- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Adaptation to edaphic stress may have a key role in plant species range expansion. _Aegilops tauschii_ Coss., the common wheat’s D-genome progenitor native to the
Transcaucasus-Middle East region, is a good model to study the relationships between soil salinity and plant distributions: one of its intraspecific sublineages, TauL1b, drove the
long-distance eastward expansion of this species range reaching semi-arid-central Asia. Salt tolerance during germination and seedling growth was evaluated in 206 _Ae. tauschii_ accessions
by treating seeds with NaCl solutions differing in concentrations. Differences in natural variation patterns were analyzed between sublineages and associated with natural edaphic condition
variables, and then compared with reproductive trait variation patterns. The natural variations observed in NaCl-induced-stress tolerance had clear geographic and genetic structure. Seedling
growth significantly increased in the TauL1b accessions that were collected from salt-affected soil habitats, whereas germinability did not. Principal component analysis suggested that the
NaCl-induced-stress tolerances and reproductive traits might have had a similar degree of influence on _Ae. tauschii_’s eastward range expansion. Adaptation to salt-affected soils through
increased seedling growth was an important factor for the species’ successful colonization of the semi-arid central Asian habitats. TauL1b accessions might provide useful genetic resources
for salt-tolerant wheat breeds. SIMILAR CONTENT BEING VIEWED BY OTHERS THE RESPONSE OF WHEAT AND ITS MICROBIOME TO CONTEMPORARY AND HISTORICAL WATER STRESS IN A FIELD EXPERIMENT Article Open
access 27 July 2022 EVALUATING HEAT AND DROUGHT RESILIENCE IN ANCIENT INDIAN DWARF WHEAT _TRITICUM SPHAEROCOCCUM_ PERCIVAL USING STRESS TOLERANCE INDICES Article Open access 30 May 2025
CLEAR EFFECTS ON ROOT SYSTEM ARCHITECTURE OF WINTER WHEAT CULTIVARS (_TRITICUM AESTIVUM_ L.) FROM CULTIVATION ENVIRONMENT AND PRACTICES Article Open access 15 May 2024 INTRODUCTION Species
ranges expand through individuals’ dispersal to novel habitats and/or adaptation to local environmental conditions. These individuals face several environmental challenges to their survival
and propagation in the new habitats, including extreme climatic and edaphic conditions, unusual competition, predation, and disease1. Foreign abiotic and biotic stresses can cause plastic
phenotypic changes in the migrants and in their early descendants and, eventually, result in the evolution of local adaptation in the following generations through genetic changes.
Peripheral populations of geographically widespread species are of great evolutionary and ecological interest because they represent model systems to study the genetic mechanisms underlying
local adaptation2. Phenotypic changes in ecologically functional traits can be essential to species range expansion through adaptation to local environments. Several studies emphasized the
importance of reproductive traits change in the evolution of plant local adaptation3. In general, adaptive phenotypic changes in the new habitats can occur rapidly within tens of
generations, particularly when the species’ standing genetic variation can provide alleles that would be favored under the new environmental conditions4,5. Climate conditions, such as
temperature, precipitation, and photoperiod, are common environmental factors that can cause adaptive phenotypic changes6,7. However, in the process of species range expansion, migrating
individuals/populations may have to overcome several stressful abiotic factors other than climate conditions in order to survive and propagate in the new habitats. Edaphic stress is one of
the key abiotic factors that may serve as a selective agent in the evolution of local adaptation8,9. In natural settings, plants’ germination, vegetative growth, root and aboveground
architecture, metabolism, and reproduction may be strongly influenced by soil’s shallowness and poor drainage (physical conditions), as well as water and nutrient deficiency, acidity,
alkalinity, salinity, and metal toxicity (chemical conditions). For this reason, edaphic factors might potentially explain the patterns of local adaptation. Nevertheless, relatively little
is known about how edaphic stress functions as a selective agent in ecological and evolutionary contexts. Particularly, much remains to be studied about the relationships between soil
salinity and plant distributions: soil chemical variables considered in biogeographic studies are usually limited to soil moisture/texture, pH, and nutrients10. This paper reports an
ecogeographic analysis of the natural variation occurring during germination and early seedling growth of the wild wheat _Aegilops tauschii_ Coss. under NaCl-induced stress. _Ae. tauschii_
(formerly known as _Aegilops squarrosa_ L.) is the D-genome progenitor of hexaploid common wheat _Triticum aestivum_ L.11,12. _Ae. tauschii_ weedy-stands often occur in wheat and barley
fields within the species range13, but the species natural habitats encompass sandy seashores, deserts margins, stony hills, steppes, wastelands, roadsides, and humid temperate forests. It
is a polymorphic self-pollinating diploid species with a wide geographic range in central Eurasia. The center of _Ae. tauschii_’s distribution is in the southern coastal region of the
Caspian Sea and Azerbaijan. From the center, the species range spreads eastward, to Pakistan and western China, via the Kopet Dag Mountains of Turkmenistan, and westward, to central Syria,
via the southeastern Turkey valleys14,15. The biogeography of _Ae. tauschii_ is intriguing. The genus _Aegilops_ has, in addition to 12 polyploid species, 10 diploid species that are thought
to have radiated some million years ago in the Transcaucasus. _Ae. tauschii_’s geographic distribution differs from that of its congeners, as this diploid species is the only one whose
distribution range expanded eastward, i.e., to the Asian part of the continent; all the other diploid species migrated toward the west and southwest of the Transcaucasus. _Ae. tauschii_ is
