- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Knowledge of the structure-property-function relationship of humic substances (HSs) is key for understanding their role in soil. Despite progress, studies on this topic are still under
discussion. We analyzed 37 humic fractions with respect to their isotopic composition, structural characteristics, and properties responsible for stimulating plant root parameters. We showed
that regardless of the source of origin of the carbon (C3 or C4), soil-extracted HSs and humic acids (HAs) are structurally similar to each other. The more labile and functionalized HS
fraction is responsible for root emission, whereas the more recalcitrant and less functionalized HA fraction is related to root growth. Labile structures promote root stimulation at lower
concentrations, while recalcitrant structures require higher concentrations to promote a similar stimulus. These findings show that lability and recalcitrance, which are derived properties
of humic fractions, are related to the type and intensity of their bioactivity. In summary, the comparison of humic fractions allowed a better understanding of the relationship between the
source of origin of plant carbon and the structure, properties, and type and intensity of the bioactivity of HSs in plants. In this study, scientific concepts are unified and the basis for
the agronomic use of HSs is established.
The functions of dissolved organic matter (DOM) in soils, specifically humic substances (DOM-HSs), are well established1,2,3,4. Several studies show that DOM-HSs regulate metabolic processes
related to plant growth, especially the emission and growth of the root system5,6,7,8,9,10,11,12. The capacity of HSs to trigger stimuli in plant metabolism is directly related to their
structure. HSs extracted from soils and with structural predominance of –CH3 and –COOH carbons stimulate carbon metabolism in Pinus nigra plants13, whereas the carbons belonging to lignin
structures and –COOH groups in vermicompost humic acids (HAs) positively correlate with the emission of lateral roots in maize plants14.
The properties related to the structure of HSs are also related to their bioactivity. HAs isolated from composted materials structurally enriched in carboxyl (–COOH) groups and hydrophobic
structures stimulated root growth in maize plants15, whereas hydrophobic structures in HSs extracted from vermicompost are responsible for stimulating the proton pumps in roots16. Zancani et
al.17 showed that embryogenic cell multiplication results from the hydrophilicity and labile conformations in soil-extracted fulvic acids, and García et al.18,19 showed that aliphatic and
oxygenated structures in vermicompost HAs are related to the protective effects on rice plants subjected to water stress.
Studies on the structure-property-function relationship of HSs in plants are of great importance in understanding their modes of action and practical use. This study aimed to investigate the
relationship between the structure of humic fractions from soils and composted materials and the properties regulating and defining the bioactivity at the root level in rice plants.
A total of 37 humic fractions (HS, HA and Humins-Hus) derived from Histosols from different sources and composted materials were characterized in this study using isotopic, chemical and
spectroscopic methods (elemental analysis, ultraviolet–visible [UV-vis] spectroscopy, Fourier transform infrared spectroscopy [FTIR] and carbon-13 cross polarization – magic angle spinning
nuclear magnetic resonance [13C-CP/MAS-NMR]). Chemometric methods were used to relate the properties with the different root parameters in plants. We also discuss the relationship between
the type and intensity of plant bioactivity and the plant carbon origin, structural characteristics and recalcitrance and lability properties.
Soil humic fractions showed the following ranking of aromaticity: HA > HS > Hu. The spectral signatures of humic fractions correspond to the presence of sp3 and sp2 carbon (see spectra
included in the Supplementary material – SM, Fig. S1). The structural characteristics of soil humic fractions showed that HAs have a higher predominance of unsubstituted C-aliphatic and
C-aromatic groups than HS and Hu fractions. HAs predominantly had unsubstituted C-aromatic groups and the most striking aromatic properties among the humic fractions extracted from composted
materials (HAs and HSs).
The structural characteristics of soil-extracted humic fractions and those extracted from composted materials indicated that unsubstituted C-aliphatic and C-aromatic groups predominate in
soil HAs. However, vermicompost HAs had aromatic characteristics that were more striking than those extracted from soils. An average predominance of unsubstituted C-aliphatic groups stood
out among the soil HS fractions (see Supplementary material Table S1). 13C-CP/MAS-NMR spectral data confirmed this observation upon multivariate analysis (Fig. 1, see Supplementary material
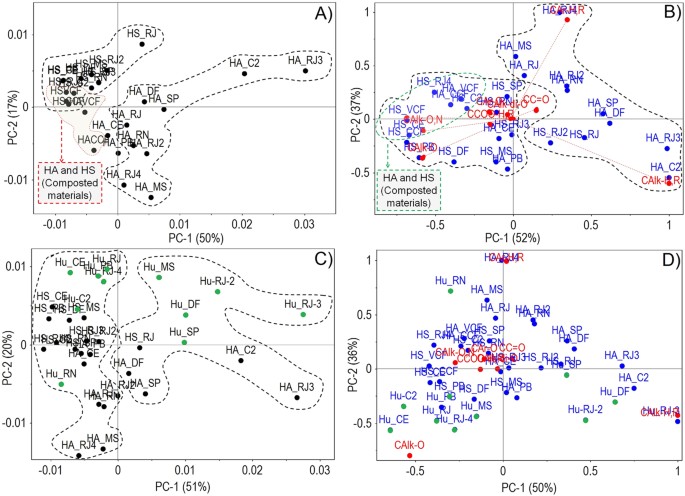
Fig. S2). The principal component analysis (PCA) plot (73% of the total variance explained) (see Supplementary material Fig. S1A) showed a clustering of ten of the thirteen studied HAs with
negative values in the PC1 (57%), wherein HAs extracted from composted materials were included. Fig. S1-A1 (see Supplementary material) shows a PCA with 92% of the total variance explained
based on the relative number of types of carbons of each HA. Six soil HAs were clustered in PC1 (60%) because of the predominance of C-alkyl-O and C-alkyl structures, whereas another five
HAs were clustered with negative values because of the predominance of C-aromatic groups. HAs extracted from composted materials were more related to substituted C-aromatic and C-aliphatic
groups.
(A,C) PCA performed using pure spectra. (B,D) PCA performed through integration of regions of pure spectra.
HS fractions were distributed into two clusters in the PCA (76% of the total variance explained) of pure spectra (see Supplementary material Fig. S1B). In contrast to the behavior of HA
fractions, six HS fractions were more related to unsubstituted C-aromatic and C-aliphatic groups in PC1 (72%). The remaining HS fractions, including those extracted from composted materials,
were closely related to substituted C-aromatic and C-aliphatic groups (see Supplementary material Fig. S1-B1).
The Hu fraction showed a distribution in the PCA with 86% of the total variance, with PC1 (72%) similar to that shown by HAs (see Supplementary material Fig. S1C). Five Hu fractions were
clustered and related to C-aliphatic groups (positive values), while the remaining fractions were clustered and related (negative values) to C-aromatic and C-aliphatic groups (PC1 72%).
Figure 1A shows the PCA (67% of the total variance explained) of pure spectra for the soluble fractions HA and HS. HA fractions were clustered in positive values, whereas HS fractions were
clustered with negative values in PC1 (50%). HAs extracted from vermicompost and compost showed a closer relationship with the soil-extracted HS fractions. In the PCA in Fig. 1B, PC1 (52%)
showed that soil HAs were related to C-aliphatic and unsubstituted C-aromatic groups, while soil HSs, HSs and HA from composted materials were related to the more functionalized C-aliphatic
and C-aromatic groups. The PCA (71% of the total variance explained) in Fig. 1C shows the three fractions studied. Five Hu fractions and three HA fractions were clustered with positive
values in PC1 (51%), while the three fractions (HS, HA and Hu) were related to negative values. The PCA summarized in Fig. 1D (86% of the total variance explained) shows a cluster in PC1
(50%), with positive values of Hus and HAs with the same origin and closely related to unsubstituted C-aliphatic groups. Another Hu group was clustered with the HSs, with positive values in
PC1 and closely related to C-functionalized groups.
HSs showed recalcitrance resulting from the unsubstituted C-aromatic and C-aliphatic groups and lability primarily resulting from the substituted C-aliphatic (C-alkyl O, N and C-alkyl-O)
groups and C of carboxyl groups (Fig. 2A). Conversely, the recalcitrance of HAs not only resulted from the unsubstituted C-aromatic and C-aliphatic groups but also showed the contribution of
C from carboxyl groups, while lability resulted from the unsubstituted C-aliphatic and C-aromatic groups (Fig. 2B). The patterns of recalcitrance and lability of Hus were significantly less
evident because the largest contribution to recalcitrance resulted from both C-aromatic groups and substituted aliphatic structures and carboxylic C. In turn, the largest contribution to
lability resulted from substituted and unsubstituted C-aliphatic groups and from carboxylic C (Fig. 2C).
The quantifications of lability and recalcitrance (%) corroborated the differences observed in the MCR of humic fractions (Fig. 2D,E,F). Soil-extracted HSs showed ~56% lability and ~24%
recalcitrance, while HAs showed ~47% lability and ~39% recalcitrance, and Hus showed 67% lability and 32% recalcitrance. The fractions showed the following ranking of recalcitrance: HA > Hu
> HS.
The spectral characteristics of humic fractions showed the presence of functional groups of different chemical natures (see spectra included in Supplementary material, Fig. S2). Figure S4
(see Supplementary material) shows the PCA (89% of the total variance explained) for the HA fraction. Nine HAs were clustered with positive values in PC1 (79%) and four with negative values.
Unlike PCAs performed using the 13C-NMR spectra, the HAs derived from composted materials showed no similarities in terms of functional groups in this analysis (see Supplementary material
Fig. S4-A). The PCA (75% of the total variance explained) for the HS fractions showed a clear separation of these fractions into two groups: seven HS fractions clustering with positive
values in PC1 (56%) and six with negative values. The HSs derived from composted materials also showed no similarities in terms of functional groups (see Supplementary material Fig. S4-B).
The PCA (86% of the total variance explained) also showed that Hus were distributed into two clusters in PC1 (65%). Six Hus were clustered with positive values and five with negative values
(see Supplementary material Fig. S4-C).
The comparison between the HA and HS fractions in the PCA (92% of the total variance explained) showed a clear separation of these fractions in PC1 (85%) (Fig. 3A). HSs and HAs showed strong
differences in terms of functional groups. HSs clustered with positive values in PC1, while HAs clustered with negative values. The PCA of the three fractions (80% of the total variance
explained) showed that Hus were similar to HAs in terms of functional groups, clustering in close relationship in PC1 (50%).
(A) soluble fractions, HAs and HS. (B) three fractions, HAs, HS and Hu.
The δ13C isotopic compositions were similar in the three soil-extracted humic fractions (see Supplementary material Table S2). In general, these humic fractions had isotopic compositions
between −20% and −30%, while the fractions isolated from composted materials had compositions between −14% and −16%. This isotopic composition showed that the plant carbon of soil humic
fractions was likely derived from C3 photosynthetic pathway plants, while the carbon of humic fractions from composted materials was derived from C4 plants20 (see Supplementary material
Table S2).
The HSs fractions extracted from composted materials had higher C values than those extracted from Histosols, whereas HS_VCF had higher quantities of N. The H/C ratio was lower in the HSs
extracted from composted materials, ν was slightly higher in HS_CCF, and δ was slightly higher in HS_VCF than in soil-extracted HSs. The fractions HS_CCF and HS_VCF had lower E4/E6 ratios.
HAs extracted from composted materials had higher levels of C and N than those in soil-extracted HAs. The HA_CCF fraction had the highest levels of O, a higher O/C ratio and a higher ω
value, while HA_VCF showed the highest values of δ and E4/E6 ratio.
Figure 4 shows how the elements were related to each soluble humic fraction (Fig. 4A) and between the three humic fractions (Fig. 4B). The PCA (67.02% of the total variance explained)
performed using the HS and HA fractions indicated the existence of a relationship between the HSs and the parameters associated with oxygenation/functionalization (O, O/C and ω), with
positive values of PC1 (41.25%) and a relationship with the parameters C/N and E4/E6 ratios, C and δ. In turn, the HAs showed a relationship with the parameters related to bond saturation
(H, H/C), ν and N content.
(A) soluble fractions, HAs and HS. (B) three fractions, HAs, HS and Hu.
The PCA (79.41% of the total variance explained) performed using the three fractions (Fig. 4B) showed that the soluble HS and HA fractions were clustered with positive values in PC1 (54.96%)
with all elements present (C, H, N, O) and with the ω, ν and E4/E6 parameters. Hus clustered independently with negative values in PC1, showing a relationship with the parameters C/N, H/C
and δ.
The effects exerted by the HS and HA humic fractions on the root system of rice plants are shown in Fig. S3 (see Supplementary material). The most promising concentrations of HSs stimulated
the root parameters in the range of 1.5–5.0 mg (C). L−1, while the HAs exerted stimulus at higher concentrations of 5.0 and 10.0 mg (C). L−1.
Figure. 5A,B show the PCAs relating the humic fractions with the root parameters, the types of carbon in 13C-CP/MAS-NMR, and the elemental composition. The PCA in Fig. 5A (68.35% of the
total variance explained) shows a clustering of HS fractions with positive values and HA fractions with negative values in PC1. The fraction of HSs extracted from soils and composted
materials showed a close relationship (stimulus) with the root parameters corresponding to surface area (S.Area), radicle length (Length) and smaller roots (0.5



