- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Molecular dynamics (MD) simulations have been performed on the interaction between carbon nanoring (CNR) and single-wall carbon nanotube (SWCNT). The results show that, the CNR can
spontaneously insert into the hollow interior of the SWCNTs to form a DNA-like double-helix, or collapse to a linked double graphitic nanoribbon and wrap in a helical manner around a tube.
Further analyses of energy components show that this unique phenomenon is the result of the Van der Waals interaction. The spiral configuration of the CNR takes the least amount of energy
and achieves the maximum occupancy. The sizes of CNR and SWCNT should meet the required conditions to guarantee the spiral form in the insertion and wrapping processes. Two CNRs can also be
encapsulated in the SWCNT to form a helix at the same time. Furthermore, we also studied the encapsulation process of CNRs modified with –OH and –H functional groups. SIMILAR CONTENT BEING
VIEWED BY OTHERS MOLECULAR DYNAMICS SIMULATION OF CARBON NANOTUBE GROWTH UNDER A TENSILE STRAIN Article Open access 07 March 2024 SELF-PROPELLED DIRECTED TRANSPORT OF C60 FULLERENE ON THE
SURFACE OF THE CONE-SHAPED CARBON NANOTUBES Article Open access 16 September 2024 ULTRASMALL SINGLE-LAYERED NBSE2 NANOTUBES FLATTENED WITHIN A CHEMICAL-DRIVEN SELF-PRESSURIZED CARBON
NANOTUBE Article Open access 11 January 2024 INTRODUCTION Carbon nanotubes (CNTs), due to their unique structure and distinctive properties, have attracted intense attention on both
theoretical researches and potential applications in many fields1. Due to their conventional hollow interior2 and large specific surface area, single-walled carbon nanotubes (SWCNTs) can
serve as a nanometer-sized mold or template3, which has aroused great interest in the research field of CNTs-based composite nanostructure fabrication. The composites integrated with SWCNTs
and other materials have considerable influence on their toughness, crystalline morphology4, mechanical strength, etc. Previous theoretical and experimental results have shown that
open-ended nanotubes could act as “molecular straws” capable of absorbing dipolar molecules through capillary action5. The presence of encapsulated “foreign” nanostructures in SWCNTs is
known to tremendously affect the properties of the pristine tube and the fillers, giving rise to unique nanostructures with exciting new applications. The encapsulation of a series of
materials within SWCNTs, including metals6,7, water8,9, fullerene10,11 and grapheme12,13, as well as the properties of the fillers have been discussed for the functionalization of CNTs. At
the same time, polymer chains such as DNA can wrap in a helical manner around a CNT with periodic pitch14,15. This non-covalent “wrapping” phenomenon can also be utilized to drive
self-assembly16,17 and alter the functionalization of the tubes18. On one hand, the fillers in the confined space possess novel properties which are quite different from those of their bulk
counterparts. On the other hand, the carbon shell can be regarded as a natural layer of fillers protecting against oxidation and shape fragmentation19. Therefore, the “CNTs-based composite”
has great potential in hydrogen storage, heterogeneous catalysis20, nanodevices, magnetic data storage, electromagnetic wave absorption, as well as drug and gene delivery in the field of
biology21,22. Although there have been large quantities of important discoveries regarding the insertion and wrapping of various materials, studies concerning the encapsulation of carbon
nanoring (CNR) into the SWCNTs and the wrapping of the CNR outside the wall of the SWCNTs have yet to be performed. The super-short carbon nanorings (CNRs) can also be considered as rolled
and enclosed narrow graphene nanoribbons (GNRs)23. CNRs with large diameters are susceptible to deform their cylindrical symmetry to various structural morphologies24, such as oval, peanut,
or even ribbon shape. Moreover, the radical deformation of the CNR can affect its mechanical25 and electrical properties26. The self-deformation and collapse27 of the CNRs make themselves
insert into SWCNTs and wrap around the SWCNTs possibly. It is always very difficult to control the length of CNRs during the synthesis process28. Recently, Sun et al. took advantage of the