the only currently known _Aegilops_ species that naturally grows in China14,15. It is not clear why and how _Ae. tauschii_ made its way to central Asia, while the other diploid species
expanded their ranges westward from Transcaucasus. The central Asian part of _Ae. tauschii_’s distribution range is characterized by cold semi-arid and continental climate conditions,
whereas the Transcaucasus-Middle East part is largely under temperate climate conditions16. Accordingly, _Ae. tauschii_ intraspecific lineages and sublineages are potentially useful to study
the ecological and evolutionary association between soil salinity tolerance patterns and species distribution ranges in plants inhabiting dry lands. Thus, this study aimed to: (1) provide a
broad picture of the natural variation patterns in _Ae. tauschii_’s NaCl-induced-stress tolerance during its germination and early seedling growth, (2) clarify if the species’ eastward
range expansion is associated with an adaptive phenotypic change in salt tolerance by comparing the natural variation patterns of lineages and sublineages in various edaphic conditions, and
(3) provide insights on the importance of salt tolerance during germination and seedling growth for _Ae. tauschii_’s eastward range expansion. MATERIALS AND METHODS STUDY SYSTEM Based on the
patterns of molecular marker variations, three genetic lineages were defined for _Ae. tauschii_: two major lineages (TauL1 and TauL2), each including two distinctive sublineages (TauL1a and
TauL1b within TauL1; TauL2a and TauL2b within TauL2), and one minor lineage (TauL3)17,18,19,20 (Supplementary Fig. S1). Of these lineages, TauL2 and TauL3 are genetically closer to the
common wheat D genome than TauL1. Geographically, TauL1 has a wide distribution range spanning from central Syria to western China, whereas TauL2 and TauL3 are restricted to the
Transcaucasus-Middle East region and Georgia, respectively (Supplementary Fig. S2). Interestingly, TauL1b sublineage, which derived from TauL1a, is the only sublineage occurring in the
central Asian part of the species range, suggesting that TauL1b drove _Ae. tauschii_’s eastward range expansion19. A population genetic analysis showed that the TauL1b accessions were
composed of two large and one small genepools and that they could be classified into three genetic groups: (1) the accessions having alleles mostly (>50%) derived from one of the large
gene pools (LowQ2); (2) the accessions having alleles mostly (>50%) derived from the other large gene pool (HighQ2); (3) the accessions having alleles almost exclusively derived from the
small gene pool (Q3) (Supplementary Fig. S1). PLANT MATERIALS Two hundred and six _Ae. tauschii_ Coss. accessions representing the entire natural habitat range and adventive populations in
the Shaanxi and Henan provinces of China were used (Supplementary Table S1). These accessions were selected because their natural variation patterns in several reproductive and morphological
traits, as well as their molecular marker genotypes, had been characterized in detail19. Only five accessions represented the TauL3 lineage. Despite that the sample size was small, we
included the TauL3 accessions to draw a species-wide picture of the natural variation patterns in _Ae. tauschii_’s NaCl-induced-stress tolerance during its germination and early seedling
growth. For each accession, we used seeds propagated by selfing from a single plant that flowered in a greenhouse during spring. Seeds were stored at 4 °C for 10–15 months before use.
EVALUATION OF SALT TOLERANCE DURING GERMINATION AND SEEDLING GROWTH Seeds of each accession were treated with 0.5% (w/v; 85.5 mM), 1.0% (w/v; 171 mM), and 1.5% (w/v; 257 mM) NaCl solutions
and with distilled water (as a control treatment). These NaCl concentrations were chosen because they were shown to be useful to study the salt tolerance during germination and seedling
growth in wild _Hordeum_ species that often occur together with _Ae. tauschii_ in natural habitats21. The electrical conductivity of 0.5%, 1.0%, and 1.5% NaCl solutions roughly corresponded
to 8.6 dS/m, 17.0 dS/m, and 26.0 dS/m, respectively. Nine seeds from each accession were used in each treatment, except for the 0.5% NaCl treatment of KU-2111 and the 1.0% NaCl treatment of
KU-2042 (eight seeds were used in both cases). For each accession, the seeds were placed on a sand layer (ca. 15 mm thick), moistened with a 0.5%, 1.0%, or 1.5% NaCl solution (or distilled
water in the case of controls), and set at the bottom of a plant tissue culture container. After two weeks at 4 °C under dark condition, germination and seedling growth took place at 22 °C
under a 12/12 h light/dark photoperiod for another two weeks. To reduce the effect of lurking environmental condition differences on phenotypes, the containers were arranged randomly in a
growth chamber. Two traits, germinability and seedling growth ability, were measured to evaluate salt tolerance during early development under a given treatment condition. Germinability was
determined by counting the number of germinated seeds in a container (germinated seed number, abbreviated as GN hereafter), whereas the seedling growth ability was calculated as the mean
length of the first leafs emerging from germinated seeds (mean length, abbreviated as ML hereafter). Seeds with a coleoptile shorter than 5.0 mm were considered non-germinated and excluded
from ML calculation. EDAPHIC AND PHENOTYPIC DATASETS Because the information on the edaphic conditions of original habitats was not available, we used the soil property estimates that were
obtained from two sources: the 30 by 30 arcsec (roughly 1 km cell) resolution salinity (electrical conductivity) dataset from the Harmonized World Soil Database ver. 1.222 and the 30 by 30
arcsec resolution available water capacity dataset from the World soil property estimates for broad-scale modeling (WISE30sec)23. The Harmonized World Soil Database combined regional and
