- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
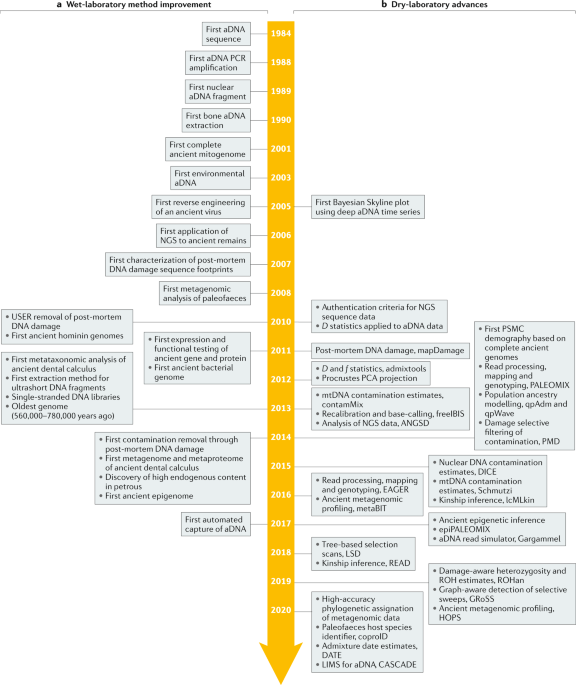
ABSTRACT Although the first ancient DNA molecules were extracted more than three decades ago, the first ancient nuclear genomes could only be characterized after high-throughput sequencing
was invented. Genome-scale data have now been gathered from thousands of ancient archaeological specimens, and the number of ancient biological tissues amenable to genome sequencing is
growing steadily. Ancient DNA fragments are typically ultrashort molecules and carry extensive amounts of chemical damage accumulated after death. Their extraction, manipulation and
authentication require specific experimental wet-laboratory and dry-laboratory procedures before patterns of genetic variation from past individuals, populations and species can be
interpreted. Ancient DNA data help to address an entire array of questions in anthropology, evolutionary biology and the environmental and archaeological sciences. The data have revealed a
considerably more dynamic past than previously appreciated and have revolutionized our understanding of many major prehistoric and historic events. This Primer provides an overview of
concepts and state-of-the-art methods underlying ancient DNA analysis and illustrates the diversity of resulting applications. The article also addresses some of the ethical challenges
associated with the destructive analysis of irreplaceable material, emphasizes the need to fully involve archaeologists and stakeholders as part of the research design and analytical
process, and discusses future perspectives. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 1 digital issues and online access to articles $119.00 per year only $119.00 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE ALLEN ANCIENT DNA RESOURCE (AADR) A CURATED COMPENDIUM OF ANCIENT HUMAN GENOMES Article Open access 10 February 2024 MORE
THAN A DECADE OF GENETIC RESEARCH ON THE DENISOVANS Article 18 September 2023 ACCURATE DETECTION OF IDENTITY-BY-DESCENT SEGMENTS IN HUMAN ANCIENT DNA Article Open access 20 December 2023
REFERENCES * Higuchi, R., Bowman, B., Freiberger, M., Ryder, O. A. & Wilson, A. C. DNA sequences from the quagga, an extinct member of the horse family. _Nature_ 312, 282–284 (1984). ADS
Google Scholar * Orlando, L. et al. Recalibrating _Equus_ evolution using the genome sequence of an early Middle Pleistocene horse. _Nature_ 499, 74–78 (2013). THIS ARTICLE PRESENTS THE
OLDEST SEQUENCED GENOME TO DATE, FROM ONE HORSE METAPODIAL PRESERVED IN PERMAFROST FOR 560,000–780,000 YEARS. ADS Google Scholar * Palkopoulou, E. et al. Complete genomes reveal signatures
of demographic and genetic declines in the woolly mammoth. _Curr. Biol._ 25, 1395–1400 (2015). Google Scholar * Barlow, A. et al. Partial genomic survival of cave bears in living brown
bears. _Nat. Ecol. Evol._ 2, 1563–1570 (2018). Google Scholar * Margaryan, A. et al. Population genomics of the Viking world. _Nature_ 585, 390–396 (2020). ADS Google Scholar * Rasmussen,
M. et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. _Nature_ 463, 757–762 (2010). THIS ARTICLE REPORTS THE FIRST ANCIENT HUMAN GENOME SEQUENCED FROM THE HAIR SHAFT OF A
4,000-YEAR-OLD PALEO-INUIT. THE AUTHORS FIND EVIDENCE FOR GENETIC DISCONTINUITY WITH MODERN GREENLANDERS, SUPPORTING MULTIPLE MIGRATION WAVES INTO ARCTIC GREENLAND. ADS Google Scholar *
Green, R. E. et al. A draft sequence of the Neandertal genome. _Science_ 328, 710–722 (2010). THIS ARTICLE REPORTS THE FIRST NEANDERTHAL GENOME OBTAINED FROM THE DNA EXTRACTS OF THREE
PALEONTOLOGICAL BONES, ESTABLISHING THE NEANDERTHAL GENETIC LEGACY WITHIN MODERN HUMAN GENOMES AND DESCRIBING IMPORTANT WET-LABORATORY AND DRY-LABORATORY METHODOLOGIES THAT HAVE SHAPED THE
FOLLOWING DECADE OF ADNA RESEARCH. ADS Google Scholar * Prüfer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. _Nature_ 505, 43–49 (2014). ADS Google
Scholar * Prüfer, K. et al. A high-coverage Neandertal genome from Vindija cave in Croatia. _Science_ 358, 655–658 (2017). ADS Google Scholar * Mafessoni, F. et al. A high-coverage
Neandertal genome from Chagyrskaya cave. _Proc. Natl Acad. Sci. USA_ 117, 15132–15136 (2020). Google Scholar * Nielsen, R. et al. Tracing the peopling of the world through genomics.
_Nature_ 541, 302–310 (2017). ADS Google Scholar * Frantz, L. A. F., Bradley, D. G., Larson, G. & Orlando, L. Animal domestication in the era of ancient genomics. _Nat. Rev. Genet._
21, 449–460 (2020). Google Scholar * Spyrou, M. A., Bos, K. I., Herbig, A. & Krause, J. Ancient pathogen genomics as an emerging tool for infectious disease research. _Nat. Rev. Genet._
20, 323–340 (2019). Google Scholar * Krause, J. et al. The complete mitochondrial DNA genome of an unknown hominin from southern Siberia. _Nature_ 464, 894–897 (2010). ADS Google Scholar
* Reich, D. et al. Genetic history of an archaic hominin group from Denisova cave in Siberia. _Nature_ 468, 1053–1060 (2010). THIS ARTICLE REPORTS THE DISCOVERY OF DENISOVANS, A PREVIOUSLY
UNKNOWN LINEAGE OF ARCHAIC HOMININS THAT LIVED IN SOUTHERN SIBERIA AT LEAST 50,000 YEARS AGO. THIS WAS THE FIRST TIME THAT A REPRESENTATIVE OF THE _HOMO_ EVOLUTIONARY TREE WAS DESCRIBED
FROM MOLECULAR DATA AND IN THE ABSENCE OF KEY MACROFOSSIL REMAINS SHOWING CLEAR MORPHOLOGICAL CHARACTERISTICS. ADS Google Scholar * Meyer, M. et al. A high-coverage genome sequence from an
archaic Denisovan individual. _Science_ 338, 222–226 (2012). THIS ARTICLE PRESENTS THE FIRST HIGH-QUALITY GENOME FROM AN ARCHAIC HOMININ AND DESCRIBES THE FIRST EXPERIMENTAL PROCEDURE FOR
DNA LIBRARY PREPARATION FROM SINGLE-STRANDED DNA TEMPLATES. THE APPROACH OUTPERFORMS COMPETING TECHNOLOGIES IN SENSITIVITY AND COMPLEXITY, AND MINIMIZES LOSS OF AUTHENTIC DNA MOLECULES. ADS
Google Scholar * Rogers, R. L. & Slatkin, M. Excess of genomic defects in a woolly mammoth on Wrangel island. _PLoS Genet._ 13, e1006601 (2017). Google Scholar * Lynch, V. J. et al.
Elephantid genomes reveal the molecular bases of woolly mammoth adaptations to the Arctic. _Cell Rep._ 12, 217–228 (2015). Google Scholar * Fry, E. et al. Functional architecture of
deleterious genetic variants in the genome of a Wrangel island mammoth. _Genome Biol. Evol._ 12, 48–58 (2020). Google Scholar * Lorenzen, E. D. et al. Species-specific responses of late
quaternary megafauna to climate and humans. _Nature_ 479, 359–364 (2011). ADS Google Scholar * Orlando, L. & Cooper, A. Using ancient DNA to understand evolutionary and ecological
processes. _Annu. Rev. Ecol. Evol. Syst._ 45, 573–598 (2014). Google Scholar * Willerslev, E. et al. Fifty thousand years of Arctic vegetation and megafaunal diet. _Nature_ 506, 47–51
(2014). ADS Google Scholar * Fortes, G. G. et al. Ancient DNA reveals differences in behaviour and sociality between brown bears and extinct cave bears. _Mol. Ecol._ 25, 4907–4918 (2016).
ADS Google Scholar * Gretzinger, J. et al. Large-scale mitogenomic analysis of the phylogeography of the Late Pleistocene cave bear. _Sci. Rep._ 9, 10700 (2019). ADS Google Scholar *
Key, F. M. et al. Emergence of human-adapted _Salmonella enterica_ is linked to the Neolithization process. _Nat. Ecol. Evol._ 4, 324–333 (2020). Google Scholar * Warinner, C. et al.
Pathogens and host immunity in the ancient human oral cavity. _Nat. Genet._ 46, 336–344 (2014). THIS ARTICLE REPORTS THE FIRST METAGENOMIC AND PALEOPROTEOMIC ANALYSIS OF ANCIENT DENTAL
PLAQUE AND DEMONSTRATES THE PRESERVATION OF ORAL MICROBIAL SIGNATURES, DIET CONTENT AND INFLAMMATION MARKERS. Google Scholar * Warinner, C. et al. A robust framework for microbial
archaeology. _Annu. Rev. Genomics Hum. Genet._ 18, 321–356 (2017). Google Scholar * Gokhman, D. et al. Reconstructing the DNA methylation maps of the Neandertal and the Denisovan. _Science_
344, 523–527 (2014). ADS Google Scholar * Pedersen, J. S. et al. Genome-wide nucleosome map and cytosine methylation levels of an ancient human genome. _Genome Res._ 24, 454–466 (2014).