confined space of the two-dimensional interlayer galleries of a layered double hydroxide (LDH) host to limit the in situ growth of CNRs, controlling the length down to the molecular scale of
~1 nm23. Therefore, a convincing model by which we may thoroughly understand the interaction between the CNRs and SWCNTs becomes very valuable. In the present study, systematic theoretical
investigations are performed to demonstrate how the CNR interacts with the SWCNT. In addition, the possible interacting mechanism is examined to establish the nature of the self-assembly of
CNRs and SWCNTs. This study is not only helpful for more thoroughly understanding the properties of CNRs at an atomistic level, but also is essential to the substantial potential application
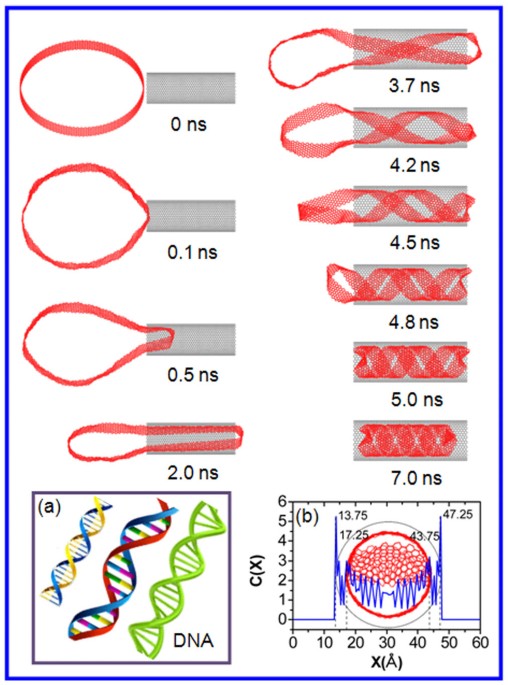
for fabricating functional nanodevices. RESULTS Figure 1 provides the representative snapshots of the CNR (100,100) with the length of 14.76 Å, inserted helically into the SWCNT (25, 25).
First, the CNR is placed at the center of the SWCNT entrances separated by 5 Å, with the axes of the CNR and SWCNT perpendicular to each other. When the simulation begins, the CNR with a
diameter of 135.6 Å tends to be thermodynamically unstable and its cylindrical symmetry is collapsed. Owing to the Van der Waals interaction29 between the CNR and SWCNT, the CNR stretches
its cross-section and approaches the SWCNT at 0.1 ns. As the simulations progress, the portion of the CNR near the entrance is captured by the inner hollow space of the SWCNT and gradually
moves forward along the inner wall of the SWCNT, in which the CNR are held tightly against SWCNT due to the interaction between them. After an initial correlation time, the CNR begins to
curl in the hollow interior of the SWCNT (at t = 3.5 ns). When the simulation time reaches 4.8 ns, the CNR displays a clear spiral conformation with a large helical pitch, attempting to
occupy the entirety of the tubes. Then the spirals become denser due to the Van der Waals interaction. Eventually, a perfect DNA-like double-helix (Figure 1(a)), with remarkably constant
pitches between neighboring spirals forms in the SWCNT. In addition, we further simulate the influence of the temperature on the final structure of the CNR-SWCNT system. It is surprising
that the final composite structure of the CNR-SWCNT system can maintain stability even when the temperature is 1000 K. This final geometric configuration of the CNR-SWCNT system can be
further characterized by the concentration distribution profile between the CNR and SWCNT in the X-direction. The separation of the adjacent layers can be obtained by the distance between
two neighboring peaks in the concentration profiles. From the peak details labeled in Figure 1(b), the separation between the inner layer of CNR and the SWCNT is about 3.5 Å, which is very
close to the wall thickness of the multiwalled CNTs (3.4 Å). We further study the insertion processes of CNRs with the same diameter, namely 135.60 Å, but different lengths, i.e. 7.38, 9.84,
12.30, 14.76, 17.22 Å. Figure 2(a) shows the diameter threshold of SWCNT successfully encapsulated by the CNR with certain lengths. We observe that the diameter of the SWCNT should be
larger than the threshold to ensure the collapse and insertion of the CNR with certain lengths. If the diameter of the nanotube is too small, the Van der Waals interaction cannot exceed the
energy barrier and the shell distance between carbon atoms cannot maintain 3.5 Å due to the confinement. Consequently, the sizes of SWCNTs should be chosen properly according to the lengths
of the CNRs to facilitate the insertion. In order to verify the final configurations of the CNR (100,100) with a length of 9.84 Å in the varied SWCNTs, a series of armchair SWCNTs (n, n) are
selected with the indices of n = 14, 15, 16, 18 and 24, yielding the tube diameters of 18.98, 20.34, 21.70, 24.41 and 32.54 Å, respectively and the tube length is set to 98.38 Å to fit the
CNR. Figure 2(b) demonstrates that a perfect helix inside the nanotube can be obtained after the CNRs are trapped by the inner wall of the tube, which is independent of the size of the tube.