national updates of soil information worldwide, whereas WISE30sec provided a dataset that was created using the soil map unit delineations of the Harmonized World Soil Database overlaid by
soil property estimates derived from analyses of the ISRIC-WISE soil profile database. ArcGIS ver. 10 (ESRI) was used to extract the estimates from these sources based on the geographic
coordinates of the sampling sites. For the 20 Caspian coastal habitats, salinity estimates and/or available water capacity estimates were extracted based on the locality information. The
_Ae. tauschii_ flowering time (i.e., days from coleoptile emergence to flower)24 and number of spikelets per spike19 datasets were taken from previous studies. STATISTICAL ANALYSIS
Statistical calculations were performed using the R Commander software ver. 2.1 for R ver. 3.2 (The R Foundation for Statistical Computing Platform)25 (for Fisher’s exact test), the R
package ‘ggplot2’26 (for data plotting), the R package ‘lawstat’27 (for Brunner-Munzel test), the R package ‘ecodist’28 (for dissimilarity matrix calculation and partial Mantel test), JMP
ver. 12.1 (SAS Institute Inc.) (for Welch’s _t_ test, Steel-Dwass test, principal component analysis (PCA), Tukey-Kramer test, and regression), and SPSS ver. 23 (IBM) (for Dunnet T3 multiple
comparisons). Dunnet T3 multiple comparisons were used when the variances in the groups were unequal. Partial Mantel tests were done using phenotypic, genetic, and latitudinal dissimilarity
(distance) matrices with 1,000 permutations. The Bayesian Information Criterion was used for model selection in stepwise multiple regression. GenAlEx ver. 6.5 was used to calculate genetic
distances29 and pairwise _F_st values30,31. RESULTS NATURAL VARIATION IN NACL-INDUCED-STRESS TOLERANCE AND ITS ASSOCIATION WITH HABITAT EDAPHIC CONDITIONS Responses to NaCl-induced stress
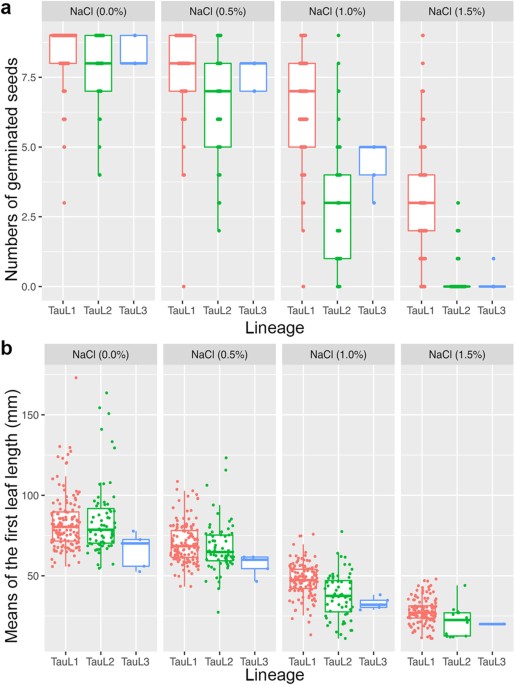
varied greatly among the tested accessions, albeit stress apparently having a negative overall effect on germination and seedling growth. As the stress increased, both GNs and MLs markedly
decreased (Fig. 1). However, GN mean values were larger in TauL1 than in TauL2 and TauL3 in all NaCl treatments (Supplementary Table S2). Because GNs (count data having a maximum value) were
not well modeled by a normal distribution, we used Brunner-Munzel test of stochastic equality to examine if the GNs values were statistically different between the lineages. As the result,
a significant difference between the TauL1 and TauL2 GN values was found in each treatment (_P_ < 0.01). In the 1.5% NaCl treatment, 119 TauL1 accessions out of the 133 (89.5%) had at
least one germinated seed (i.e., they showed seedling growth ability), whereas only 20.6% (14 out of 68) of the TauL2 accessions and 20.0% (one out of five) of the TauL3 accessions showed
seedling growth ability. Furthermore, the TauL1 accessions outperformed the TauL2 and TauL3 accessions in seedling growth ability under all NaCl stress conditions, as revealed by TauL1
larger ML mean values compared to TauL2 and TauL3 (Supplementary Table S2). Differences between TauL1 and TauL2 mean ML values were statistically significant in the 1.0% and 1.5% NaCl
treatments (Welch’s _t_ test). All these findings showed that _Ae. tauschii_’s NaCl-induced-stress tolerance natural variations have a clear genetic structure and that the geographically
widespread TauL1 lineage is distinct from the others with respect to under-stress germinability and seedling growth ability. In general, increases in environmental NaCl concentrations may
cause osmotic stress that disturbs plants’ water uptake, as well as ionic stress that interferes with metabolic processes through the activity of excess Na+ and Cl− 32. As so, we compared
soil salinity and available water capacity conditions between TauL1, TauL2, and TauL3 habitats. Soil salinity estimates were available for the habitats of 197 out of the 206 accessions
analyzed, whereas available water capacity estimates were available for 205 accessions (Supplementary Table S1). Several soils of the TauL1 habitats had salinity (i.e., electrical
conductivity) values >1 dS/m, whereas salinity value ranges widely overlapped between lineages (Fig. 2a). The soils of two Caspian coastal TauL2 habitats had high electrical conductivity
values (5.9 dS/m). Electrical conductivity mean values were larger in TauL1 (0.60 dS/m) than in TauL2 (0.46 dS/m) and TauL3 (0.10 dS/m). A Brunner-Munzel test showed a significant stochastic
difference between the TauL1 and TauL2 electrical conductivity values (_P_ = 0.00). The ranges of soil available water capacity values also widely overlapped between lineages, but the
proportion of low available water capacity (<15 cm/m) habitats (i.e., high osmotic stress habitats) was higher in TauL1 (67/132, 51%) than in TauL2 (15/68, 22%) (Fig. 2b). The proportion
was 3/5 (60%) in TauL3. Available water capacity mean values were larger in TauL2 (16.9 cm/m) than in TauL1 (15.3 cm/m) and TauL3 (15.0 cm/m). A Brunner-Munzel test showed a significant
stochastic difference between the TauL1 and TauL2 available water capacity values (_P_ < 0.00). These findings suggested an association between TauL1 accessions’ increased
NaCl-induced-stress tolerances and the edaphic conditions of their natural habitats. INCREASED SEEDLING GROWTH IN TAUL1B To address if _Ae. tauschii_’s eastward range expansion is associated
with an adaptive change in salt tolerance, the NaCl-induced-stress tolerances of the TauL1 sublineages/groups were compared. To simplify the NaCl-induced-stress tolerance comparisons, a PCA
based on the among-accession covariance matrix was performed to produce a single virtual trait (i.e., the first principal component that defined the axis of maximum phenotypic divergence
among the accessions in a multivariate space) for each under-stress germinability (based on the GNs under 0.5%, 1.0%, and 1.5% NaCl conditions) and early seedling growth (based on the MLs
under 0.5% and 1.0% NaCl conditions). The ML dataset under the 1.5% NaCl treatment was not used due to the large number of accessions (72 in total) not showing the trait. In addition, two