THIS ARTICLE IS THE FIRST REPORT OF ANCIENT EPIGENOMES, LEVERAGING POST-MORTEM DNA DEGRADATION SIGNATURES TO STATISTICALLY INFER DNA METHYLATION AND NUCLEOSOME POSITIONING. Google Scholar *
Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. _Nat. Rev. Genet._ 17, 333–351 (2016). Google Scholar * Poinar, H.
N. et al. Metagenomics to paleogenomics: large-scale sequencing of mammoth DNA. _Science_ 311, 392–394 (2006). ADS Google Scholar * Green, R. E. et al. Analysis of one million base pairs
of Neanderthal DNA. _Nature_ 444, 330–336 (2006). ADS Google Scholar * Orlando, L., Gilbert, M. T. P. & Willerslev, E. Reconstructing ancient genomes and epigenomes. _Nat. Rev. Genet._
16, 395–408 (2015). Google Scholar * Giguet-Covex, C. et al. Long livestock farming history and human landscape shaping revealed by lake sediment DNA. _Nat. Commun._ 5, 3211 (2014). ADS
Google Scholar * Avila-Arcos, M. C. et al. Application and comparison of large-scale solution-based DNA capture-enrichment methods on ancient DNA. _Sci. Rep._ 1, 74 (2011). Google Scholar
* Maricic, T., Whitten, M. & Pääbo, S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. _PLoS ONE_ 5, e14004 (2010). ADS Google Scholar * Fu, Q. et al. DNA
analysis of an early modern human from Tianyuan cave, China. _Proc. Natl Acad. Sci. USA_ 110, 2223–2227 (2013). THIS STUDY PRESENTS THE FIRST APPLICATION OF IN-SOLUTION TARGET ENRICHMENT AT
THE GENOME SCALE AND DESCRIBES SEQUENCE FOR A FULL CHROMOSOME OF AN APPROXIMATELY 40,000-YEAR-OLD ANATOMICALLY MODERN HUMAN FROM CHINA. ADS Google Scholar * Carpenter, M. L. et al.
Pulling out the 1%: whole-genome capture for the targeted enrichment of ancient DNA sequencing libraries. _Am. J. Hum. Genet._ 93, 852–864 (2013). Google Scholar * Dabney, J. et al.
Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. _Proc. Natl Acad. Sci. USA_ 110, 15758–15763 (2013). THIS STUDY REPORTS
A NEW DNA EXTRACTION METHOD FROM ANCIENT OSSEOUS REMAINS THAT IS TAILORED TO THE ULTRASHORT AND EXTENSIVELY DAMAGED NATURE OF ADNA MOLECULES. THIS METHODOLOGY ALLOWED THE RETRIEVAL OF FULL
MITOCHONDRIAL GENOME SEQUENCES FROM 300,000-YEAR-OLD CAVE BEAR SPECIMENS PRESERVED IN ATAPUERCA, SPAIN. ADS Google Scholar * Korlević, P. et al. Reducing microbial and human contamination
in DNA extractions from ancient bones and teeth. _BioTechniques_ 59, 87–93 (2015). Google Scholar * Gamba, C. et al. Comparing the performance of three ancient DNA extraction methods for
high-throughput sequencing. _Mol. Ecol. Resour._ 16, 459–469 (2016). Google Scholar * Boessenkool, S. et al. Combining bleach and mild predigestion improves ancient DNA recovery from bones.
_Mol. Ecol. Resour._ 17, 742–751 (2017). Google Scholar * Gansauge, M.-T. & Meyer, M. Selective enrichment of damaged DNA molecules for ancient genome sequencing. _Genome Res._ 24,
1543–1549 (2014). Google Scholar * Adler, C. J. et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and industrial
revolutions. _Nat. Genet._ 45, 450–455, 455e1 (2013). Google Scholar * Wagner, S. et al. High-throughput DNA sequencing of ancient wood. _Mol. Ecol._ 27, 1138–1154 (2018). Google Scholar *
Lendvay, B. et al. Improved recovery of ancient DNA from subfossil wood—application to the world’s oldest Late Glacial pine forest. _New Phytol._ 217, 1737–1748 (2018). Google Scholar *
Der Sarkissian, C. et al. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. _Mol. Ecol. Resour._ 17, 835–853 (2017). Google Scholar * Pedersen,
M. W. et al. Postglacial viability and colonization in North America’s ice-free corridor. _Nature_ 537, 45–49 (2016). ADS Google Scholar * Slon, V. et al. Neandertal and Denisovan DNA from
Pleistocene sediments. _Science_ 356, 605–608 (2017). ADS Google Scholar * Teasdale, M. D. et al. Paging through history: parchment as a reservoir of ancient DNA for next generation
sequencing. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 370, 20130379 (2015). Google Scholar * O’Sullivan, N. J. et al. A whole mitochondria analysis of the Tyrolean Iceman’s leather
provides insights into the animal sources of Copper Age clothing. _Sci. Rep._ 6, 31279 (2016). ADS Google Scholar * Gamba, C. et al. Genome flux and stasis in a five millennium transect of
European prehistory. _Nat. Commun._ 5, 5257 (2014). THIS STUDY REPORTS THE FIRST MATCHED EMPIRICAL EVIDENCE ESTABLISHING BETTER DNA PRESERVATION RATES IN PETROSAL BONES, WHICH PAVED THE WAY
FOR FUTURE STUDIES AT THE POPULATION SCALE, AND ALSO PRESENTS COMPLETE GENOMES OF MESOLITHIC HUNTER–GATHERERS AND NEOLITHIC FARMERS IN THE CAUCASUS, SUPPORTING DIRECT EARLY CONTACT BETWEEN
BOTH GROUPS. ADS Google Scholar * Damgaard, P. B. et al. Improving access to endogenous DNA in ancient bones and teeth. _Sci. Rep._ 5, 11184 (2015). ADS Google Scholar * Krause, J. et
al. A complete mtDNA genome of an early modern human from Kostenki, Russia. _Curr. Biol._ 20, 231–236 (2010). THIS ARTICLE ESTABLISHES IMPORTANT CRITERIA FOR ADNA DATA AUTHENTICATION,
INCLUDING THE PRESENCE OF POST-MORTEM CYTOSINE DEAMINATION SIGNATURES AND DNA FRAGMENTATION THROUGH DEPURINATION, AND REPORTS A COMPLETE MITOCHONDRIAL GENOME FROM AN APPROXIMATELY
32,000-YEAR-OLD ANATOMICALLY MODERN HUMAN FROM KOSTENKI, RUSSIA. Google Scholar * Skoglund, P. et al. Separating endogenous ancient DNA from modern day contamination in a Siberian
Neandertal. _Proc. Natl Acad. Sci. USA_ 111, 2229–2234 (2014). ADS Google Scholar * Renaud, G., Schubert, M., Sawyer, S. & Orlando, L. Authentication and assessment of contamination in
ancient DNA. _Methods Mol. Biol._ 1963, 163–194 (2019). Google Scholar * Jónsson, H., Ginolhac, A., Schubert, M., Johnson, P. L. F. & Orlando, L. mapDamage2.0: fast approximate
Bayesian estimates of ancient DNA damage parameters. _Bioinformatics_ 29, 1682–1684 (2013). Google Scholar * Leonardi, M. et al. Evolutionary patterns and processes: lessons from ancient
DNA. _Syst. Biol._ 66, e1–e29 (2017). Google Scholar * Pääbo, S., Gifford, J. A. & Wilson, A. C. Mitochondrial DNA sequences from a 7000-year old brain. _Nucleic Acids Res._ 16,
9775–9787 (1988). Google Scholar * Miller, W. et al. Sequencing the nuclear genome of the extinct woolly mammoth. _Nature_ 456, 387–390 (2008). ADS Google Scholar * Gilbert, M. T. P. et
al. Resistance of degraded hair shafts to contaminant DNA. _Forensic Sci. Int._ 156, 208–212 (2006). Google Scholar * Seguin-Orlando, A. et al. Pros and cons of methylation-based enrichment
methods for ancient DNA. _Sci. Rep._ 5, 11826 (2015). ADS Google Scholar * Hagelberg, E., Sykes, B. & Hedges, R. Ancient bone DNA amplified. _Nature_ 342, 485 (1989). ADS Google
Scholar * Sirak, K. et al. Human auditory ossicles as an alternative optimal source of ancient DNA. _Genome Res._ 30, 427–436 (2020). Google Scholar * Bos, K. I. et al. A draft genome of
Yersinia pestis from victims of the Black Death. _Nature_ 478, 506–510 (2011). THIS ARTICLE REPORTS THE FIRST COMPLETE GENOME OF AN ANCIENT BACTERIAL PATHOGEN, _Y. PESTIS_, FROM OSSEOUS
HUMAN REMAINS OF INDIVIDUALS WHO DIED FROM THE BLACK DEATH IN 1347–1348. ADS Google Scholar * Margaryan, A. et al. Ancient pathogen DNA in human teeth and petrous bones. _Ecol. Evol._ 8,
3534–3542 (2018). Google Scholar * Brown, T. A., Allaby, R. G., Brown, K. A., O’Donoghue, K. & Sallares, R. DNA in wheat seeds from European archaeological sites. _Experientia_ 50,
571–575 (1994). Google Scholar * Suyama, Y. et al. DNA sequence from a fossil pollen of _Abies_ spp. from Pleistocene peat. _Genes. Genet. Syst._ 71, 145–149 (1996). Google Scholar *
Jaenicke-Després, V. et al. Early allelic selection in maize as revealed by ancient DNA. _Science_ 302, 1206–1208 (2003). ADS Google Scholar * Vallebueno-Estrada, M. et al. The earliest
maize from San Marcos Tehuacán is a partial domesticate with genomic evidence of inbreeding. _Proc. Natl Acad. Sci. USA_ 113, 14151–14156 (2016). Google Scholar * Ramos-Madrigal, J. et al.
Genome sequence of a 5,310-year-old maize cob provides insights into the early stages of maize domestication. _Curr. Biol._ 26, 3195–3201 (2016). Google Scholar * Ramos-Madrigal, J. et al.