What would happen if the length of the SWCNT is too short? Figure 3 shows the overall insertion process of the CNR (100,100) with the length of 14.76 Å into the SWCNT (25, 25). The length
of the SWCNT is chosen as 24.60 Å. It is worth noting that the CNR can insert into the SWCNT successfully with the trapped portion to form a helix. When the simulation proceeds, the front
helical structure is pushed out and the portion outside the tube continuously enters the SWCNT in a helical manner. Finally, the CNR attempts to be symmetric to the tube, with the middle
portion of the CNR in the inner wall of the tube forming a perfect helical configuration. In the above discoveries, we have mainly focused on the insertion of one CNR into the SWCNT. Next,
we will clarify how two CNRs insert into the SWCNT. As shown in Figure 4, the two CNRs with the same diameter and length are placed at the two ends of the SWCNT, with their axes
perpendicular to each other. When the simulation begins, the two CNRs are captured by the inner hollow of the SWCNT and move forward along the wall of the tube, due to the Van der Waals
interaction. When they contact with each other, one of them is pushed down to a graphitic nanoribbon, as a result of the competition between the two CNRs. When the simulation time reaches
2.25 ns, helices arise to both of the CNRs. When the simulation time t = 5.0 ns, the CNRs display a clear helical conformation. The decreased total potential energy EP and negative Van der
Waals interaction energyΔEvdW, which are synchronous to each other, indicate that the self-assembly course is spontaneous and the adhesion is strong. The unique spontaneous encapsulation of
CNRs into SWCNTs arouses our interest to investigate the delivery of the materials, as this can be utilized to deliver substances into the nanoscale confined space without any other external
force. In order to explore the possible applications, we simulate the interaction between the SWCNTs and CNRs modified with different functional groups. We select the –OH modified CNR
(100,100) with the length of 14.76 Å encapsulated into the SWCNT (30, 30). As shown in Figure 5, the CNR modified with the –OH can spontaneously be inserted into the SWCNT to form a helix.
It is found that the chemical groups –OH have a certain influence on the interval between the neighboring segments. As the CNR (100,100) without modification has 200 dangling σ-orbitals at
one end, the possible number of the functional groups to modify the CNR is 0–400. Then a series of simulations are performed on the CNRs (100,100) modified with different number of –OH, as
shown in Figure 6(a). When one end of the CNR is modified with 50,100 and 133 –OH, the CNR can form a helix in the SWCNT. In contrast, the CNR fully modified with 200 –OH at one end cannot
form a helix and the CNR modified with 400 -OH even cannot be trapped by the hollow interior of the SWCNT. In addition to the –OH, we also chemically attach –H to the CNR (100,100) with the
length of 14.76 Å uniformly. In Figure 6(a), it is shown that when the dangling σ-orbitals on the carbon atoms are all saturated by hydrogen atoms, the CNR can still be encapsulated into the
SWCNT and form a helix, which is quite different from the final configuration of the CNR modified with –OH. In addition, the helix-formation of the CNR without any dangling σ-orbitals also
suggests that the Van der Waals interaction is the main driving force in forming a helical configuration, rather than the dangling σ-orbitals on carbon atoms at the end of CNRs30. We also
study the stability of final configuration in different systems, in which the CNR is modified with a different number of –OH. As illustrated in Figure 6(b), we find that the whole potential
of the 200 –OH system decreases to a certain value and maintains at this level for 7 ns, which indicates that the configuration without helix is comparably stable. The potential in the
system of CNR modified with 200 –OH is very different from that in the system of a helix configuration (Figure 6(c)). The potential energy in the helix-forming process has evident periods:
two “rapid drop” periods, a “platform” period and the equilibrium state. It may be suspected that the modification of the CNR with different functional groups can influence the final
configuration in the encapsulation process. The above studies mainly focus on the insertion feature of the CNRs into the hollow SWCNTs. In this section, we will investigate the collapse and
wrapping characteristic of the CNRs around the SWCNT. Figure 7(a) shows the typical snapshots of the CNR (80, 80) with the length of 14.76 Å spontaneously wrapping on the SWCNT (15, 15).
Initially, the CNR positioned in the center of the SWCNT, with the axis of the CNR aligned parallel to that of the SWCNT and the separation distance of about 5 Å above the SWCNT. As shown in
Figure 7(a), the CNR approaches the SWCNT rapidly due to the strong attractive force. During the approaching process, the CNR gradually stretches its cross-section from a circle to an oval,
because the carbon atoms close to the SWCNT can endure stronger Van der Waals force than the upper one. After the CNR contacts with the SWCNT, the upper atoms continue approaching the
SWCNT, inducing the oval cross-section to collapse to a “Δ” shape31, completely covering the surface of the SWCNT. Then the axes of the CNR and SWCNT are staggered and the cross-section
changes into a bow-shape. During this process, the Van der Waals interaction on the top semicircular tube is so weak that it makes the CNR collapse rather slowly. Eventually, at 9 ns, the
collapse of the entire CNR is further accelerated due to the increase of the contact area32. The sequential collapse of these rings forms a linked double graphitic nanoribbon, showing a
double-walled helix wrapping around the SWCNT with a large helical pitch. Our results show that regardless of whether the axes of the CNR and SWCNT are perpendicular or parallel to each
other in the initial models, the CNR can wrap around the SWCNT helically. The helix of the CNR adhering on the wall of the SWCNT resembles the tendril of a morning glory climbing around a
tree trunk. This growth pattern helps the plant maximize the sunlight absorption in the minimum space and grow strong. It will be difficult to separate it with the trunk even in the strong
wind. We also study the concentration distribution profiles of the wrapping process in the X-direction and total potential energy EP of the CNR-SWCNT system as a function of time. As shown
in Figure 7(b), the distance between two layers of the CNR outside the tube is also about 3.5 Å. The potential energy shown in Figure 7(c) has a decreasing tendency with the simulation time
and reaches the mimimum at equilibrium. The final configurations of the CNRs with different diameters adhering on the SWCNTs are summarized in Table 1. We set the lengths of all SWCNTs (15,
15) as the same figure, i.e. 73.79 Å and set the lengths of all CNRs to be 14.76 Å. As illustrated in Table 1, all CNRs with diameters larger than the threshold of 12.20 Å are collapsed.
However, CNRs with diameters between 50.17 and 73.22 Å cannot fully collapse and instead simply deform their cylindrical symmetry to bow-shapes. During this process, the Van der Waals energy
cannot overcome the energy required for the fully mechanical deformation of the CNRs, leading to a mechanically bistable configuration33. However, what we wish to know is how the CNRs
spontaneously collapse to form ribbons and wrap on the SWCNT helically. In order to explore the possible effects of the length of the SWCNTs on the final configuration, SWCNTs (15, 15) with
different length and CNRs (45, 45) with length of 14.76 Å are selected. As shown in Figure 8, it is clear that the CNRs with diameters of 61.02 Å cannot collapse fully when the length of the
SWCNTs reaches 63.95 Å. Due to the fact that we cannot determine all the CNRs with different diameters and SWCNTs with different lengths, Table 1 and Figure 8 suggest that if the lengths of
the SWCNTs are smaller than the diameters of the CNRs, the perfect helix can be guaranteed. Figure 8(d) also illustrated that the SWCNTs with sufficient lengths can cause the CNRs to
collapse as a linked double graphitic layer paralleled to the axe of the SWCNTs. DISCUSSION In this study, a series of atomistic simulations have been performed to study the self-assembly of
the CNR-SWCNT systems. The CNR can spontaneously insert into the inner cavity of the SWCNT to form a DNA-like double-helix. Furthermore, the CNR can also collapse to a double enclosed
graphitic nanoribbon adhering on the outside wall in a spiral form. In principle, the geometric structure transformation is determined by the competition between the van der Waals
interaction energy and the bending strain energy of the CNR32. The competition between the Van der Waals energy, which provide an attractive force to collapse the CNR and the elastic energy,
which usually tends to keep the CNR in a circular form33, could lead to the circle configuration, fully collapsed configuration, or even mechanically bistable configuration of a CNR in
which the two competitive energies are comparable. During the self-assembly process, it is the Van der Waals interaction between the CNR and the SWCNT, which helps the CNR overcome the
energy barrier so that it may collapse30,34 and drives the CNR to be trapped in the tube and undergo self-scrolling. Taking the insertion process as an example, as shown in Figure 9(a), the
negative ΔEvdW, indicating an attractive force, suggests that the Van der Waals interaction between the CNR and SWCNT plays a dominant role in driving the continuous collapse of the CNR and
forming spirals. Throughout the helix-forming course, the Van der Waals energy decreases significantly and finally reaches its minimum. The Van der Waals energy has partially transformed to
the internal energy for mechanical deformation of CNR and partially converted into kinetic energy, thus sustaining the structure transition. As a result, the collapsed CNRs have the largest
area to contact with the SWCNT, which reduces the systemic potential energy and enhances the stability of the CNR-SWCNT system. We can explain well why the CNR can be trapped by the inner
wall of the SWCNTs and collapse around the tube, but what surprises us is why the CNR enters or adheres on the SWCNT to form spirals. It can be speculated that two possible reasons are
responsible for the spirals-forming process. The first reason is that the spiral pattern is the lowest energy state of the CNR-SWCNT system. To reveal it quantitatively, the total potential
energy EP of the insertion process is shown in Figure 9(b). The decreased potential energy of the CNR-SWCNT system indicates that the helix-forming course is spontaneous and that the system
gradually reaches a more stable state. When the energy reaches its minimum, the entire system is in equilibrium and the perfect spirals are formed. It is worth noting that, during the
inserting process, the curve has an evident “platform” in the period of 2 to 4 ns, indicating that the contact area is virtually unchanged after the tube is fully filled between 2 and 4 ns.
As far as the wrapping process is concerned, a “rapid drop”, which occurred at about 9 ns, is caused by the increase of the contact area, which corresponds to the accelerated collapse
process. The second reason for this phenomenon is that the compact spirals of the CNR are the natural space savers which can achieve the maximum occupancy of the confined SWCNT. The spiral
form of CNR encapsulated in the SWCNT is similar to the ordered, helical conformations of long molecular chains such as DNA and protein in the confined cell and the wrapping process of the
CNR resembles to the tendrils of the climbing plants. To better understand the helical configuration in the crowed environment, Snir and Kamien35 constructed a simulation system, in which a
solid, impenetrable but flexible tube immersed in a solution of hard spheres. It is amazing that the flexible tube will take on a regular helix, which takes the least amount of energy and
takes up the least space. Similarly, the spiral phenomenon is a relatively common but rather interesting phenomenon in the nature, such as spiral arrangement of florets in the sunflower
(Figure 9(c)), the spiral nucleation of SiC crystal in a limited space and the spiral arrangement of thunderstorms in the center of a tropical cyclone. What's more, Chinese old Tai Chi
totem (Figure 9(c)) also emphasizes the philosophy of helical configuration, which implies that the universe derives from a series of helix. It is believed that helix is one of the essential
features of materials and life even though its origin remains unclear. To conclude, the CNR can be helically inserted into the SWCNT or wrap around the tube in a spiral form. It is
suspected that the spiral configuration of the CNR takes the least amount of energy and takes up the least space. It is shown that the Van der Waals interaction between the CNR and the SWCNT
plays a dominant role in driving the CNR to insert into the nanotube or wrap around the tube. The decrease of the potential energy in the CNR-SWCNT systems suggests that the helical
insertion and wrapping processes are spontaneous. The sizes of the SWCNTs should exceed a certain threshold, to ensure the insertion of CNRs with certain lengths. Moreover, the diameters and
lengths of the nanotubes have negligible effects on the encapsulation of the CNR to form a perfect helix. Two CNRs can also be encapsulated into the SWCNT spontaneously to form a helix. We
also manifest that the modification of the CNR can influence the final configuration in the encapsulation process. Meanwhile, the results of our study also reveal that the sizes of the CNRs
and SWCNTs should meet some required conditions to guarantee wrapping on the tube in a helical form. The above-mentioned findings are of great importance toward the better understanding of
CNT-SWCNT systems. The unique phenomenon of self-assembly in CNRs-SWCNTs systems has potential advantages in the application as nanocontainer for drug delivery, molecular transportation.
This composite structure can be utilized to fabricate functional nanodevices, such as sensor, nanoelectronic components, integrated circuits and optoelectronic devices. METHODS In this
paper, the force field of condensed-phased optimized molecular potentials for atomistic simulation studies (COMPASS)36 is used to model the atomic interaction. COMPASS is an ab initio force
field which has been parameterized and validated using condensed-phase properties in addition to various ab initio calculations and experimental data, with a functional form that includes
covalent terms as well as long-range, non-bond (Van der Waals(VdW)) interactions and electrostatic forces. The aims of the force field are to achieve high accuracy in predicting the
properties of very complex mixtures37 and it has been widely used due to its potential to obtain reasonable results in terms of the mechanical properties of CNTs38,39. The van der Waals
energy is described by LJ-96 function37 in COMPASS forcefield, the functional forms of which is listed as: , where _D_0 = 0.064 Kal/mol and _R_0 = 4.01 Å40,41. A constant volume and constant
temperature dynamics (NVT) ensemble is used in our molecular dynamics simulations with temperature of 300 K. The Andersen method42 in the thermostat is employed to control the temperature
and generate the correct statistical ensemble. For temperature control, the thermodynamic temperature is kept constant by allowing the simulated system to exchange energy using a “heat
bath”. The Verlet algorithm is adopted to integrate the equations of motion for the entire system. The time step is chosen to be 1.0 fs and the data are collected every 5 ps to record the
full-precision trajectory for further analysis. The SWCNTs are fixed as rigid tube structures. The CNRs are super-short CNTs with a length down to the molecular scale. REFERENCES * Zhao, X.
C. & Johnson, J. K. Simulation of adsorption of DNA on carbon nanotubes. J. Am. Chem. Soc. 129, 10438–10445 (2007). Article CAS PubMed Google Scholar * Tsang, S. C., Chen, Y. K.,
Harris, P. J. F. & Green, M. L. H. A simple chemical method of opening and filling carbon nanotubes. Nature 372, 159–162 (1994). Article CAS ADS Google Scholar * Ajayan, P. M.,
Stephan, O., Redlich, P. & Colliex, C. Carbon nanotubes as removable templates for metal oxide nanocomposites and nanostructure. Nature 375, 564–567 (1995). Article CAS ADS Google
Scholar * Li, H. et al. Carbon nanotube seeded silicon crystal growth. Appl. Phys. Lett. 95, 063106 (2009). Article PubMed Central ADS CAS Google Scholar * Pederson, M. R. &
Broughton, J. Q. Nanocapillarity in fullerene tubules. Phys. Rev. Lett. 69, 2689–2692 (1992). Article CAS PubMed ADS Google Scholar * Ajayan, P. M., Ebbesen, T. W., Ichihashi, T.,
Iijima, S. & Tanigaki, K. Opening carbon nanotubes with oxygen and implications for filling. Nature 362, 522–525 (1993). Article CAS ADS Google Scholar * Borawiak-Palen, E. et al.
Iron filled single-wall carbon nanotubes-a novel ferromagnetic medium. Chem. Phys. Lett. 421, 129–133 (2006). Article ADS CAS Google Scholar * Maniwa, Y. et al. Water-filled single-wall
carbon nanotubes as molecular nanovalves. Nat. Mater. 6, 135–141 (2007). Article CAS PubMed ADS Google Scholar * Zou, J., Ji, B. H., Feng, X. Q. & Gao, H. J. Molecular-dynamic
studies of carbon-water-carbon composite nanotubes. Small 2, 1348–1355 (2006). Article CAS PubMed Google Scholar * Smith, B. W., Monthioux, M. & Luzzi, D. E. Encapsulated C60 in
carbon nanotubes. Nature 396, 323–324 (1998). Article CAS Google Scholar * Troche, K. S. et al. Prediction of ordered phases of encapsulated C60, C70 and C78 inside carbon nanotubes. Nano
Lett. 5, 349–355 (2005). Article CAS PubMed ADS Google Scholar * Jiang, Y. Y., Li, H. & Li, Y. F. Helical encapsulation of graphene nanoribbon into carbon nanotube. ACS Nano 5,
2126–2133 (2011). Article CAS PubMed Google Scholar * Chuvilin, A. et al. Self-assembly of a sulphur-terminated graphene nanoribbon within a single-walled carbon nanotube. Nature 10,
687–692 (2011). Article CAS Google Scholar * Zheng, M., Anand, J. & Michael, S. S. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 302, 1545–1548
(2003). Article CAS PubMed ADS Google Scholar * Brittany, G., Brenda, S., Donald, S. B., Robert, M. S. & Jennifer, N. C. Sequence-independent helical wrapping of single-walled
carbon nanotubes by long genomic DNA. Nano Lett. 6, 159–164 (2006). Article CAS Google Scholar * Baskaran, D., Mays, J. W. & Bratcher, M. S. Noncovalent and nonspecific molecular
interactions of polymers with multiwalled carbon nanotube. Chem. Mater. 17, 3389–3397 (2005). Article CAS Google Scholar * Nish, A., Hwang, J. Y., Doig, J. & Nicholas, R. J. Highly
selective dispersion of single-walled carbon nanotubes using aromatic polymers. Nat. Nanotechnol. 2, 640–646 (2007). Article CAS PubMed ADS Google Scholar * Chen, R. J., Zhang, Y.,
Wang, D. & Dai, H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 123, 3838–3839 (2001). Article CAS PubMed
Google Scholar * Choi, W. Y., Kang, J. W. & Hwang, H. J. Structure of ultrathin copper nonawires encapsulated in carbon nanotubes. Phys. Rev. B 68, 193405 (2003). Article ADS CAS
Google Scholar * Planeix, J. M. et al. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 116, 7935–7936 (1994). Article CAS Google Scholar *
Singh, R. et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 127,
4388–4396 (2005). Article CAS PubMed Google Scholar * Sun, F. W. & Li, H. Torsional strain energy evolution of carbon nanotubes and their stability with encapsulated helical copper
nanowires. Carbon 49, 1408–1451 (2011). Article CAS Google Scholar * Sun, J. et al. Carbon nanorings and their enhanced lithium storage properties. Adv. Mater. 25, 1125–1130 (2013).
Article CAS PubMed ADS Google Scholar * Elliott, J. A. et al. Collapse of single-wall carbon nanotubes is diameter dependent. Phy. Rev. Lett. 92, 095501 (2004). Article ADS CAS
Google Scholar * Gao, G. H., Cagin, T. & Goddard, W. A. Energetics, structure, mechanical and vibrational properties of single-walled carbon nanotubes. Nanotechnology 9, 184–191 (1998).
Article CAS ADS Google Scholar * Lu, J. Q. et al. Metal-to-semiconductor transition in squashed armchair carbon nanotubes. Phys. Rev. Lett. 90, 156601 (2003). Article PubMed ADS CAS
Google Scholar * Chopra, N. G. et al. Fully collapsed carbon nanotubes. Nature 377, 135–138 (1995). Article CAS ADS Google Scholar * Sun, X. M. et al. Optical properties of ultrashort
semiconducting single-walled carbon nanotube capsules down to sub-10 nm. J. Am. Chem. Soc. 130, 6551–6555 (2008). Article CAS PubMed Google Scholar * Ruoff, R. S., Tersoff, J., Lorents,
D. C., Subramoney, S. & Chan, B. Radial deformation of carbon nanotubes by van der Waals forces. Nature 364, 514–516 (1993). Article CAS ADS Google Scholar * Li, Y. F., Sun, F. W.
& Li, H. Helical wrapping and insertion of graphene nanoribbon to single-walled carbon nanotube. J. Phys. Chem. C 115, 18459–18467 (2011). Article CAS Google Scholar * Yan, K. Y. et
al. Radial collapse of single-walled carbon nanotubes induced by the Cu2O surface. J. Phys. Chem. C 113, 3120–3126 (2009). Article CAS Google Scholar * Yan, K. Y. et al. The core/shell
composite nanowires produced by self-scrolling carbon nanotubes onto copper nanowires. ACS NANO 3, 2235–2240 (2009). Article CAS PubMed Google Scholar * Lu, W. B. & Chou, T. W.
Radial deformation and its related energy variations of single-walled carbon nanotubes. Phys. Rev. B 83, 134113 (2011). Article ADS CAS Google Scholar * Zhang, S. L., Khare, R. &
Belytschko, T. Transition states and minimum energy pathways for the collapse of carbon nanotubes. Phy. Rev. B 73, 075423 (2006). Article ADS CAS Google Scholar * Snir, Y. & Kamien,
R. D. Entropically driven helix formation. Science 307, 1067 (2005). Article CAS PubMed Google Scholar * Sun, H. COMPASS: an ab initio force-field optimized for condensed-phase
applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998). Article CAS Google Scholar * Bunte, S. W. & Sun, H. Molecular modeling of
energetic materials: the parameterization and validation of nitrate esters in the compass force field. J. Phys. Chem. B 104, 2477–2489 (2000). Article CAS Google Scholar * Wang, Q., Duan,
W. H., Liew, K. M. & He, X. Q. Inelastic buckling of carbon nanotubes. Appl. Phys. Lett. 90, 033110-1-3 (2007). Article ADS CAS Google Scholar * Wang, Q. Atomic transportation via
carbon nanotubes. Nano. Lett. 9, 245–249 (2009). Article CAS PubMed ADS Google Scholar * Sun, H., Mumby, S. J., Maple, J. R. & Hagler, A. T. An ab initio CFF93 all-atom force field
for polycarbonates. J. Am. Chem. Soc. 116, 2918–2981 (1994). Google Scholar * Rigby, D., Sun, H. & Eichinger, B. E. Computer simulations of poly(ethylene oxide): force field, PVT
diagram and cyclization behaviour. Polym. Int. 44, 311–330 (1998). Article Google Scholar * Andersen, H. C. MDs simulations at constant pressure and/or temperature. J. Chem. Phys. 72,
2384–2393 (1980). Article CAS ADS Google Scholar Download references ACKNOWLEDGEMENTS We would like to acknowledge the support from the National Natural Science Foundation of China
(Grant No. 51271100) and the National Basic Research Program of China (Grant No.2012CB825702). This work is also supported by the Special Funding in the Project of the Taishan Scholar
Construction Engineering. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials, Ministry of Education, Shandong
University, Jinan, 250061, People's Republic of China Wei Chen & Hui Li Authors * Wei Chen View author publications You can also search for this author inPubMed Google Scholar * Hui
Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.C. performed all simulations and prepared the manuscript. W.C. and H.L. designed the
research, discussed the results, drew conclusions and edited the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC
SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION Video 1: The insertion and helix-forming process of the CNR (100,100) into the SWCNT (25,25). SUPPLEMENTARY INFORMATION Video 2: The two CNRs
(100,100) inserted into the SWCNT (30,30) at the same time. SUPPLEMENTARY INFORMATION Video 3: The wrapping and helix-forming process of the CNR (80,80) around the SWCNT (15,15). RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, W., Li, H. How Does Carbon Nanoring Deform to Spiral Induced by Carbon
Nanotube?. _Sci Rep_ 4, 3865 (2014). https://doi.org/10.1038/srep03865 Download citation * Received: 25 November 2013 * Accepted: 07 January 2014 * Published: 27 January 2014 * DOI:
https://doi.org/10.1038/srep03865 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(749x0:751x2)/alyssa-milano-122022-1-tout-26075d5a552a468aabc4a95683256e66.jpg)