accessions, KU-2001 and KU-2003, were excluded from the PCA for under-stress early seedling growth, due to germination failure under 0.5% and/or 1.0% NaCl conditions. The under-stress
germinability PCA’s PC1 (named the TauL1-germinability PC) and the under-stress early seedling growth ability PCA’s PC1 (named the TauL1-seedling PC) explained 61.7% and 79.8% of the total
variance, respectively. All eigenvectors of both under-stress PCs were positive: 0.24 (GN, 0.5% NaCl, PC loading = 0.42), 0.61 (GN, 1.0% NaCl, PC loading = 0.81), and 0.76 (GN, 1.5% NaCl, PC
loading = 0.89) for the TauL1-germinability PC; 0.81 (ML, 0.5% NaCl, PC loading = 0.94) and 0.59 (ML, 1.0% NaCl, PC loading = 0.82) for the TauL1-seedling PC. Accordingly, the accessions’
TauL1-germinability PC and TauL1-seedling PC values provided useful phenotypic indices that positively correlated with the levels of the NaCl-induced-stress tolerances in TauL1. Mean
TauL1-germinability PC values were not significantly different between TauL1a, TauL1b, and TauL1x (Tukey-Kramer test, _P_ > 0.05) (Fig. 3; Supplementary Table S3). In contrast, mean
TauL1-seedling PC values were larger in TauL1b (0.33) than in TauL1a (−0.35) and TauL1x (−0.73), the difference between TauL1a and TauL1b values was significant (Dunnett T3 multiple
comparisons, _P_ = 0.013). Control (distilled water) experiments showed that the differences of mean germinability values of TauL1a, TauL1b, and TauL1x were not statistically significant
(Supplementary Fig. S3; Supplementary Table S4). ML mean values of these sublineages/group varied, but only TauL1a (78.2 mm) and TauL1b (87.4 mm) MLs were significantly different (Dunnett T3
multiple comparisons, _P_ = 0.004), indicating that seedling growth was increased in TauL1b relative to TauL1a under control conditions. These results showed that seedling growth ability
was larger in TauL1b than in TauL1a under NaCl-induced-stress conditions as well as under NaCl-free condition, whereas germinability was not markedly different. STRUCTURE OF THE TAUL1B
UNDER-STRESS SEEDLING GROWTH VARIATION To study the genetic structure of TauL1b’s under-stress seedling growth variation, we compared TauL1-seedling PC values between HighQ2, LowQ2, and Q3
groups. Within-group mean genetic distances were 104.1 (HighQ2), 128.2 (LowQ2), and 13.6 (Q3) and pairwise _F_ST values showed low to moderate degrees of genetic differentiation between
these groups (Supplementary Table S5). Within TauL1b, mean TauL1-seedling PC values were larger in HighQ2 (1.23) than in LowQ2 (−0.25) and Q3 (0.38) and the difference between HighQ2 and
LowQ2 means was significant (Turkey-Kramer test, _P_ < 0.05) (Fig. 4; Supplementary Table S3). The under-stress TauL1b seedling growth phenotypes were classified into two groups: long
first leaf (TauL1-seedling PC values ≥ 0) and short first leaf (TauL1-seedling PC values < 0). In HighQ2, 23 out of 26 accessions (88.5%) had long first-leaf phenotypes. This proportion
was lower in LowQ2 (15/41, 36.6%) and in Q3 (3/5, 60.0%). A partial Mantel test showed that phenotypically similar TauL1b accessions had similar genetic compositions, whereas dissimilar
accessions had dissimilar genetic compositions, when the effect of latitudinal distances was controlled (Mantel _r_ statistic = 0.12, _P_ = 0.00). Most long first-leaf accessions (37/41,
90.2%) occurred in the southern portion of TauL1b’s geographic range (latitude < 37.5°). In contrast, the proportion of short first-leaf accessions in northern (13/31, 42.0%) and south
(18/31, 58.1%) habitats were comparable (Supplementary Table S6). No significant association was found between the phenotypic and latitudinal dissimilarity (distance) matrices, when the
effect of genetic dissimilarity was controlled (partial Mantel test, Mantel _r_ statistic = 0.01, _P_ = 0.41). Overall, the distributions of long first-leaf and short first-leaf accessions
were significantly biased between north and south (Fisher’s exact test, _P_ = 0.00) and among TauL1b groups (Fisher’s exact test, _P_ < 0.00) (Fig. 4b). These findings showed that
under-stress TauL1b seedling growth variations had clear genetic and geographic structures. ECOLOGICAL FUNCTIONS OF NACL-INDUCED-STRESS TOLERANCE DURING SEEDLING GROWTH IN TAUL1B The natural
variation patterns found under the NaCl conditions showed that seedling growth ability was larger in the HighQ2 accessions than in the LowQ2 and Q3 accessions. However, the ecological
functions of this larger seedling growth ability were not clear and, as so, the TauL1b accessions’ seedling phenotypes association with natural habitat soil salinity and available water
capacity was assessed. Of the 72 TauL1b accessions for which TauL1-seedling PC values were obtained, 41 displayed the long first-leaf phenotypes, whereas 31 displayed the short first-leaf
phenotypes (Supplementary Table S6). Of these, the habitat of 35 long first-leaf and 29 short first-leaf accessions had usable soil salinity estimates. Available water capacity estimates
were obtained for 41 long first-leaf and 31 short first-leaf accessions. Soil salinity estimates showed notable patterns: 13 out of 35 long first-leaf accessions (37.1%) were sampled in
increased salinity soil habitats (electrical conductivity values > 1 dS/m), whereas only two out of 29 short first-leaf accessions (6.9%) were sampled in such habitats (Fig. 5a). The
patterns found for the available water capacity estimates were less obvious, but the proportion of accessions sampled in reduced available water capacity soil habitats (<15 cm/m) was
higher in long first-leaf accessions (28 out of 41, 68.3%) than in short first-leaf accessions (13 out of 31, 41.9%) (Fig. 5b). A stepwise multiple regression that used the 64 accessions
having no missing values selected electrical conductivity, but not available water capacity and the interaction, as the independent predictor of TauL1-seedling PC. A simple linear regression
showed that electrical conductivity had significant effect on the TauL1-seedling PC (percentage of explained variance _R_2 = 0.13, _F_-value = 9.49, parameter estimate = 0.62, _P_ = 0.00).
The edaphic conditions of the habitats were classified into three categories: (1) increased salinity (electrical conductivity values > 1 dS/m) and reduced available water capacity (<15
cm/m) (IR); (2) reduced salinity (electrical conductivity values ≤ 1 dS/m) and reduced available water capacity (<15 cm/m) (RR); and (3) reduced salinity (electrical conductivity values
≤ 1 dS/m) and increased available water capacity (≥15 cm/m) (RI). Examination of the distribution of the seedling phenotypes among these categories revealed that 28 out of 35 long first-leaf
accessions (80.0%) were sampled in the relatively stressful habitats, i.e., IR and RR soil habitats (Fig. 5c; Supplementary Table S7). Almost all long first-leaf accessions sampled from IR
habitats were belonged to HighQ2. Samples collected in RR habitats had similar numbers of long first-leaf (15) and short first-leaf (11) accessions, whereas most accessions sampled from RI
habitats had the short first-leaf phenotype (16/23, 69.6%). Thus, the distribution of long first-leaf and short first-leaf accessions was significantly different between habitats differing
in edaphic conditions (Fisher’s exact test, _P_ = 0.00). These findings indicated that increased seedling growth ability under NaCl-induced-stress conditions likely had an ecological role in
TauL1b’s adaptation to natural edaphic stress. Furthermore, we found that the long first-leaf TauL1b accessions sampled in the IR and RR soil habitats had discontinuous north-south genetic
structure, whereas their phenotypic variation had no obvious geographic patterns (Supplementary Table S7; Supplementary Fig. S4). Similar numbers of HighQ2 and LowQ2 accessions were sampled
in RR soil habitats (nine and six, respectively) (Supplementary Table S7). The nine HighQ2 accessions had generally high Q2 values (>0.8) (i.e., the proportion of alleles inferred to have
originated from one of the two large TauL1b genepools) and all but one occurred in the south, whereas six LowQ2 accessions had low Q2 values (<0.05) and occurred in the north
(Supplementary Fig. S4). The TauL1-seedling PC values of these accessions varied from 0.25 to 3.95 (mean = 1.35) in the north (latitude ≥ 35°) and from 0.12 to 3.00 (mean = 1.25) in the
south (latitude < 35°). Among the 13 long first-leaf accessions sampled in IR soil habitats, 12 belonged to HighQ2 and their genetic variation was geographically structured: Q2 values of
the southern accessions were usually higher than those of the northern accessions (Supplementary Fig. S4). The TauL1-seedling PC values of these accessions ranged from 1.06 to 2.85 (mean =
1.69) in the north (latitude ≥ 35°) and from 0.63 to 2.58 (mean = 1.48) in the south (latitude < 35°). IMPACT OF SALT TOLERANCE DURING SEEDLING GROWTH ON SPECIES RANGE EXPANSION Given
that NaCl-induced stress tolerance during seedling growth is ecologically functional, adaptation to salt-affected soils might have had an important role in TauL1 accessions’ migration and
colonization of central Asian habitats. If so, what impact did salt tolerance have on _Ae. tauschii_ eastward range expansion relative to other traits of ecological importance such as
flowering time and seed numbers? To address this question, we first examined the association of TauL1b seedling phenotypes with flowering time and the numbers of spikelets per spike.
Accessions presenting the long first-leaf phenotype under NaCl-induced-stress conditions mostly showed early flowering, whereas short first-leaf accessions tended to show increased flowering
time; this negative association was statistically significant (_P_ = 0.02) (Fig. 6a). Interestingly, this negative association was less evident and not significant for the regression
performed on MLs under the distilled-water condition (Fig. 6b). These findings suggested that the NaCl-induced-tolerance during seedling growth might be involved with the expression of rapid
life cycle in the natural salt-affected soil habitats. No apparent association was found between the numbers of spikelets per spike and the NaCl-stressed and distilled-water seedling
phenotypes (Fig. 6c,d). Next, we evaluated the impact of NaCl-induced-stress tolerance during seedling growth through a PCA of the TauL1 accessions. In this PCA, the among-accession
correlation matrix was used. This allowed obtaining the distribution of TauL1a, TauL1b, and TauL1x accessions in a multidimensional space defined by the rectangular coordinate axes of
TauL1-seedling PC, flowering time, and the number of spikelets per spike. This PCA considered only the 127 accessions that had no missing values for these traits. The first and second
principal components (PC1 and PC2) explained 50.2% and 33.1% of the correlation structure, respectively. The eigenvectors of PC1 were −0.63 (TauL1-seedling PC, PC loading = −0.77), 0.70
(flowering time, PC loading = 0.86), and 0.33 (number of spikelets per spike, PC loading = 0.40), whereas the eigenvectors of PC2 were 0.45 (TauL1-seedling PC, PC loading = 0.45), −0.01
(flowering time, PC loading = −0.01), and 0.89 (number of spikelets per spike, PC loading = 0.89). The plot of the 127 accessions in the space defined by PC1 and PC2 showed that the TauL1b
accessions, particularly the HighQ2 and Q3 accessions, had generally smaller PC1 values than other accessions (Fig. 7). The eigenvectors of PC1 indicated that TauL1b accessions had
characteristically higher under-stress seedling growth ability, earlier flowering, and larger per-spike spikelet numbers than TauL1a and TauL1x accessions. Comparable absolute PC loadings
were found for TauL1-seedling PC (0.77) and flowering time (0.86), and both loadings were larger than that found for the number of spikelets per spike (0.40). These results suggested that
salt tolerance during seedling growth and flowering time might have been important factors for _Ae. tauschii_’s eastward range expansion. DISCUSSION In this study, the habitat soil property
estimates were taken from the 30 by 30 arcsec resolution soil datasets. The electrical conductivity and available water capacity values were not validated using soil samples where the plants
actually grow. Therefore, the use of these soil datasets may have inherent limitations on analyses in terms of accuracy. Nevertheless, results pointed out a probable association between
natural variations in the patterns of _Ae. tauschii_ NaCl-induced-stress tolerances and local edaphic variables. Thus, this work highlighted the potential of these soil datasets for use in
the ecogeographic studies of _Ae. tauschii_. Results also suggested that stress tolerances might indeed have ecological functions that are involved in plant’s adaptation to salt-affected
soils. Lineages’ pairwise comparisons revealed the higher germinability and seedling growth ability of the geographically widespread TauL1 under stress conditions in relation to other
lineages. Seedling growth ability of TauL1b accessions, which were responsible for the eastward range expansion of the TauL1 lineage, was higher than that of TauL1a accessions under control
and NaCl-induced-stress conditions, whereas the germinability of both sublineages was similar. The distinct effects of NaCl-induced stress on germination and seedling growth ability are
consistent with the genetic evidence that different quantitative trait loci control these two traits in wheat33. Therefore, the increased seedling growth ability of TauL1b might be one of
the key phenotypes that facilitated the colonization of the semi-arid salt-affected central Asian habitats. Both genetic and geographic factors influenced the variation patterns of TauL1b
seedling growth ability under stress (Supplementary Table S6). Because long first-leaf accessions mostly occurred in the southern part of this lineage distribution range, their increased
seedling growth ability might have been involved in the adaptation to the edaphic conditions of the southern habitats (Fig. 4). The electrical conductivity estimates of the _Ae. tauschii_
habitats are lower than the soil salinity (electrical conductivity) threshold of yield decline for common wheat (6.0 dS/m)34. The threshold of yield decline is not known for _Ae. tauschii_,
but one obvious question here would be whether the habitats’ edaphic conditions actually had an impact on the distribution of _Ae. tauschii_, given such low electrical conductivity
estimates. In general, low salinity levels can exert physiological stress on plants. Furthermore, plant responses to salinity vary with the developmental stage at time of exposure10.
Therefore, the findings of this study may suggest that _Ae. tauschii_ plants are relatively sensitive to soil salts during early developmental stages under natural conditions. The fact that
common wheat is less salt-tolerant during emergence and seedling stage is consistent with this view34. Each TauL1b genetic group contained long first-leaf accessions (Supplementary Table
S6). In addition, the genetic variations of the long first-leaf accessions had clear patterns of differentiation and geographic structure (Supplementary Table S5; Supplementary Fig. S4).
Given that _Ae. tauschii_ is a selfing species, therefore reproducing in a scenario of reduced gene flow, one possible explanation for these observations would be that, in _Ae. tauschii_,
NaCl-induced-stress tolerance evolved through multiple pathways of adaptation to the natural edaphic stresses encountered during the species range expansion. For example, an increased
capacity of water uptake might be a mechanism responsible for TauL1b’s increased seedling growth ability. In fact, the TauL1b accessions growing in semi-arid habitats also had longer MLs
than the TauL1a and TauL1x accessions in control conditions (Supplementary Fig. S3), suggesting that tolerance to osmotic stress was a critical factor in the species’ range expansion toward
east. Furthermore, ionic stress tolerance might be involved in TauL1b’s increased seedling growth ability under stress: salinity stress might be more intense in IR than in RR habitats, but
the proportion of long first-leaf accessions was not lower in IR habitats (13/15, 86.7%) than in RR habitats (15/26, 57.7%) (Fisher’s Exact Test, _P_ = 0.08) (Supplementary Table S7). It is
not clear why there were no obvious geographic patterns in the phenotypic variations of the long first-leaf TauL1b accessions (Supplementary Table S7; Supplementary Fig. S4). Use of high
resolution soil datasets might solve this issue. Refined experimental approaches taking into account complex phenotypic effects, such as transgenerational plasticity, would enable to better
assess the degree of salt tolerance providing suitable phenotype data for inferring the genes controlling seedling growth ability under natural salt-stress conditions35. Detailed knowledge
on the genetic mechanism(s) underlying the evolution of seedling growth ability would contribute to better understanding of the adaptation of _Ae. tauschii_ to salt-affected soils during its
eastward range expansion. _Ae. tauschii_’s life history and phenological traits, particularly seed production and flowering time, are thought to have had a role in this species dispersal
and adaptation in central Asia19. The PCA performed using seedling growth ability, flowering time, and spikelet number per-spike datasets provided information on traits/phenotypes
contributions to _Ae. tauschii_’s eastward range expansion. The influence of seedling growth ability under stress was large and similar to that found for flowering time, suggesting that
adaptive phenotypic changes in physiological traits might have been important factors in the eastward range expansion of _Ae. tauschii_. Because seedling growth traits are expressed early in
development, they are usually exposed to natural selection pressures before other traits are expressed. For this reason, early seedling growth can have a particular adaptive importance
under natural conditions. Because the seedling growth ability was assessed using individuals undergoing early post-germination stages, the influence its performance under stress conditions
had on the eastward range expansion of _Ae. tauschii_ might be consistent with the influence that has been attributed to germination behavior in determining species ecological and geographic
ranges36. We note that the observed association between seedling growth ability under stress and early flowering in _Ae. tauschii_ parallels a case in _Medicago_ in which flowering time was
shown to play a major role in adaptation to saline soils9. _Ae. tauschii_’s eastward range expansion was probably a complex evolutionary process that involved adaptive changes in multiple
ecologically functional traits, including phenological, life history, and physiological traits. Given that _Ae. tauschii_ is one of the progenitors of common wheat11,12, a widely accepted
scenario for its evolution is that in which male _Ae. tauschii_ crossed with a cultivated female form of the tetraploid wheat _Triticum turgidum_ L. under natural conditions. This cross gave
rise to triploid F1 hybrids, which, in turn, produced F2 hybrids, the direct ancestors of current common wheat, through natural genome duplication via union of male and female unreduced 2n
gametes37. Previous work indicated that the _T. turgidum_ - _Ae. tauschii_ natural hybridization involved a few _Ae. tauschii_ populations that were genetically close to the TauL2 lineage17.
This means that an extensive portion of _Ae. tauschii_ genetic variation has literally been left in the wild. Furthermore, the _Ae. tauschii_ germplasm can be readily incorporated into the
common wheat germplasm using synthetic common wheats that can be produced by artificially crossing _T. turgidum_ with _Ae. tauschii_38. Hence, the _Ae. tauschii_ germplasm represents one of
the most important genetic resources for common wheat breeding. Several studies regarding _Ae. tauschii_’s salt tolerance characterization and use in common wheat breeding programs have been
performed39,40,41,42,43. In wheat, salt tolerance is a rather complex trait associated with the ability to maintain low Na+ and high K+ concentrations, which is accomplished by limiting the
concentration of Na+ that enters the xylem44,45,46,47. _Ae. tauschii_ contributed a locus (_Kna1_) that controls the concentration of Na+ entering the xylem to bread wheat (a form of common
wheat) through a natural hybridization event. Therefore, it is a great source of natural genetic variation for controlling Na+ accumulation in leaves43,48,49. In contrast, little was known
about how salt stress affects _Ae. tauschii_’s germination behavior and early seedling growth. Results of the present study suggest that TauL1b accessions may be useful for salt-tolerant
wheat breeds, because, in general, uniform and rapid seedling growth are traits of great agronomic value. TauL1b accessions also have phenological (early flowering) and reproductive (high
seed productivity) traits suitable for wheat production, and may serve as a pool of novel alleles for controlling these traits, as the TauL1b genepool is relatively distantly related to the
D-genome of common wheat (Supplementary Fig. S1). Further studies are needed to provide practical knowledge on the ecology and genetics of _Ae. tauschii_’s germination and seedling salt
tolerances. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Saisho, D. _et al_. Salt tolerance during germination and seedling growth of wild wheat _Aegilops tauschii_ and its impact on the
species range expansion. _Sci. Rep._ 6, 38554; doi: 10.1038/srep38554 (2016). PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. REFERENCES * Bridle, J. R. & Vines, T. H. Limits to evolution at range margins: when and why does adaptation fail? Trend. Ecol. Evol. 22, 140–147 (2007).
Article Google Scholar * Eckert, C. G., Samis, K. E. & Lougheed, S. C. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol. Ecol. 17,
1170–1188 (2008). Article CAS Google Scholar * Lee, C. E. Evolutionary genetics of invasive species. Trend. Ecol. Evol. 17, 386–391 (2002). Article Google Scholar * Hendry, A. P.,
Nosil, P. & Rieseberg, L. H. The speed of ecological speciation. Funct. Ecol. 21, 455–464 (2007). Article Google Scholar * Prentis, P. J., Wilson, J. R. U., Dormontt, E. E.,
Richardson, D. M. & Lowe, A. J. Adaptive evolution in invasive species. Trend. Plant Sci. 13, 288–294 (2008). Article CAS Google Scholar * Bradley, N. L., Leopold, A. C., Ross, J.
& Huffaker, W. Phenological changes reflect climate change in Wisconsin. Proc. Natl. Acad. Sci. USA 96, 9701–9704 (1999). Article ADS CAS Google Scholar * Franks, S. J. & Weis,
A. E. A change in climate causes rapid evolution of multiple life-history traits and their interactions in an annual plant. J. Evol. Biol. 21, 1321–1334 (2008). Article CAS Google Scholar
* Baxter, I. et al. A coastal cline in sodium accumulation in _Arabidopsis thaliana_ is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet. 6, e1001193, doi:
10.1371/journal.pgen.1001193 (2010). Article CAS PubMed PubMed Central Google Scholar * Friesen, M. L. et al. The ecological genomic basis of salinity adaptation in Tunisian _Medicago
truncatula_. BMC Genomics 15, 1160, doi: 10.1186/1471-2164-15-1160 (2014). Article PubMed PubMed Central Google Scholar * Bui, E. N. Soil salinity: a neglected factor in plant ecology
and biogeography. J. Arid Environ. 92, 14–25 (2013). Article ADS Google Scholar * Kihara, H. Discovery of the DD-analyser, one of the ancestors of _Triticum vulgare_ (abstr). Agric.
Hortic. 19, 889–890 (in Japanese) (1944). Google Scholar * McFadden, E. S. & Sears, E. R. The artificial synthesis of _Triticum spelta_. Rec. Genet. Soc. Am. 13, 26–27 (1944). Google
Scholar * Matsuoka, Y. et al. Durum wheat cultivation associated with _Aegilops tauschii_ in northern Iran. Genet. Resour. Crop Evol. 55, 861–868 (2007). Article Google Scholar * van
Slageren, M. W. Wild Wheats: A Monograph Of Aegilops L. And Amblyopyrum (Jaub. and Spach) Eig (Poaceae). (Wageningen Agricultural University, 1994). * Kilian, B. et al. Aegilops in Wild Crop
Relatives: Genomic And Breeding Resources, Cereals (ed. Kole, C. ) 1–76 (Springer-Verlag Berlin Heidelberg, 2011). * Peel, M. C., Finlayson, B. L. & McMahon, T. A. Updated world map of
the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644 (2007). Article ADS Google Scholar * Matsuoka, Y. et al. Genetic basis for spontaneous hybrid genome
doubling during allopolyploid speciation of common wheat shown by natural variation analyses of the paternal species. PLoS One 8, e68310, doi: 10.1371/journal.pone.0068310 (2013). Article
ADS CAS PubMed PubMed Central Google Scholar * Wang, J. et al. _Aegilops tauschii_ single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and
pinpoint the geographic origin of hexaploid wheat. New Phytol. 198, 925–937 (2013). Article CAS Google Scholar * Matsuoka, Y., Takumi, S. & Kawahara, T. Intraspecific lineage
divergence and its association with reproductive trait change during species range expansion in central Eurasian wild wheat _Aegilops tauschii_ Coss. (Poaceae). BMC Evol. Biol. 15, 213, doi:
10.1186/s12862-015-0496-9 (2015). Article CAS PubMed PubMed Central Google Scholar * Gogniashvili, M. et al. Complete chloroplast genomes of _Aegilops tauschii_ Coss. and _Ae.
cylindrica_ Host sheds light on plasmon D evolution. Curr. Genet. 62, 791, doi: 10.1007/s00294-016-0583-5 (2016). Article CAS PubMed Google Scholar * Mano, Y. & Takeda, K. Genetic
resources of salt tolerance in wild _Hordeum_ species. Euphytica 103, 137–141 (1998). Article Google Scholar * FAO, IIASA, ISRIC, ISSCAS & JRC. _Harmonized World Soil Database (version
1.2_) http://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/harmonized-world-soil-database-v12 (Date of access:01/03/2016) (2012). * Batjes, N. H. World soil property
estim_ates_ fo_r broad-scale modelling (WISE30sec_) ISRIC – World Soil Information Report 2015/01 (with data set, available at www.isric.org)
http://www.isric.org/data/isric-wise-derived-soil-property-estimates-30-30-arcsec-global-grid-wise30sec (Date of access:01/03/2016) (2015). * Matsuoka, Y., Takumi, S. & Kawahara, T.
Flowering time diversification and dispersal in central Eurasian wild wheat _Aegilops tauschii_ Coss.: genealogical and ecological framework. PLoS One 3, e3138, doi:
10.1371/journal.pone.0003138 (2008). Article ADS CAS PubMed PubMed Central Google Scholar * Fox, J. The R Commander: a basic-statistics graphical user interface to R. J. Stat. Softw.