Palaeogenomic insights into the origins of French grapevine diversity. _Nat. Plants_ 5, 595–603 (2019). Google Scholar * Weiß, C. L. et al. Temporal patterns of damage and decay kinetics of
DNA retrieved from plant herbarium specimens. _R. Soc. Open Sci._ 3, 160239 (2016). ADS Google Scholar * Goloubinoff, P., Pääbo, S. & Wilson, A. C. Evolution of maize inferred from
sequence diversity of an _Adh2_ gene segment from archaeological specimens. _Proc. Natl Acad. Sci. USA_ 90, 1997–2001 (1993). ADS Google Scholar * Mascher, M. et al. Genebank genomics
bridges the gap between the conservation of crop diversity and plant breeding. _Nat. Genet._ 51, 1076–1081 (2019). Google Scholar * Fordyce, S. L. et al. Deep sequencing of RNA from ancient
maize kernels. _PLoS ONE_ 8, e50961 (2013). ADS Google Scholar * Smith, O. et al. Small RNA activity in archeological barley shows novel germination inhibition in response to environment.
_Mol. Biol. Evol._ 34, 2555–2562 (2017). Google Scholar * Goldstein, P. Z. & Desalle, R. Calibrating phyfDabnlogenetic species formation in a threatened insect using DNA from
historical specimens. _Mol. Ecol._ 12, 1993–1998 (2003). Google Scholar * Rawlence, N. J., Wood, J. R., Armstrong, K. N. & Cooper, A. DNA content and distribution in ancient feathers
and potential to reconstruct the plumage of extinct avian taxa. _Proc. Biol. Sci._ 276, 3395–3402 (2009). Google Scholar * Oskam, C. L. et al. Fossil avian eggshell preserves ancient DNA.
_Proc. Biol. Sci._ 277, 1991–2000 (2010). Google Scholar * Bro-Jørgensen, M. H. et al. Ancient DNA analysis of Scandinavian medieval drinking horns and the horn of the last aurochs bull.
_J. Archaeol. Sci._ 99, 47–54 (2018). Google Scholar * Foley, B. P., Hansson, M. C., Kourkoumelis, D. P. & Theodoulou, T. A. Aspects of ancient Greek trade re-evaluated with amphora DNA
evidence. _J. Archaeol. Sci._ 39, 389–398 (2012). Google Scholar * Kashuba, N. et al. Ancient DNA from mastics solidifies connection between material culture and genetics of mesolithic
hunter-gatherers in Scandinavia. _Commun. Biol._ 2, 185 (2019). Google Scholar * Jensen, T. Z. T. et al. A 5700 year-old human genome and oral microbiome from chewed birch pitch. _Nat.
Commun._ 10, 5520 (2019). ADS Google Scholar * Tito, R. Y. et al. Phylotyping and functional analysis of two ancient human microbiomes. _PLoS ONE_ 3, e3703 (2008). ADS Google Scholar *
Hagan, R. W. et al. Comparison of extraction methods for recovering ancient microbial DNA from paleofeces. _Am. J. Phys. Anthropol._ 171, 275–284 (2020). Google Scholar * Søe, M. J. et al.
Ancient DNA from latrines in Northern Europe and the Middle East (500 BC–1700 AD) reveals past parasites and diet. _PLoS ONE_ 13, e0195481 (2018). Google Scholar * Poinar, H. N. et al.
Molecular coproscopy: dung and diet of the extinct ground sloth _Nothrotheriops shastensis_. _Science_ 281, 402–406 (1998). THIS ARTICLE REPORTS THE FIRST APPLICATION OF NEXT-GENERATION DNA
SEQUENCING TO ANCIENT SPECIMENS AND DESCRIBES THE METAGENOMIC NATURE OF PALEONTOLOGICAL REMAINS TOGETHER WITH MEGABASE-SCALE DATA FROM THE WOOLLY MAMMOTH GENOME. ADS Google Scholar * Bon,
C. et al. Coprolites as a source of information on the genome and diet of the cave hyena. _Proc. Biol. Sci._ 279, 2825–2830 (2012). Google Scholar * Willerslev, E. et al. Diverse plant and
animal genetic records from Holocene and Pleistocene sediments. _Science_ 300, 791–795 (2003). THIS ARTICLE REPORTS THE FIRST ANALYSIS OF ENVIRONMENTAL DNA PRIOR TO THE ADVENT OF
NEXT-GENERATION DNA SEQUENCING AND ESTABLISHES THE LONG-TERM DNA PERSISTENCE OF PALEOCOMMUNITIES WITHIN SEDIMENTS. ADS Google Scholar * Willerslev, E. et al. Ancient biomolecules from deep
ice cores reveal a forested southern Greenland. _Science_ 317, 111–114 (2007). ADS Google Scholar * Coolen, M. J. & Overmann, J. Analysis of subfossil molecular remains of purple
sulfur bacteria in a lake sediment. _Appl. Environ. Microbiol._ 64, 4513–4521 (1998). Google Scholar * Bardill, J. et al. Advancing the ethics of paleogenomics. _Science_ 360, 384–385
(2018). ADS Google Scholar * Prendergast, M. E. & Sawchuk, E. Boots on the ground in Africa’s ancient DNA ‘revolution’: archaeological perspectives on ethics and best practices.
_Antiquity_ 92, 803–815 (2018). Google Scholar * Alberti, F. et al. Optimized DNA sampling of ancient bones using computed tomography scans. _Mol. Ecol. Resour._ 18, 1196–1208 (2018).
Google Scholar * Sirak, K. A. et al. A minimally-invasive method for sampling human petrous bones from the cranial base for ancient DNA analysis. _BioTechniques_ 62, 283–289 (2017). Google
Scholar * Harney, É. et al. A minimally destructive protocol for DNA extraction from ancient teeth. Preprint at _bioRxiv_ https://doi.org/10.1101/2020.08.19.256412(2020). * Patzold, F.,
Zilli, A. & Hundsdoerfer, A. K. Advantages of an easy-to-use DNA extraction method for minimal-destructive analysis of collection specimens. _PLoS ONE_ 15, e0235222 (2020). Google
Scholar * Sugita, N. et al. Non-destructive DNA extraction from herbarium specimens: a method particularly suitable for plants with small and fragile leaves. _J. Plant Res._ 133, 133–141
(2020). Google Scholar * Austin, R. M., Sholts, S. B., Williams, L., Kistler, L. & Hofman, C. A. Opinion: to curate the molecular past, museums need a carefully considered set of best
practices. _Proc. Natl Acad. Sci. USA_ 116, 1471–1474 (2019). Google Scholar * Pálsdóttir, A. H., Bläuer, A., Rannamäe, E., Boessenkool, S. & Hallsson, J. H. Not a limitless resource:
ethics and guidelines for destructive sampling of archaeofaunal remains. _R. Soc. Open Sci._ 6, 191059 (2019). ADS Google Scholar * Claw, K. G. et al. A framework for enhancing ethical
genomic research with Indigenous communities. _Nat. Commun._ 9, 2957 (2018). ADS Google Scholar * Wagner, J. K. et al. Fostering responsible research on ancient DNA. _Am. J. Hum. Genet._
107, 183–195 (2020). Google Scholar * Eisenmann, S. et al. Reconciling material cultures in archaeology with genetic data: the nomenclature of clusters emerging from archaeogenomic
analysis. _Sci. Rep._ 8, 13003 (2018). ADS Google Scholar * Frieman, C. J. & Hofmann, D. Present pasts in the archaeology of genetics, identity, and migration in Europe: a critical
essay. _World Archaeol._ 51, 528–545 (2019). Google Scholar * Furholt, M. De-contaminating the aDNA — archaeology dialogue on mobility and migration: discussing the culture-historical
legacy. _CSA_ 27, 53–68 (2019). Google Scholar * Hakenbeck, S. E. Genetics, archaeology and the far right: an unholy Trinity. _World Archaeol._ 51, 517–527 (2019). Google Scholar * Fulton,
T. L. & Shapiro, B. Setting up an ancient DNA laboratory. _Methods Mol. Biol._ 1963, 1–13 (2019). Google Scholar * Dabney, J., Meyer, M. & Pääbo, S. Ancient DNA damage. _Cold
Spring Harb. Perspect. Biol._ 5, a012567 (2013). Google Scholar * Höss, M. & Pääbo, S. DNA extraction from Pleistocene bones by a silica-based purification method. _Nucleic Acids Res._
21, 3913–3914 (1993). Google Scholar * Green, R. E. et al. The Neandertal genome and ancient DNA authenticity. _EMBO J._ 28, 2494–2502 (2009). Google Scholar * Korlević, P., Talamo, S.
& Meyer, M. A combined method for DNA analysis and radiocarbon dating from a single sample. _Sci. Rep._ 8, 4127 (2018). ADS Google Scholar * Fagernäs, Z. et al. A unified protocol for
simultaneous extraction of DNA and proteins from archaeological dental calculus. _J. Archaeol. Sci._ 118, 105135 (2020). Google Scholar * Rohland, N. & Hofreiter, M. Ancient DNA
extraction from bones and teeth. _Nat. Protoc._ 2, 1756–1762 (2007). Google Scholar * Rohland, N., Glocke, I., Aximu-Petri, A. & Meyer, M. Extraction of highly degraded DNA from ancient
bones, teeth and sediments for high-throughput sequencing. _Nat. Protoc._ 13, 2447–2461 (2018). Google Scholar * Glocke, I. & Meyer, M. Extending the spectrum of DNA sequences
retrieved from ancient bones and teeth. _Genome Res._ 27, 1230–1237 (2017). Google Scholar * Wales, N., Andersen, K., Cappellini, E., Ávila-Arcos, M. C. & Gilbert, M. T. P. Optimization
of DNA recovery and amplification from non-carbonized archaeobotanical remains. _PLoS ONE_ 9, e86827 (2014). ADS Google Scholar * Wales, N. et al. in _Ancient DNA: Methods and Protocols_
(eds. Shapiro, B. et al.) 45–55 (Springer, 2019). * Meyer, M. et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. _Nature_ 505, 403–406 (2014). ADS Google Scholar
* Höss, M., Jaruga, P., Zastawny, T. H., Dizdaroglu, M. & Pääbo, S. DNA damage and DNA sequence retrieval from ancient tissues. _Nucleic Acids Res._ 24, 1304–1307 (1996). Google Scholar
* Briggs, A. W. et al. Patterns of damage in genomic DNA sequences from a Neandertal. _Proc. Natl Acad. Sci. USA_ 104, 14616–14621 (2007). THIS ARTICLE PRESENTS THE FIRST STATISTICAL MODEL
OF POST-MORTEM DNA DEGRADATION AND ITS IMPACT ON NUCLEOTIDE MISINCORPORATION PATTERNS, WHICH PROVIDES THE BASIS OF IMPORTANT CRITERIA FOR DATA AUTHENTICATION. ADS Google Scholar * Sawyer,
S., Krause, J., Guschanski, K., Savolainen, V. & Pääbo, S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. _PLoS ONE_ 7, e34131 (2012). ADS
Google Scholar * Ho, S. Y. W., Heupink, T. H., Rambaut, A. & Shapiro, B. Bayesian estimation of sequence damage in ancient DNA. _Mol. Biol. Evol._ 24, 1416–1422 (2007). Google Scholar
* Axelsson, E., Willerslev, E., Gilbert, M. T. P. & Nielsen, R. The effect of ancient DNA damage on inferences of demographic histories. _Mol. Biol. Evol._ 25, 2181–2187 (2008). Google
Scholar * Briggs, A. W. et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. _Nucleic Acids Res._ 38, e87 (2010). THIS ARTICLE PRESENTS AN
EXPERIMENTAL PROCEDURE BASED ON ENZYMATIC DIGESTION THAT ELIMINATES NUCLEOTIDE MISINCORPORATIONS FROM ANCIENT SEQUENCE DATA, WHICH PROVIDED THE BASIS FOR ROBUST STATISTICAL INFERENCE,
MINIMALLY IMPACTED BY SPURIOUS ALLELIC VARIATION, AND HAS PAVED THE WAY TO ADNA METHYLATION MAPS. Google Scholar * Rohland, N., Harney, E., Mallick, S., Nordenfelt, S. & Reich, D.
Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 370, 20130624 (2015). THIS STUDY REPORTS THE FIRST METHOD FOR DNA LIBRARY
PREPARATION THAT IS COMPATIBLE WITH FULL AUTOMATION AND ALSO DESCRIBES VARIOUS APPROACHES AIMED AT AUTHENTICATING DATA WHILE MINIMIZING THE IMPACT OF POST-MORTEM DNA MISINCORPORATION ON
DOWNSTREAM ANALYSES. Google Scholar * Hebert, P. D. N., Cywinska, A., Ball, S. L. & deWaard, J. R. Biological identifications through DNA barcodes. _Proc. Biol. Sci._ 270, 313–321
(2003). Google Scholar * Taberlet, P. et al. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. _Nucleic Acids Res._ 35, e14 (2007). Google Scholar *
Hofreiter, M., Mead, J. I., Martin, P. & Poinar, H. N. Molecular caving. _Curr. Biol._ 13, R693–R695 (2003). Google Scholar * Parducci, L. et al. Glacial survival of boreal trees in
northern Scandinavia. _Science_ 335, 1083–1086 (2012). ADS Google Scholar * Alsos, I. G. et al. The role of sea ice for vascular plant dispersal in the Arctic. _Biol. Lett._ 12, 20160264
(2016). Google Scholar * Crump, S. E. et al. Arctic shrub colonization lagged peak postglacial warmth: molecular evidence in lake sediment from Arctic Canada. _Glob. Chang. Biol._ 25,
4244–4256 (2019). ADS Google Scholar * Ziesemer, K. A. et al. Intrinsic challenges in ancient microbiome reconstruction using 16S rRNA gene amplification. _Sci. Rep._ 5, 16498 (2015). ADS
Google Scholar * Smith, O. et al. Archaeology. Sedimentary DNA from a submerged site reveals wheat in the British Isles 8000 years ago. _Science_ 347, 998–1001 (2015). ADS Google Scholar
* Parducci, L. et al. Shotgun environmental DNA, pollen, and macrofossil analysis of lateglacial lake sediments from southern Sweden. _Front. Ecol. Evol._ 7, 189 (2019). Google Scholar *
Gaffney, V. et al. Multi-proxy characterisation of the Storegga tsunami and its impact on the early holocene landscapes of the southern North Sea. _Geosciences_ 10, 270 (2020). ADS Google
Scholar * Cribdon, B., Ware, R., Smith, O., Gaffney, V. & Allaby, R. G. PIA: more accurate taxonomic assignment of metagenomic data demonstrated on sedaDNA from the North Sea. _Front.
Ecol. Evol._ 8, 84 (2020). Google Scholar * Noonan, J. P. et al. Genomic sequencing of Pleistocene cave bears. _Science_ 309, 597–599 (2005). ADS Google Scholar * Noonan, J. P. et al.
Sequencing and analysis of Neanderthal genomic DNA. _Science_ 314, 1113–1118 (2006). ADS Google Scholar * Bentley, D. R. et al. Accurate whole human genome sequencing using reversible
terminator chemistry. _Nature_ 456, 53–59 (2008). ADS Google Scholar * Meyer, M. & Kircher, M. Illumina sequencing library preparation for highly multiplexed target capture and
sequencing. _Cold Spring Harb Protoc_ 2010, pdb.prot5448 (2010). Google Scholar * Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on
the Illumina platform. _Nucleic Acids Res._ 40, e3 (2012). Google Scholar * Gansauge, M.-T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged
DNA. _Nat. Protoc._ 8, 737–748 (2013). Google Scholar * Gansauge, M.-T., Aximu-Petri, A., Nagel, S. & Meyer, M. Manual and automated preparation of single-stranded DNA libraries for the
sequencing of DNA from ancient biological remains and other sources of highly degraded DNA. _Nat. Protoc._ 15, 2279–2300 (2020). Google Scholar * Gansauge, M.-T. et al. Single-stranded DNA
library preparation from highly degraded DNA using T4 DNA ligase. _Nucleic Acids Res._ 45, e79 (2017). Google Scholar * Harkins, K. M. et al. A novel NGS library preparation method to
characterize native termini of fragmented DNA. _Nucleic Acids Res._ 48, e47 (2020). Google Scholar * Carøe, C. et al. Single-tube library preparation for degraded DNA. _Methods Ecol. Evol._
9, 410–419 (2018). Google Scholar * Fages, A. et al. Tracking five millennia of horse management with extensive ancient genome time series. _Cell_ 177, 1419–1435.e31 (2019). Google Scholar
* van der Valk, T., Vezzi, F., Ormestad, M., Dalén, L. & Guschanski, K. Index hopping on the Illumina HiseqX platform and its consequences for ancient DNA studies. _Mol. Ecol. Resour._
20, 1171–1181 (2019). Google Scholar * Dabney, J. & Meyer, M. Length and GC-biases during sequencing library amplification: a comparison of various polymerase-buffer systems with
ancient and modern DNA sequencing libraries. _BioTechniques_ 52, 87–94 (2012). Google Scholar * Seguin-Orlando, A. et al. Amplification of TruSeq ancient DNA libraries with AccuPrime Pfx:
consequences on nucleotide misincorporation and methylation patterns. _STAR: Sci. Technol. Archaeol. Res._ 1, 1–9 (2015). Google Scholar * Meyer, M. et al. From micrograms to picograms:
quantitative PCR reduces the material demands of high-throughput sequencing. _Nucleic Acids Res._ 36, e5 (2008). Google Scholar * Thompson, J. R., Marcelino, L. A. & Polz, M. F.
Heteroduplexes in mixed-template amplifications: formation, consequence and elimination by ‘reconditioning PCR’. _Nucleic Acids Res._ 30, 2083–2088 (2002). Google Scholar * Max Planck
Institute for the Science of Human History. MPI-SHH archaeogenetics. _protocols.io_ https://www.protocols.io/workspaces/mpishh-archaeogenetics (2020). * Burbano, H. A. et al. Targeted
investigation of the Neandertal genome by array-based sequence capture. _Science_ 328, 723–725 (2010). ADS Google Scholar * Devault, A. M. et al. Second-pandemic strain of _Vibrio
cholerae_ from the Philadelphia cholera outbreak of 1849. _N. Engl. J. Med._ 370, 334–340 (2014). Google Scholar * Enk, J. M. et al. Ancient whole genome enrichment using baits built from
modern DNA. _Mol. Biol. Evol._ 31, 1292–1294 (2014). Google Scholar * Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. _Nature_ 522,
207–211 (2015). ADS Google Scholar * Cruz-Dávalos, D. I. et al. Experimental conditions improving in-solution target enrichment for ancient DNA. _Mol. Ecol. Resour._ 17, 508–522 (2017).
Google Scholar * Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. _Nature_ 528, 499–503 (2015). ADS Google Scholar * Cruz-Dávalos, D. I. et al. In-solution
Y-chromosome capture-enrichment on ancient DNA libraries. _BMC Genomics_ 19, 608 (2018). Google Scholar * Günther, T. & Nettelblad, C. The presence and impact of reference bias on
population genomic studies of prehistoric human populations. _PLoS Genet._ 15, e1008302 (2019). Google Scholar * Lachance, J. & Tishkoff, S. A. SNP ascertainment bias in population
genetic analyses: why it is important, and how to correct it. _Bioessays_ 35, 780–786 (2013). Google Scholar * Lang, P. L. M. et al. Hybridization ddRAD-sequencing for population genomics
of nonmodel plants using highly degraded historical specimen DNA. _Mol. Ecol. Resour._ 20, 1228–1247 (2020). Google Scholar * Suchan, T. et al. Hybridization capture using RAD probes
(hyRAD), a new tool for performing genomic analyses on collection specimens. _PLoS ONE_ 11, e0151651 (2016). Google Scholar * Hernandez-Rodriguez, J. et al. The impact of endogenous
content, replicates and pooling on genome capture from faecal samples. _Mol. Ecol. Resour._ 18, 319–333 (2018). Google Scholar * Smith, O. et al. Genomic methylation patterns in
archaeological barley show de-methylation as a time-dependent diagenetic process. _Sci. Rep._ 4, 5559 (2014). Google Scholar * Keller, A. et al. New insights into the Tyrolean Iceman’s
origin and phenotype as inferred by whole-genome sequencing. _Nat. Commun._ 3, 698 (2012). ADS Google Scholar * Mak, S. S. T. et al. Comparative performance of the BGISEQ-500 vs Illumina
HiSeq2500 sequencing platforms for palaeogenomic sequencing. _Gigascience_ 6, 1–13 (2017). ADS Google Scholar * Martins, R. F., Kampmann, M.-L. & Förster, D. W. Sequencing library
preparation from degraded samples for non-illumina sequencing platforms. _Methods Mol. Biol._ 1963, 85–92 (2019). Google Scholar * Kircher, M., Stenzel, U. & Kelso, J. Improved base
calling for the Illumina Genome Analyzer using machine learning strategies. _Genome Biol._ 10, R83 (2009). Google Scholar * Renaud, G., Kircher, M., Stenzel, U. & Kelso, J. freeIbis: an
efficient basecaller with calibrated quality scores for Illumina sequencers. _Bioinformatics_ 29, 1208–1209 (2013). Google Scholar * Costello, M. et al. Characterization and remediation of
sample index swaps by non-redundant dual indexing on massively parallel sequencing platforms. _BMC Genomics_ 19, 332 (2018). Google Scholar * Dolle, D. et al. CASCADE: a custom-made
archiving system for the conservation of ancient DNA experimental data. _Front. Ecol. Evol._ 8, 185 (2020). ADS Google Scholar * Renaud, G., Stenzel, U. & Kelso, J. leeHom: adaptor
trimming and merging for Illumina sequencing reads. _Nucleic Acids Res._ 42, e141 (2014). Google Scholar * Schubert, M., Lindgreen, S. & Orlando, L. AdapterRemoval v2: rapid adapter
trimming, identification, and read merging. _BMC Res. Notes_ 9, 88 (2016). Google Scholar * Schuenemann, V. J. et al. Genome-wide comparison of medieval and modern _Mycobacterium leprae_.
_Science_ 341, 179–183 (2013). ADS Google Scholar * Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. _Bioinformatics_ 26, 589–595 (2010).
Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Google Scholar * Renaud, G., Hanghøj, K., Willerslev, E.
& Orlando, L. gargammel: a sequence simulator for ancient DNA. _Bioinformatics_ 33, 577–579 (2017). Google Scholar * Schubert, M. et al. Improving ancient DNA read mapping against
modern reference genomes. _BMC Genomics_ 13, 178 (2012). Google Scholar * Shapiro, B. & Hofreiter, M. A paleogenomic perspective on evolution and gene function: new insights from
ancient DNA. _Science_ 343, 1236573 (2014). Google Scholar * Cahill, J. A. et al. Genomic evidence of widespread admixture from polar bears into brown bears during the last ice age. _Mol.
Biol. Evol._ 35, 1120–1129 (2018). Google Scholar * Poullet, M. & Orlando, L. Assessing DNA sequence alignment methods for characterizing ancient genomes and methylomes. _Front. Ecol.
Evolut._ 8, 105 (2020). Google Scholar * Seguin-Orlando, A. et al. Ligation bias in Illumina next-generation DNA libraries: implications for sequencing ancient genomes. _PLoS ONE_ 8, e78575
(2013). ADS Google Scholar * Garrison, E. et al. Variation graph toolkit improves read mapping by representing genetic variation in the reference. _Nat. Biotechnol._ 36, 875–879 (2018).
Google Scholar * Schubert, M. et al. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. _Nat. Protoc._ 9, 1056–1082
(2014). Google Scholar * Peltzer, A. et al. EAGER: efficient ancient genome reconstruction. _Genome Biol._ 17, 60 (2016). Google Scholar * Renaud, G., Slon, V., Duggan, A. T. & Kelso,
J. Schmutzi: estimation of contamination and endogenous mitochondrial consensus calling for ancient DNA. _Genome Biol._ 16, 224 (2015). Google Scholar * Rasmussen, M. et al. An aboriginal
Australian genome reveals separate human dispersals into Asia. _Science_ 334, 94–98 (2011). ADS Google Scholar * Korneliussen, T. S., Moltke, I., Albrechtsen, A. & Nielsen, R.
Calculation of Tajima’s _D_ and other neutrality test statistics from low depth next-generation sequencing data. _BMC Bioinforma._ 14, 289 (2013). Google Scholar * Peyrègne, S. & Peter,
B. M. AuthentiCT: a model of ancient DNA damage to estimate the proportion of present-day DNA contamination. _Genome Biol._ 21, 246 (2020). Google Scholar * Meyer, M. et al. Nuclear DNA
sequences from the Middle Pleistocene Sima de los Huesos hominins. _Nature_ 531, 504–507 (2016). ADS Google Scholar * Skoglund, P. et al. Origins and genetic legacy of Neolithic farmers
and hunter-gatherers in Europe. _Science_ 336, 466–469 (2012). ADS Google Scholar * Nakatsuka, N. et al. A Paleogenomic reconstruction of the deep population history of the Andes. _Cell_
181, 1131–1145.e21 (2020). Google Scholar * Skoglund, P., Ersmark, E., Palkopoulou, E. & Dalén, L. Ancient wolf genome reveals an early divergence of domestic dog ancestors and
admixture into high-latitude breeds. _Curr. Biol._ 25, 1515–1519 (2015). Google Scholar * Soraggi, S., Wiuf, C. & Albrechtsen, A. Powerful inference with the _D_-statistic on
low-coverage whole-genome data. _G3_ 8, 551–566 (2018). Google Scholar * Skoglund, P., Storå, J., Götherström, A. & Jakobsson, M. Accurate sex identification of ancient human remains
using DNA shotgun sequencing. _J. Archaeol. Sci._ 40, 4477–4482 (2013). Google Scholar * Mittnik, A., Wang, C.-C., Svoboda, J. & Krause, J. A molecular approach to the sexing of the
triple burial at the upper Paleolithic site of Dolní Věstonice. _PLoS ONE_ 11, e0163019 (2016). Google Scholar * Schubert, M. et al. Zonkey: a simple, accurate and sensitive pipeline to
genetically identify equine F1-hybrids in archaeological assemblages. _J. Archaeol. Sci._ 78, 147–157 (2017). Google Scholar * Lipatov, M., Sanjeev, K., Patro, R. & Veeramah, K. Maximum
likelihood estimation of biological relatedness from low coverage sequencing data. Preprint at _bioRxiv_ https://doi.org/10.1101/023374 (2015). Article Google Scholar * Kennett, D. J. et
al. Archaeogenomic evidence reveals prehistoric matrilineal dynasty. _Nat. Commun._ 8, 14115 (2017). ADS Google Scholar * Monroy Kuhn, J. M., Jakobsson, M. & Günther, T. Estimating
genetic kin relationships in prehistoric populations. _PLoS ONE_ 13, e0195491 (2018). Google Scholar * Ziesemer, K. A. et al. The efficacy of whole human genome capture on ancient dental
calculus and dentin. _Am. J. Phys. Anthropol._ 168, 496–509 (2019). Google Scholar * Mittnik, A. et al. Kinship-based social inequality in Bronze Age Europe. _Science_ 366, 731–734 (2019).
ADS Google Scholar * Olalde, I. et al. The Beaker phenomenon and the genomic transformation of northwest Europe. _Nature_ 555, 190–196 (2018); erratum 555, 543 (2018). ADS Google Scholar
* Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. _PLoS Genet._ 2, e190 (2006). Google Scholar * McVean, G. A genealogical interpretation of principal
components analysis. _PLoS Genet._ 5, e1000686 (2009). Google Scholar * Meisner, J. & Albrechtsen, A. Inferring population structure and admixture proportions in low-depth NGS data.
_Genetics_ 210, 719–731 (2018). Google Scholar * Lazaridis, I. et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. _Nature_ 513, 409–413 (2014). ADS
Google Scholar * Engelhardt, B. E. & Stephens, M. Analysis of population structure: a unifying framework and novel methods based on sparse factor analysis. _PLoS Genet._ 6, e1001117
(2010). Google Scholar * Skoglund, P., Sjödin, P., Skoglund, T., Lascoux, M. & Jakobsson, M. Investigating population history using temporal genetic differentiation. _Mol. Biol. Evol._
31, 2516–2527 (2014). Google Scholar * Malaspinas, A.-S. et al. bammds: a tool for assessing the ancestry of low-depth whole-genome data using multidimensional scaling (MDS).
_Bioinformatics_ 30, 2962–2964 (2014). Google Scholar * Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. _Genome Res._ 19,
1655–1664 (2009). Google Scholar * Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. _Genetics_ 155, 945–959 (2000). Google
Scholar * Skotte, L., Korneliussen, T. S. & Albrechtsen, A. Estimating individual admixture proportions from next generation sequencing data. _Genetics_ 195, 693–702 (2013). Google
Scholar * Joseph, T. A. & Pe’er, I. Inference of population structure from time-series genotype data. _Am. J. Hum. Genet._ 105, 317–333 (2019). Google Scholar * Reich, D., Thangaraj,
K., Patterson, N., Price, A. L. & Singh, L. Reconstructing Indian population history. _Nature_ 461, 489–494 (2009). ADS Google Scholar * Patterson, N. et al. Ancient admixture in human
history. _Genetics_ 192, 1065–1093 (2012). THIS ARTICLE DESCRIBES THE STATISTICAL METHODOLOGY FORMING THE CORE OF MOST SUBSEQUENT ADNA STUDIES AND AIMS AT INVESTIGATING POPULATION STRUCTURE
AND ADMIXTURE PATTERNS. Google Scholar * Raghavan, M. et al. Upper Palaeolithic Siberian genome reveals dual ancestry of native Americans. _Nature_ 505, 87–91 (2014). ADS Google Scholar
* Reich, D. et al. Denisova admixture and the first modern human dispersals into Southeast Asia and Oceania. _Am. J. Hum. Genet._ 89, 516–528 (2011). Google Scholar * Cassidy, L. M. et al.
A dynastic elite in monumental Neolithic society. _Nature_ 582, 384–388 (2020). ADS Google Scholar * McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation.
_Nat. Genet._ 48, 1279–1283 (2016). Google Scholar * Antonio, M. L. et al. Ancient Rome: a genetic crossroads of Europe and the Mediterranean. _Science_ 366, 708–714 (2019). ADS Google
Scholar * Sirugo, G., Williams, S. M. & Tishkoff, S. A. The missing diversity in human genetic studies. _Cell_ 177, 26–31 (2019). Google Scholar * Allentoft, M. E. et al. Population
genomics of Bronze Age Eurasia. _Nature_ 522, 167–172 (2015). ADS Google Scholar * Scheib, C. L. et al. East Anglian early Neolithic monument burial linked to contemporary Megaliths. _Ann.
Hum. Biol._ 46, 145–149 (2019). Google Scholar * Ringbauer, H., Novembre, J. & Steinrücken, M. Detecting runs of homozygosity from low-coverage ancient DNA. Preprint at _bioRxiv_
https://doi.org/10.1101/2020.05.31.126912 (2020). Article Google Scholar * Lawson, D. J., Hellenthal, G., Myers, S. & Falush, D. Inference of population structure using dense haplotype
data. _PLoS Genet._ 8, e1002453 (2012). Google Scholar * Burger, J. et al. Low prevalence of lactase persistence in Bronze Age Europe indicates ongoing strong selection over the last 3,000
years. _Curr. Biol._ 30, 1–9 (2020). Google Scholar * Maixner, F. et al. The 5,300-year-old _Helicobacter pylori_ genome of the Iceman. _Science_ 351, 162–165 (2016). ADS Google Scholar
* Lugli, G. A. et al. Ancient bacteria of the Ötzi’s microbiome: a genomic tale from the Copper Age. _Microbiome_ 5, 5 (2017). Google Scholar * Borry, M. et al. CoproID predicts the source
of coprolites and paleofeces using microbiome composition and host DNA content. _PeerJ_ 8, e9001 (2020). Google Scholar * Rifkin, R. F. et al. Multi-proxy analyses of a mid-15th century
Middle Iron Age Bantu-speaker palaeo-faecal specimen elucidates the configuration of the ‘ancestral’ sub-Saharan African intestinal microbiome. _Microbiome_ 8, 62 (2020). Google Scholar *
Tett, A. et al. The _Prevotella copri_ complex comprises four distinct clades underrepresented in westernized populations. _Cell Host Microbe_ 26, 666–679.e7 (2019). Google Scholar *
Vågene, Å. J. et al. _Salmonella enterica_ genomes from victims of a major sixteenth-century epidemic in Mexico. _Nat. Ecol. Evol._ 2, 520–528 (2018). Google Scholar * Wood, D. E. &
Salzberg, S. L. Kraken: ultrafast metagenomic sequence classification using exact alignments. _Genome Biol._ 15, R46 (2014). Google Scholar * Velsko, I. M., Frantz, L. A. F., Herbig, A.,
Larson, G. & Warinner, C. Selection of appropriate metagenome taxonomic classifiers for ancient microbiome research. _mSystems_ 3, e00080-18 (2018). Google Scholar * Eisenhofer, R.
& Weyrich, L. S. Assessing alignment-based taxonomic classification of ancient microbial DNA. _PeerJ_ 7, e6594 (2019). Google Scholar * Segata, N. et al. Metagenomic microbial community
profiling using unique clade-specific marker genes. _Nat. Methods_ 9, 811–814 (2012). Google Scholar * Hübler, R. et al. HOPS: automated detection and authentication of pathogen DNA in
archaeological remains. _Genome Biol._ 20, 280 (2019). Google Scholar * Louvel, G., Der Sarkissian, C., Hanghøj, K. & Orlando, L. metaBIT, an integrative and automated metagenomic
pipeline for analysing microbial profiles from high-throughput sequencing shotgun data. _Mol. Ecol. Resour._ 16, 1415–1427 (2016). Google Scholar * Knights, D. et al. Bayesian
community-wide culture-independent microbial source tracking. _Nat. Methods_ 8, 761–763 (2011). Google Scholar * Llamas, B. et al. High-resolution analysis of cytosine methylation in
ancient DNA. _PLoS ONE_ 7, e30226 (2012). ADS Google Scholar * Smith, R. W. A., Monroe, C. & Bolnick, D. A. Detection of cytosine methylation in ancient DNA from five Native American
populations using bisulfite sequencing. _PLoS ONE_ 10, e0125344 (2015). Google Scholar * Wagner, S., Plomion, C. & Orlando, L. Uncovering signatures of DNA methylation in ancient plant
remains from patterns of post-mortem DNA damage. _Front. Ecol. Evol._ 8, 11 (2020). Google Scholar * Hanghøj, K. et al. Fast, accurate and automatic ancient nucleosome and methylation maps
with epiPALEOMIX. _Mol. Biol. Evol._ 33, 3284–3298 (2016). Google Scholar * Hanghøj, K., Renaud, G., Albrechtsen, A. & Orlando, L. DamMet: ancient methylome mapping accounting for
errors, true variants, and post-mortem DNA damage. _Gigascience_ 8, giz025 (2019). Google Scholar * Pääbo, S. et al. Genetic analyses from ancient DNA. _Annu. Rev. Genet._ 38, 645–679
(2004). Google Scholar * Racimo, F., Marnetto, D. & Huerta-Sánchez, E. Signatures of archaic adaptive introgression in present-day human populations. _Mol. Biol. Evol._ 34, 296–317
(2017). Google Scholar * de Barros Damgaard, P. et al. The first horse herders and the impact of early Bronze Age steppe expansions into Asia. _Science_ 360, eaar7711 (2018). Google Scholar
* Damgaard, PdeB. et al. 137 ancient human genomes from across the Eurasian steppes. _Nature_ 557, 369–374 (2018); author correction 563, E16 (2018). ADS Google Scholar * Narasimhan, V.
M. et al. The formation of human populations in South and Central Asia. _Science_ 365, eaat7487 (2019). Google Scholar * Seguin-Orlando, A. et al. Paleogenomics. Genomic structure in
Europeans dating back at least 36,200 years. _Science_ 346, 1113–1118 (2014). ADS Google Scholar * Sikora, M. et al. Ancient genomes show social and reproductive behavior of early Upper
Paleolithic foragers. _Science_ 358, 659–662 (2017). ADS Google Scholar * Fu, Q. et al. Genome sequence of a 45,000-year-old modern human from western Siberia. _Nature_ 514, 445–449
(2014). ADS Google Scholar * Yang, M. A. et al. 40,000-year-old individual from Asia provides insight into early population structure in Eurasia. _Curr. Biol._ 27, 3202–3208.e9 (2017).
Google Scholar * Olalde, I. & Posth, C. Latest trends in archaeogenetic research of west Eurasians. _Curr. Opin. Genet. Dev._ 62, 36–43 (2020). Google Scholar * Yang, M. A. et al.
Ancient DNA indicates human population shifts and admixture in northern and southern China. _Science_ 369, 282–288 (2020). ADS Google Scholar * Lipson, M. et al. Population turnover in
remote Oceania shortly after initial settlement. _Curr. Biol._ 28, 1157–1165.e7 (2018). Google Scholar * Lipson, M. et al. Ancient genomes document multiple waves of migration in Southeast
Asian prehistory. _Science_ 361, 92–95 (2018). ADS Google Scholar * McColl, H. et al. The prehistoric peopling of Southeast Asia. _Science_ 361, 88–92 (2018). ADS Google Scholar *
Lazaridis, I. et al. Genetic origins of the Minoans and Mycenaeans. _Nature_ 548, 214–218 (2017). ADS Google Scholar * Wang, K. et al. Ancient genomes reveal complex patterns of population
movement, interaction, and replacement in sub-Saharan Africa. _Sci. Adv._ 6, eaaz0183 (2020). ADS Google Scholar * Sánchez-Quinto, F. et al. Genomic affinities of two 7,000-year-old
Iberian hunter-gatherers. _Curr. Biol._ 22, 1494–1499 (2012). Google Scholar * Lipson, M. et al. Parallel palaeogenomic transects reveal complex genetic history of early European farmers.
_Nature_ 551, 368–372 (2017). ADS Google Scholar * Jeong, C. et al. The genetic history of admixture across inner Eurasia. _Nat. Ecol. Evol._ 3, 966–976 (2019). Google Scholar *
Moreno-Mayar, J. V. et al. Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. _Nature_ 553, 203–207 (2018). ADS Google Scholar * Moreno-Mayar, J. V.
et al. Early human dispersals within the Americas. _Science_ 362, eaav2621 (2018). ADS Google Scholar * Flegontov, P. et al. Palaeo-Eskimo genetic ancestry and the peopling of Chukotka
and North America. _Nature_ 570, 236–240 (2019). ADS Google Scholar * Nieves-Colón, M. A. et al. Ancient DNA reconstructs the genetic legacies of precontact Puerto Rico communities. _Mol.
Biol. Evol._ 37, 611–626 (2020). Google Scholar * Nägele, K. et al. Genomic insights into the early peopling of the Caribbean. _Science_ 369, 456–460 (2020). ADS Google Scholar *
Schroeder, H. et al. Genome-wide ancestry of 17th-century enslaved Africans from the Caribbean. _Proc. Natl Acad. Sci. USA_ 112, 3669–3673 (2015). ADS Google Scholar * Barquera, R. et al.
Origin and health status of first-generation Africans from early Colonial Mexico. _Curr. Biol._ 30, 2078–2091.e11 (2020). Google Scholar * Larson, G. et al. Current perspectives and the
future of domestication studies. _Proc. Natl Acad. Sci. USA_ 111, 6139–6146 (2014). ADS Google Scholar * Larson, G. et al. Phylogeny and ancient DNA of Sus provides insights into neolithic
expansion in island Southeast Asia and Oceania. _Proc. Natl Acad. Sci. USA_ 104, 4834–4839 (2007). ADS Google Scholar * Kistler, L. et al. Ancient plant genomics in archaeology, herbaria,
and the environment. _Annu. Rev. Plant. Biol._ 71, 605–629 (2020). Google Scholar * Ní Leathlobhair, M. et al. The evolutionary history of dogs in the Americas. _Science_ 361, 81–85
(2018). ADS Google Scholar * Frantz, L. A. F. et al. Genomic and archaeological evidence suggest a dual origin of domestic dogs. _Science_ 352, 1228–1231 (2016). ADS Google Scholar *
Sinding, M.-H. S. et al. Arctic-adapted dogs emerged at the Pleistocene–Holocene transition. _Science_ 368, 1495–1499 (2020). ADS Google Scholar * Gaunitz, C. et al. Ancient genomes
revisit the ancestry of domestic and Przewalski’s horses. _Science_ 360, 111–114 (2018). ADS Google Scholar * Frantz, L. A. F. et al. Ancient pigs reveal a near-complete genomic turnover
following their introduction to Europe. _Proc. Natl Acad. Sci. USA_ 116, 17231–17238 (2019). Google Scholar * Gutaker, R. M. et al. The origins and adaptation of European potatoes
reconstructed from historical genomes. _Nat. Ecol. Evol._ 3, 1093–1101 (2019). Google Scholar * Ameen, C. et al. Specialized sledge dogs accompanied Inuit dispersal across the North
American Arctic. _Proc. R. Soc. Lond. B Biol. Sci._ 286, 20191929 (2019). Google Scholar * da Fonseca, R. R. et al. The origin and evolution of maize in the Southwestern United States.
_Nat. Plants_ 1, 14003 (2015). MathSciNet Google Scholar * Gutaker, R. M. et al. Flax latitudinal adaptation at LuTFL1 altered architecture and promoted fiber production. _Sci. Rep._ 9,
976 (2019). ADS Google Scholar * Park, S. D. E. et al. Genome sequencing of the extinct Eurasian wild aurochs, _Bos primigenius_, illuminates the phylogeography and evolution of cattle.
_Genome Biol._ 16, 234 (2015). Google Scholar * Verdugo, M. P. et al. Ancient cattle genomics, origins, and rapid turnover in the Fertile Crescent. _Science_ 365, 173–176 (2019). ADS
Google Scholar * Daly, K. G. et al. Ancient goat genomes reveal mosaic domestication in the Fertile Crescent. _Science_ 361, 85–88 (2018). ADS Google Scholar * Kistler, L. et al.
Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. _Science_ 362, 1309–1313 (2018). ADS Google Scholar * Allaby, R. G., Ware, R. L. & Kistler, L. A
re-evaluation of the domestication bottleneck from archaeogenomic evidence. _Evol. Appl._ 12, 29–37 (2019). Google Scholar * Scott, M. F. et al. A 3,000-year-old Egyptian emmer wheat
genome reveals dispersal and domestication history. _Nat. Plants_ 5, 1120–1128 (2019). Google Scholar * Wales, N. et al. Ancient DNA reveals the timing and persistence of organellar genetic
bottlenecks over 3,000 years of sunflower domestication and improvement. _Evol. Appl._ 12, 38–53 (2018). Google Scholar * Smith, O. et al. A domestication history of dynamic adaptation and
genomic deterioration in sorghum. _Nat. Plants_ 5, 369–379 (2019). Google Scholar * Swarts, K. et al. Genomic estimation of complex traits reveals ancient maize adaptation to temperate
North America. _Science_ 357, 512–515 (2017). ADS Google Scholar * Girdland Flink, L. et al. Establishing the validity of domestication genes using DNA from ancient chickens. _Proc. Natl
Acad. Sci. USA_ 111, 6184–6189 (2014). ADS Google Scholar * Loog, L. et al. Inferring allele frequency trajectories from ancient DNA indicates that selection on a chicken gene coincided
with changes in medieval husbandry practices. _Mol. Biol. Evol._ 34, 1981–1990 (2017). Google Scholar * Librado, P. et al. Ancient genomic changes associated with domestication of the
horse. _Science_ 356, 442–445 (2017). ADS Google Scholar * Salo, W. L., Aufderheide, A. C., Buikstra, J. & Holcomb, T. A. Identification of _Mycobacterium tuberculosis_ DNA in a
pre-Columbian Peruvian mummy. _Proc. Natl Acad. Sci. USA_ 91, 2091–2094 (1994). ADS Google Scholar * Taubenberger, J. K. et al. Characterization of the 1918 influenza virus polymerase
genes. _Nature_ 437, 889–893 (2005). ADS Google Scholar * Wilbur, A. K. et al. Deficiencies and challenges in the study of ancient tuberculosis DNA. _J. Archaeol. Sci._ 36, 1990–1997
(2009). Google Scholar * Wagner, D. M. et al. _Yersinia pestis_ and the plague of Justinian 541–543 AD: a genomic analysis. _Lancet Infect. Dis._ 14, 319–326 (2014). Google Scholar *
Spyrou, M. A. et al. Phylogeography of the second plague pandemic revealed through analysis of historical _Yersinia pestis_ genomes. _Nat. Commun._ 10, 4470 (2019). ADS Google Scholar *
Keller, M. et al. Ancient _Yersinia pestis_ genomes from across Western Europe reveal early diversification during the first Pandemic (541–750). _Proc. Natl Acad. Sci. USA_ 116, 12363–12372
(2019). Google Scholar * Susat, J. et al. _Yersinia pestis_ strains from Latvia show depletion of the pla virulence gene at the end of the second plague pandemic. _Sci. Rep._ 10, 14628
(2020). ADS Google Scholar * Bos, K. I. et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. _Nature_ 514, 494–497 (2014). ADS Google
Scholar * Sabin, S. et al. A seventeenth-century _Mycobacterium tuberculosis_ genome supports a Neolithic emergence of the _Mycobacterium tuberculosis_ complex. _Genome Biol._ 21, 201
(2020). Google Scholar * Shillito, L.-M., Blong, J. C., Green, E. J. & van Asperen, E. N. The what, how and why of archaeological coprolite analysis. _Earth Sci. Rev._ 207, 103196
(2020). Google Scholar * Warinner, C., Speller, C. & Collins, M. J. A new era in palaeomicrobiology: prospects for ancient dental calculus as a long-term record of the human oral
microbiome. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 370, 20130376 (2015). Google Scholar * Velsko, I. M. et al. Microbial differences between dental plaque and historic dental calculus
are related to oral biofilm maturation stage. _Microbiome_ 7, 102 (2019). Google Scholar * Farrer, A. G. et al. Biological and cultural drivers of oral microbiota in Medieval and
Post-Medieval London, UK. Preprint at _bioRxiv_ https://doi.org/10.1101/343889 (2018). Article Google Scholar * Ho, S. Y. W. & Shapiro, B. Skyline-plot methods for estimating
demographic history from nucleotide sequences. _Mol. Ecol. Resour._ 11, 423–434 (2011). Google Scholar * Allentoft, M. E. et al. Extinct New Zealand megafauna were not in decline before
human colonization. _Proc. Natl Acad. Sci. USA_ 111, 4922–4927 (2014). ADS Google Scholar * Thomas, J. E. et al. Demographic reconstruction from ancient DNA supports rapid extinction of
the great auk. _eLife_ 8, e47509 (2019). Google Scholar * Dussex, N. et al. Complete genomes of two extinct New Zealand passerines show responses to climate fluctuations but no evidence for
genomic erosion prior to extinction. _Biol. Lett._ 15, 20190491 (2019). Google Scholar * Li, H. & Durbin, R. Inference of human population history from whole genome sequence of a
single individual. _Nature_ 475, 493–496 (2011). Google Scholar * Schiffels, S. & Durbin, R. Inferring human population size and separation history from multiple genome sequences. _Nat.
Genet._ 46, 919–925 (2014). Google Scholar * Murray, G. G. R. et al. Natural selection shaped the rise and fall of passenger pigeon genomic diversity. _Science_ 358, 951–954 (2017). ADS
Google Scholar * Díez-Del-Molino, D., Sánchez-Barreiro, F., Barnes, I., Gilbert, M. T. P. & Dalén, L. Quantifying temporal genomic erosion in endangered species. _Trends Ecol. Evol._
33, 176–185 (2018). Google Scholar * Der Sarkissian, C. et al. Evolutionary genomics and conservation of the endangered Przewalski’s horse. _Curr. Biol._ 25, 2577–2583 (2015). Google
Scholar * Norén, K., Godoy, E., Dalén, L., Meijer, T. & Angerbjörn, A. Inbreeding depression in a critically endangered carnivore. _Mol. Ecol._ 25, 3309–3318 (2016). Google Scholar *
Pedersen, M. W. et al. Ancient and modern environmental DNA. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 370, 20130383 (2015). Google Scholar * Haile, J. et al. Ancient DNA chronology
within sediment deposits: are paleobiological reconstructions possible and is DNA leaching a factor? _Mol. Biol. Evol._ 24, 982–989 (2007). Google Scholar * Binladen, J. et al. The use of
coded PCR primers enables high-throughput sequencing of multiple homolog amplification products by 454 parallel sequencing. _PLoS ONE_ 2, e197 (2007). ADS Google Scholar * Murchie, T. J.
et al. Optimizing extraction and targeted capture of ancient environmental DNA for reconstructing past environments using the PalaeoChip Arctic-1.0 bait-set. _Quat. Res_.
https://doi.org/10.1017/qua.2020.59 (2020). * Haile, J. et al. Ancient DNA reveals late survival of mammoth and horse in interior Alaska. _Proc. Natl Acad. Sci. USA_ 106, 22352–22357 (2009).
ADS Google Scholar * Seersholm, F. V. et al. Rapid range shifts and megafaunal extinctions associated with late Pleistocene climate change. _Nat. Commun._ 11, 2770 (2020). ADS Google
Scholar * Key, F. M., Posth, C., Krause, J., Herbig, A. & Bos, K. I. Mining metagenomic data sets for ancient DNA: recommended protocols for authentication. _Trends Genet._ 33, 508–520
(2017). Google Scholar * Günther, T. & Jakobsson, M. in _Handbook of Statistical Genomics_ (eds Balding, D., Moltke, I. & Marioni, J.) 295–340 (Wiley, 2019). * Skourtanioti, E. et
al. Genomic history of Neolithic to bronze age Anatolia, Northern Levant, and Southern Caucasus. _Cell_ 181, 1158–1175.e28 (2020). Google Scholar * Burmeister, S. Archaeological research on
migration as a multidisciplinary challenge. _medieval worlds_ 4, 42–64 (2016). Google Scholar * Knipper, C. et al. Female exogamy and gene pool diversification at the transition from the
final Neolithic to the early bronze age in central Europe. _Proc. Natl Acad. Sci. USA_ 114, 10083–10088 (2017). Google Scholar * Zarrillo, S. et al. The use and domestication of _Theobroma
cacao_ during the mid-Holocene in the upper Amazon. _Nat. Ecol. Evol._ 2, 1879–1888 (2018). Google Scholar * Hendy, J. et al. Proteomic evidence of dietary sources in ancient dental
calculus. _Proc. Biol. Sci._ 285, 20180977 (2018). Google Scholar * Roberts, P. et al. Calling all archaeologists: guidelines for terminology, methodology, data handling, and reporting when
undertaking and reviewing stable isotope applications in archaeology. _Rapid Commun. Mass. Spectrom._ 32, 361–372 (2018). ADS Google Scholar * Radini, A. et al. Medieval women’s early
involvement in manuscript production suggested by lapis lazuli identification in dental calculus. _Sci. Adv._ 5, eaau7126 (2019). ADS Google Scholar * Ren, M. et al. The origins of
cannabis smoking: chemical residue evidence from the first millennium BCE in the Pamirs. _Sci. Adv._ 5, eaaw1391 (2019). ADS Google Scholar * Reitsema, L. J. & Holder, S. Stable
isotope analysis and the study of human stress, disease, and nutrition. _Bioarc. Int._ 2, 63–74 (2018). Google Scholar * Maixner, F. et al. The Iceman’s last meal consisted of fat, wild
meat, and cereals. _Curr. Biol._ 28, 2348–2355.e9 (2018). Google Scholar * Lejzerowicz, F. et al. Ancient DNA complements microfossil record in deep-sea subsurface sediments. _Biol. Lett._
9, 20130283 (2013). Google Scholar * van Dijk, E. L., Jaszczyszyn, Y., Naquin, D. & Thermes, C. The third revolution in sequencing technology. _Trends Genet._ 34, 666–681 (2018). Google
Scholar * Komor, A. C., Badran, A. H. & Liu, D. R. CRISPR-based technologies for the manipulation of eukaryotic genomes. _Cell_ 168, 20–36 (2017). Google Scholar * Li, H. L., Gee, P.,
Ishida, K. & Hotta, A. Efficient genomic correction methods in human iPS cells using CRISPR–Cas9 system. _Methods_ 101, 27–35 (2016). Google Scholar * Muchnik, S. K., Lorente-Galdos,
B., Santpere, G. & Sestan, N. Modeling the evolution of human brain development using organoids. _Cell_ 179, 1250–1253 (2019). Google Scholar * Kanton, S. et al. Organoid single-cell
genomic atlas uncovers human-specific features of brain development. _Nature_ 574, 418–422 (2019). ADS Google Scholar * Racimo, F., Renaud, G. & Slatkin, M. Joint estimation of
contamination, error and demography for nuclear DNA from ancient humans. _PLoS Genet._ 12, e1005972 (2016). Google Scholar * Jun, G. et al. Detecting and estimating contamination of human
DNA samples in sequencing and array-based genotype data. _Am. J. Hum. Genet._ 91, 839–848 (2012). Google Scholar * Link, V. et al. ATLAS: analysis tools for low-depth and ancient samples.
Preprint at _bioRxiv_ https://doi.org/10.1101/105346 (2017). Article Google Scholar * Librado, P. & Orlando, L. Detecting signatures of positive selection along defined branches of a
population tree using LSD. _Mol. Biol. Evol._ 35, 1520–1535 (2018). Google Scholar * Refoyo-Martínez, A. et al. Identifying loci under positive selection in complex population histories.
_Genome Res._ 29, 1506–1520 (2019). Google Scholar * Renaud, G., Hanghøj, K., Korneliussen, T. S., Willerslev, E. & Orlando, L. Joint estimates of heterozygosity and runs of
homozygosity for modern and ancient samples. _Genetics_ 212, 587–614 (2019). Google Scholar * Brace, S. et al. Ancient genomes indicate population replacement in early neolithic britain.
_Nat. Ecol. Evol._ 3, 765–771 (2019). Google Scholar * Yates, J. A. F. et al. Community-curated and standardised metadata of published ancient metagenomic samples with AncientMetagenomeDir.
Preprint at _bioRxiv_ https://doi.org/10.1101/2020.09.02.279570 (2020). Article Google Scholar Download references ACKNOWLEDGEMENTS The authors thank A. Hübner for assistance with figure
4c. L.O., P.S., P.W.S. and C.W. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreements
ERC-2015-CoG 681605-PEGASUS, ERC-2018-StG 852558-AGRICON, ERC-2015-StG 678901-FoodTransforms and ERC-2017-StG 804844-DAIRYCULTURES, respectively). L.O. was also supported by ANR (LifeChange)
and the Simone et Cino Del Duca Foundation (HealthTimeTravel). P.S. was also supported by the Francis Crick Institute core funding (FC001595) from Cancer Research UK, the UK Medical
Research Council and the Wellcome Trust, a Wellcome Trust Investigator award (217223/Z/19/Z) and the Vallee Foundation. C.W. also received funding from the Max Planck Society, the Deutsche
Forschungsgemeinschaft (EXC 2051 #390713860) and the Siemens Foundation (Paleobiochemistry). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre d’Anthropobiologie et de Génomique de
Toulouse, Université Paul Sabatier Toulouse III, Faculté de Médecine Purpan, Toulouse, France Ludovic Orlando & Clio Der Sarkissian * School of Life Sciences, University of Warwick,
Coventry, UK Robin Allaby * Ancient Genomics Laboratory, Francis Crick Institute, London, UK Pontus Skoglund * Institute for Pre- and Protohistoric Archaeology and Archaeology of the Roman
Provinces, Ludwig Maximilian University, Munich, Germany Philipp W. Stockhammer * Department of Archaeogenetics, Max Planck Institute for the Science of Human History, Jena, Germany Philipp
W. Stockhammer, Johannes Krause & Christina Warinner * International Laboratory for Human Genome Research, National Autonomous University of Mexico, Queretaro, Mexico María C.
Ávila-Arcos * Key Laboratory of Vertebrate Evolution and Human Origins, Institute of Vertebrate Paleontology and Paleoanthropology, Center for Excellence in Life and Paleoenvironment,
Chinese Academy of Sciences, Beijing, China Qiaomei Fu * Lundbeck Foundation GeoGenetics Center, GLOBE Institute, University of Copenhagen, Copenhagen, Denmark Eske Willerslev * Welcome
Trust, Sanger Institute, Hinxton, UK Eske Willerslev * The Danish Institute for Advanced Study, University of Southern Denmark, Odense, Denmark Eske Willerslev * Department of Zoology,
University of Cambridge, Cambridge, UK Eske Willerslev * School of Human Evolution and Social Change, Arizona State University, Tempe, AZ, USA Anne C. Stone * Department of Anthropology,
Harvard University, Cambridge, MA, USA Christina Warinner Authors * Ludovic Orlando View author publications You can also search for this author inPubMed Google Scholar * Robin Allaby View
author publications You can also search for this author inPubMed Google Scholar * Pontus Skoglund View author publications You can also search for this author inPubMed Google Scholar * Clio
Der Sarkissian View author publications You can also search for this author inPubMed Google Scholar * Philipp W. Stockhammer View author publications You can also search for this author
inPubMed Google Scholar * María C. Ávila-Arcos View author publications You can also search for this author inPubMed Google Scholar * Qiaomei Fu View author publications You can also search
for this author inPubMed Google Scholar * Johannes Krause View author publications You can also search for this author inPubMed Google Scholar * Eske Willerslev View author publications You
can also search for this author inPubMed Google Scholar * Anne C. Stone View author publications You can also search for this author inPubMed Google Scholar * Christina Warinner View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Introduction (L.O., A.C.S. and C.W.); Experimentation (L.O., R.A., C.D.S., P.W.S., A.C.S. and C.W.);
Results (L.O., P.S., R.A., P.W.S., M.C.A.-A. and C.W.); Applications (L.O., R.A., P.W.S., C.D.S., M.C.A.-A., Q.F., J.K., E.W., A.C.S. and C.W.); Reproducibility and data deposition (L.O. and
M.C.A.-A.); Limitations and optimizations (L.O.); Outlook (L.O., P.W.S., A.C.S. and C.W.); Overview of the Primer (L.O. and C.W.). CORRESPONDING AUTHOR Correspondence to Ludovic Orlando.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Reviews Methods Primers_ thanks T. Günther, L.
Matisoo-Smith, R. Pinhasi, N. Rawlence, A. Zink and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS ANCIENT HUMAN DNA UMAP:
https://umap.openstreetmap.fr/en/map/ancient-human-dna_41837#6/51.000/2.000 BITBUCKET: https://bitbucket.org/product EUROPEAN NUCLEOTIDE ARCHIVE (ENA): https://www.ebi.ac.uk/ena GITHUB:
https://github.com INTERNATIONAL SYMPOSIUM FOR BIOMOLECULAR ARCHAEOLOGY (ISBA): https://isba9.sciencesconf.org MAX PLANCK HARVARD RESEARCH CENTER FOR THE ARCHAEOSCIENCE OF THE ANCIENT
MEDITERRANEAN: https://www.archaeoscience.org/ PROTOCOLS.IO: https://www.protocols.io REICH LABORATORY: https://reich.hms.harvard.edu/datasets SEQUENCE READ ARCHIVE (SRA):
https://www.ncbi.nlm.nih.gov/sra STANDARDS AND PRECAUTIONS AND ADVANCES IN ANCIENT METAGENOMICS (SPAAM):https://github.com/SPAAM-workshop GLOSSARY * Ancient DNA (aDNA). Ultrashort and
degraded DNA fragments that are preserved in subfossil material, including hard tissues, such as bones, teeth and shells, and soft tissues, such as mummified skin and hair, as well as
sediments. * Holobiomes The total sum of the DNA fragments making up the genome of a host organism and all of its microbiota. * DNA library A molecular construction in which DNA fragments
are ligated to DNA adapters of known sequences in order to be amplified and optionally captured prior to sequencing; different sequencing platforms require different library constructs. *
DNA barcoding The taxonomic assignment of metagenomic DNA content on the basis of DNA fragments that show limited intra-specific sequence diversity but large inter-specific sequence
diversity. * Shotgun sequencing Non-targeted sequencing of DNA library content. * DNA ligases A class of enzymes that are capable of stitching together different DNA fragments. *
Ascertainment bias Statistical bias resulting from the collection of genetic data at a subset of loci that do not reflect the overall genetic diversity present at the whole-genome scale. *
Demultiplexing A process by which pools of sequences originating from different DNA libraries are assigned back to their original samples on the basis of short synthetic sequences added
during library indexing. * Outgroup An individual, a population or a group of populations and/or species that are genetically close but different from those under study. * Identity by
descent DNA segments between two or more individuals are identical by descent when they are inherited from a common ancestor in the absence of recombination. * Procrustes analysis Also known
as Procrustes superimposition. A statistical method allowing the translation, rotation and scaling of multidimensional objects within a single analytical space where they can be compared. *
16S meta-barcodes Selected variable regions of the 16S ribosomal RNA gene whose sequence provides taxonomic resolution amongst bacteria and archaea. * DNA methylation A biological process
by which the activity of a DNA segment is modified without changing the underlying sequence but by adding methyl groups to the DNA molecule. * Bisulfite conversion A chemical reaction using
sodium bisulfite that converts unmethylated CpG dinucleotides into UpGs but leaves methylated CpGs intact, thereby allowing the detection of DNA methylation by sequencing. *
Immunoprecipitation A molecular laboratory technique by which specific molecules are purified on the basis of their chemical affinities for particular protein groups, such as antibodies. *
Population replacement A population process by which the gene pool of one local population is at least partially replaced by that coming from another, genetically distinct, population. *
Environmental DNA (eDNA). Fragments of DNA that are preserved within sediments and water that can be used for a fast, cost-effective monitoring of the ecology of a given region. *
Stratigraphic leaching The migration of DNA across strata in sediments caused by water movement, microorganism growth or bioturbation and compromising the reliability of the stratigraphy,
that is, the order, position and age of the geological layers formed by the different piles of sediments. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Orlando, L., Allaby, R., Skoglund, P. _et al._ Ancient DNA analysis. _Nat Rev Methods Primers_ 1, 14 (2021). https://doi.org/10.1038/s43586-020-00011-0 Download citation * Accepted: 16
December 2020 * Published: 11 February 2021 * DOI: https://doi.org/10.1038/s43586-020-00011-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative