly and homostyly in _Turnera ulmifolia_ L. (Turneraceae). _Heredity_ 55: 167–174. Article Google Scholar * Sokal, R. R. and Rohlf, F. J. (1995). _Biometry_ 3rd edn. W. H. Freeman, San
Francisco. Google Scholar * Webb, C. J. and Lloyd, D. G. (1986). The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. N. Z. J. Bot.,
24: 163–178. Download references ACKNOWLEDGEMENTS We thank Jan Molinar, Henri Michaud and Max Debussche for help in locating populations; Lawrence Harder and Anne Worley for valuable
discussion and assistance in the field; Linley Jesson, Bill Cole and John Richards for helpful comments on the manuscript. This work was funded by an operating grant from NSERC to S.C.H.B.
and a grant from CNRS to J.D.T. A.M.B. was supported by graduate scholarships from NSERC and the Ontario Government. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Botany,
University of Toronto, 25 Willcocks Street, Toronto, M5S 3B2, Ontario, Canada Angela M Baker & Spencer C H Barrett * Centre d’Ecologie Fonctionnelle et Evolutive, CNRS, 1919 Route de
Mende, Montpellier, Cedex 5, 34293, France John D Thompson Authors * Angela M Baker View author publications You can also search for this author inPubMed Google Scholar * John D Thompson
View author publications You can also search for this author inPubMed Google Scholar * Spencer C H Barrett View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Angela M Baker. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Baker, A., Thompson, J. & Barrett, S.
Evolution and maintenance of stigma-height dimorphism in _Narcissus_. I. Floral variation and style-morph ratios. _Heredity_ 84, 502–513 (2000).
https://doi.org/10.1046/j.1365-2540.2000.00651.x Download citation * Received: 25 May 1999 * Accepted: 04 October 1999 * Published: 01 May 2000 * Issue Date: 01 May 2000 * DOI:
https://doi.org/10.1046/j.1365-2540.2000.00651.x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Amaryllidaceae * disassortative mating *
heterostyly * morph ratios * population size * stigma-height dimorphism