14, 1–42 (2005). Google Scholar * Wickham, H. ggplot2: elegant graphics for data analysis (Springer-Verlag, 2009). * Hui, W., Gel, Y. R. & Gastwirth, J. L. Lawstat: an R package for
law, public policy and biostatistics. J. Stat. Softw. 28, 3, doi: 10.18637/jss.v028.i03 (2007). Article Google Scholar * Goslee, S. C. & Urban, D. L. The ecodist package for
dissimilarity-based analysis of ecological data. J. Stat. Softw. 22, 7, doi: 10.18637/jss.v022.i07 (2007). Article Google Scholar * Smouse, P. & Peakall, R. Spatial autocorrelation
analysis of individual multiallele and multilocus genetic structure. Heredity 82, 561–573 (1999). Article Google Scholar * Peakall, R. & Smouse, P. E. GENALEX 6: genetic analysis in
Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (2006). Article Google Scholar * Peakall, R. & Smouse, P. E. GenAlEx 6.5: genetic analysis in
Excel. Population genetic software for teaching and research-an update. Bioinformatics 28, 2537–2539 (2012). Article CAS Google Scholar * Munns, R. & Tester, M. Mechanisms of salinity
tolerance. Annu. Rev. Plant Biol. 59, 651–681 (2008). Article CAS Google Scholar * Landjeva, S., Lohwasser, U. & Börner, A. Genetic mapping within the wheat D genome reveals QTL for
germination, seed vigour and longevity, and early seedling growth. Euphytica 171, 129–143 (2010). Article Google Scholar * Maas, E. V. & Hoffman, G. J. Crop salt tolerance – current
assessment. J. Irr. Drain. Div. 103, 115–134 (1977). Google Scholar * Vu, W. T., Chang, P. L., Moriuchi, K. S. & Friesen, M. L. Genetic variation of transgenerational plasticity of
offspring germination in response to salinity stress and the seed transcriptome of _Medicago truncatula_. BMC Evol. Biol. 15, 59, doi: 10.1186/s12862-015-0322-4 (2015). Article CAS PubMed
PubMed Central Google Scholar * Donohue, K., de Casas, R. R., Burghardt, L., Kovach, K. & Willis, C. G. Germination, postgermination adaptation, and species ecological ranges. Annu.
Rev. Ecol. Evol. Syst. 41, 293–319 (2010). Article Google Scholar * Matsuoka, Y. Evolution of polyploid _Triticum_ wheats under cultivation: the role of domestication, natural
hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol. 52, 750–764 (2011). Article CAS Google Scholar * Kihara, H. & Lilienfeld, F. A new synthesized
6x-wheat. Hereditas Suppl. Vol, 307–319 (1949). * Gorham, J., Hardy, C., Wyn Jones, R. G., Joppa, L. R. & Law, C. N. Chromosomal location of a K/Na discrimination character in the D
genome of wheat. Theor. Appl. Genet. 74, 584–588 (1987). Article CAS Google Scholar * Farooq, S., Niazi, M. L. K., Iqbal, N. & Shah, T. M. Salt tolerance potential of wild resources
of the tribe Triticeae. II. Screening of species of the genus _Aegilops_. Plant Soil 119, 255–260 (1989). Article CAS Google Scholar * Gorham, J. Salt tolerance in the Triticeae: K/Na
discrimination in _Aegilops_ species. J. Exp. Bot. 41, 615–621 (1990). Article CAS Google Scholar * Schachtman, D. P., Munns, R. & Whitecross, M. I. Variation in sodium exclusion and
salt tolerance in _Triticum tauschii_. Crop Sci. 31, 992–997 (1991). Article CAS Google Scholar * Schachtman, D. P., Lagudah, E. S. & Munns, R. The expression of salt tolerance from
_Triticum tauschii_ in hexaploid wheat. Theor. Appl. Genet. 84, 714–719 (1992). Article CAS Google Scholar * Colmer, T. D., Flowers, T. J. & Munns, R. Use of wild relatives to improve
salt tolerance in wheat. J. Exp. Bot. 57, 1059–1078 (2006). Article CAS Google Scholar * Munns, R. et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter
gene. Nat. Biotech. 30, 360–364 (2012). Article CAS Google Scholar * Byrt, C. S. et al. The Na+ transporter, TaHKT1;5‐D, limits shoot Na+ accumulation in bread wheat. Plant J. 80, 516–526
(2014). Article CAS Google Scholar * Zhu, M. et al. Nax loci affect SOS1-like Na+/H+ exchanger expression and activity in wheat. J. Exp. Bot. 67, 835–844 (2016). Article Google Scholar
* Dvořak, J., Noaman, M. M., Goyal, S. & Gorham, J. Enhancement of the salt tolerance of _Triticum turgidum_ L. by the _Kna1_ locus transferred from the _Triticum aestivum_ L.
chromosome 4D by homoeologous recombination. Theor. Appl. Genet. 87, 872–877 (1994). Article Google Scholar * Pritchard, D. J. et al. K+/Na+ discrimination in synthetic hexaploid wheat
lines: transfer of the trait for K+Na+ discrimination from _Aegilops tauschii_ into a _Triticum turgidum_ background. Cereal Res. Commun. 30, 261–267 (2002). CAS Google Scholar Download
references ACKNOWLEDGEMENTS We thank Taihachi Kawahara and Kenji Kato for the seeds of _Ae. tauschii_ accessions, Junko Shibata for technical assistance, and Kazuhiro Sato for practical
advice and support. This research was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) as part of a Joint Research Program implemented at the Institute
of Plant Science and Resources, Okayama University, Japan and by JSPS KAKENHI Grant Number 15H04439. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Plant Science and Resources,
Okayama University, Kurashiki, 710-0046, Japan Daisuke Saisho * Laboratory of Plant Genetics, Graduate School of Agricultural Science, Kobe University, Nada-ku, 657-8501, Kobe, Japan Shigeo
Takumi * Fukui Prefectural University, Matsuoka, Eiheiji, Yoshida, Fukui, 910-1195, Japan Yoshihiro Matsuoka Authors * Daisuke Saisho View author publications You can also search for this
author inPubMed Google Scholar * Shigeo Takumi View author publications You can also search for this author inPubMed Google Scholar * Yoshihiro Matsuoka View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS D.S. conceived, designed, and conducted the experiments. S.T. supplied trait data. Y.M. conceived and designed the experiments,
contributed materials, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International
License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is
not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saisho, D., Takumi, S. & Matsuoka, Y. Salt tolerance during germination and
seedling growth of wild wheat _Aegilops tauschii_ and its impact on the species range expansion. _Sci Rep_ 6, 38554 (2016). https://doi.org/10.1038/srep38554 Download citation * Received: 11
August 2016 * Accepted: 09 November 2016 * Published: 08 December 2016 * DOI: https://doi.org/10.1038/srep38554 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative









