- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Psychological stress changes both behaviour and metabolism to protect organisms. Adrenaline is an important driver of this response. Anxiety correlates with circulating free fatty
acid levels and can be alleviated by a peripherally restricted β-blocker, suggesting a peripheral signal linking metabolism with behaviour. Here we show that adrenaline, the β3 agonist
CL316,243 and acute restraint stress induce growth differentiation factor 15 (GDF15) secretion in white adipose tissue of mice. Genetic inhibition of adipose triglyceride lipase or genetic
deletion of β-adrenergic receptors blocks β-adrenergic-induced increases in GDF15. Increases in circulating GDF15 require lipolysis-induced free fatty acid stimulation of M2-like macrophages
within white adipose tissue. Anxiety-like behaviour elicited by adrenaline or restraint stress is eliminated in mice lacking the GDF15 receptor GFRAL. These data provide molecular insights
into the mechanisms linking metabolism and behaviour and suggest that inhibition of GDF15–GFRAL signalling might reduce acute anxiety. SIMILAR CONTENT BEING VIEWED BY OTHERS STRESS INCREASES
HEPATIC RELEASE OF LIPOCALIN 2 WHICH CONTRIBUTES TO ANXIETY-LIKE BEHAVIOR IN MICE Article Open access 08 April 2024 SIRT1 IN THE BNST MODULATES CHRONIC STRESS-INDUCED ANXIETY OF MALE MICE
VIA FKBP5 AND CORTICOTROPIN-RELEASING FACTOR SIGNALING Article 29 June 2023 EBI2 IS A NEGATIVE MODULATOR OF BROWN ADIPOSE TISSUE ENERGY EXPENDITURE IN MICE AND HUMAN BROWN ADIPOCYTES Article
Open access 29 March 2022 MAIN The incidence of anxiety disorders has rapidly increased over the last two decades, and anxiety is now the most prevalent psychiatric disorder, affecting
nearly 30% of the Western population at some point1. Acute stress-induced anxiety helps maintain the arousal and vigilance required to avoid repeated exposures to dangerous conditions2,3.
Catecholamines, namely noradrenaline and adrenaline (international nonproprietary names: norepinephrine and epinephrine, respectively), are critical to coordinating the central and
peripheral responses to psychological stress3,4,5,6. Central noradrenaline, particularly from the locus coeruleus, is known to control acute anxiety-like behaviour3,4. Interestingly, acute
peripheral administration of adrenaline also produces anxiety5,6,7,8,9, despite adrenaline not crossing the blood–brain barrier, and this effect is abolished by the β-blocker
propranolol10,11,12,13. Central mechanisms could explain the effects of propranolol, which readily crosses the blood–brain barrier; however, treatment with practolol, a β-blocker that does
not cross the blood–brain barrier, elicits similar anxiolytic effects14. These studies suggest that there may be peripheral endocrine signals linking β-adrenergic signalling with behaviour
that have yet to be identified. Growth differentiation factor 15 (GDF15) is a distant member of the transforming growth factor-β superfamily that circulates at low levels under normal
physiological conditions but is elevated during cellular stress and mitochondrial diseases15,16. GDF15 signals through its receptor, glial-cell-derived neurotrophic factor receptor α-like
(GFRAL), which is found exclusively in the brainstem17. GDF15–GFRAL signalling leads to behavioural changes, including reduced food intake18, nausea19 and aversive19,20 and anxiety-like
behaviours20,21,22. These effects may involve activation of the sympathetic and hypothalamic–pituitary–adrenal (HPA) axes23,24,25,26, both of which are classic responses to psychological
stress. In this study, we aimed to identify potential links between peripheral β-adrenergic activity and anxiety. We show that β-adrenergic stimulation activates adipocyte lipolysis, which
promotes the secretion of GDF15 from adipose tissue-resident macrophages, ultimately linking changes in peripheral metabolism with behaviour. RESULTS PSYCHOLOGICAL STRESS INCREASES GDF15
THROUGH Β-ADRENERGIC SIGNALLING Adipose tissue is a major endocrine organ that links metabolism with neuronal circuits and is acutely sensitive to changes in β-adrenergic
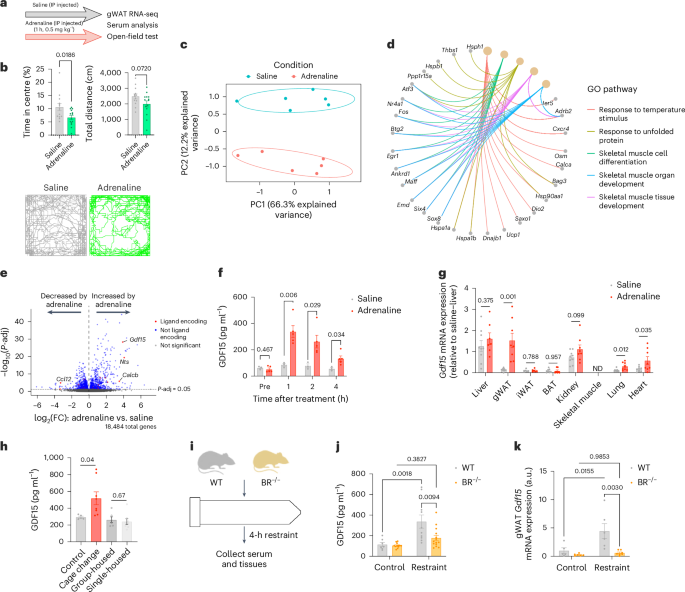
signalling5,6,7,8,9,10,13,27. We treated mice with saline or adrenaline injected intraperitoneally (IP) (Fig. 1a). In agreement with previous studies28, adrenaline induced anxiety-like
behaviour without affecting overall activity levels (Fig. 1b). To identify adipose-derived endocrine signals, we collected gonadal white adipose tissue (gWAT) and serum 1 h after treatment
with saline or the same dose of adrenaline from another cohort of mice. As anticipated29, adrenaline produced marked and distinct separation in principal component analysis (Fig. 1c), with
the most upregulated differentially expressed genes (DEGs) associated with the unfolded protein response and thermogenic and adaptive gene signatures (Fig. 1d and Extended Data Fig. 1a).
From this gene set, we then isolated genes encoding secreted protein products (that is, ligands)30 and found that _Gdf15_ was the most highly upregulated (_P_ = 6.59 × 10−29) secreted
factor, showing a >4 log2-fold increase in adrenaline-treated mice compared with saline-treated mice (Fig. 1e and Extended Data Fig. 1b); indeed, _Gdf15_ was one of the most upregulated
genes in gWAT. Corresponding with elevations in gWAT _Gdf15_ mRNA, GDF15 protein in serum was increased nearly five-fold within 1 h and remained elevated up to 4 h after injection (Fig. 1f).
Reverse transcription followed by qPCR confirmed that adrenaline produced an approximately eight-fold increase in _Gdf15_ in gWAT without affecting _Gdf15_ in the liver, kidney,
subcutaneous inguinal WAT (iWAT) or brown adipose tissue (Fig. 1g). _Gdf15_ expression was slightly increased in the lung and heart but was undetectable in skeletal muscle (Fig. 1g).
Consistent with lower gWAT mass, serum GDF15 levels were lower in age-matched female mice than in male mice, but the relative response to adrenaline was similar between sexes (Extended Data
Fig. 1c). Adrenaline also induced a similar increase in GDF15 whether mice were housed at room temperature or thermoneutrality (Extended Data Fig. 1d). These data indicate that adrenaline
leads to marked increases in gWAT and serum GDF15 in mice. Previous studies characterizing GDF15-null mice found that these animals exhibit less anxiety-like behaviour in open-field and
elevated-plus tests21,22. Psychological stress can be acutely induced in mice by changing their cages, whereas chronic single housing for 3 days is a form of long-term psychological
stress31. We found that, consistent with adrenaline injection, acute cage change increased serum GDF15 levels; however, single housing did not (Fig. 1h). Consistent with our observations in
mice that chronic stress and GDF15 are not linked, two-sample Mendelian randomization (2SMR) of GDF15 within the UK Biobank and serum GDF15 in children who were overweight and obese did not
show associations with anxiety (Extended Data Fig. 2a–c). Although coding polymorphisms affect the measurement of circulating GDF15 in humans32, these data suggest that adrenergic-induced
GDF15 may be involved in acute but not chronic psychological stress. An acute psychological stress that reliably increases adrenaline in mice is physical restraint33. We observed increased
circulating adrenaline following acute physical restraint, although the levels were lower than after treatment with IP injected adrenaline (Extended Data Fig. 1e). Adrenaline signals in
adipose tissue through β-adrenergic receptors34. Therefore, to examine whether psychological stress induces GDF15 through β-adrenergic receptors, we physically restrained wild-type (WT) mice
and mice lacking β1, β2 and β3 adrenergic receptors (_Adrb1_, _Adrb2_ and _Adrb3_ triple knockout (BR−/−)) in 50-ml conical tubes (Fig. 1i). Restraint of WT mice increased serum and gWAT
GDF15; however, this response was eliminated in BR−/− mice (Fig. 1j,k). Baseline GDF15 levels were indistinguishable between genotypes and, importantly, lipopolysaccharide (LPS) increased
serum GDF15 levels35 and lowered blood glucose levels similarly in WT and BR−/− mice (Extended Data Fig. 3a,b), demonstrating that these mice have an intact GDF15 response to
non-β-adrenergic stimuli. These results show that psychological stress increases GDF15 and that this is dependent on β-adrenergic signalling. ATGL IS CRITICAL FOR PROMOTING
Β-ADRENERGIC-INDUCED GDF15 Lipolysis is a major regulator of the transcriptional responses within adipose tissue29 and is increased by β-adrenergic-induced activation of adipose triglyceride
lipase (ATGL), the rate-limiting enzyme for the release of free fatty acids from WAT36. To determine whether ATGL is responsible for regulating GDF15 secretion from adipose tissue, we
treated adipocyte-specific ATGL-knockout mice (AdATGL−/−) and their floxed littermate controls (AdATGLflox/flox) with IP injected saline or CL316,243 (CL), a potent and selective
β3-adrenergic agonist. ATGL expression was ~90% lower in gWAT of AdATGL−/− mice (Fig. 2a). CL increased serum nonesterified fatty acids and markers of the unfolded protein response, _Atf4_
and _Chop_, in gWAT of AdATGLflox/flox animals (Fig. 2b–d). These responses were eliminated in AdATGL−/− mice despite β-adrenergic signalling remaining intact, as evidenced by the similar
increase in _Ppargc1a_ expression and phosphorylation of cAMP-dependent protein kinase (PKA) substrates in AdATGL−/− and floxed mice (Fig. 2e,f). Consistent with our observations in BR−/−
mice, basal GDF15 levels were similar between AdATGLflox/flox and AdATGL−/− mice; however, CL-induced increases in circulating and gWAT GDF15 were eliminated in AdATGL−/− mice (Fig. 2g,h).
Treatment with IP injected cilostamide, a phosphodiesterase-3 inhibitor that leads to increased cellular cAMP levels, exaggerated the effects of CL on circulating GDF15 levels (Fig. 2i),
supporting the involvement of the β3 adrenergic receptor–cAMP–ATGL pathway in stimulating GDF15. Physical restraint stress promotes adipose tissue lipolysis37. Serum glycerol was increased
in AdATGLflox/flox but not in AdATGL−/− mice in response to acute tube restraint (Fig. 2j). Similarly, circulating GDF15 levels were increased in AdATGLflox/flox but not in AdATGL−/− mice
(Fig. 2k). We tested the behavioural response to acute restraint stress in these mice, but AdATGL−/− mice became hypoglycaemic following restraint, and this was associated with marked
reductions in overall activity during open-field tests (Extended Data Fig. 4a–c), an effect that was likely secondary to the defect in adipose tissue lipolysis. To evaluate whether
β-adrenergic signalling increases GDF15 in a cell-autonomous manner, we cultured and differentiated mouse white adipocytes before treating them with CL and the ATGL inhibitor ATGListatin38.
As expected, CL increased glycerol release in the medium, and this was blocked by ATGListatin; however, neither CL nor ATGListatin altered GDF15 levels in the medium (Fig. 2l,m). These data
suggest that GDF15 is unlikely to be derived from adipocytes in response to adrenergic stimulation. As adipocyte-specific ablation of ATGL inhibited GDF15 secretion but isolated adipocytes
did not secrete GDF15 in response to CL, we hypothesized that another cell type within adipose tissue might be responsible for GDF15 secretion. To test this, we fractioned gWAT and iWAT into
adipocytes and the stromal vascular fraction (SVF). Consistent with our hypothesis and recent studies39, _Gdf15_ expression was higher in the SVF of both WAT depots but particularly in gWAT
(Fig. 2n). Moreover, in response to IP injected adrenaline, _Gdf15_ was increased in only the SVF (Fig. 2o), whereas the related cytokine, _Fgf21_, was increased in only the adipocyte
fraction (Extended Data Fig. 5a). Appropriate separation was confirmed as leptin was found only in the adipocyte fraction (Extended Data Fig. 5b). These results point towards ATGL-dependent
intercellular communication mediating β-adrenergic-induced adipose tissue GDF15 secretion. MACROPHAGES ARE THE PRIMARY SOURCE OF SERUM GDF15 To determine the probable cell type secreting
GDF15 in adipose tissue, we analysed publicly available single-cell RNA-sequencing (scRNA-seq) data of cells from gWAT of CL-treated mice40. Cells were divided into either lineage marker
positive (Lin+) (including monocytes, macrophages and B cells) or Lin− (including fibroblasts and adipocytes). _Gdf15_ was primarily expressed in Lin+ cell types, especially in macrophages
(Fig. 3a,b), with negligible expression in any Lin− cell type, including adipocytes (Extended Data Fig. 5c). In response to CL treatment, there was increased _Gdf15_ expression only in the
macrophage population (Fig. 3a). Because macrophage populations in adipose tissue are highly heterogeneous41, we used established gene signatures to identify three major clusters of
macrophage populations: classically activated M1-like (_Adgre1_/_F480_ and _iNos_/_Cd86_/_Cd80_), alternatively activated M2-like (_Adgre1_/_F480_ and _Cd163_/_Arg1_) and macrophages that
express _Adgre1_/_F480_ but no other markers for M1- or M2-like activation (Fig. 3c). We observed that CL led to the accumulation of both M1- and M2-like macrophages in gWAT, as noted in the
original report40. CL increased _Gdf15_ in M2-like _Adgre1_/_F480_- and _Cd163_/_Arg1_-positive macrophages to a greater degree than observed in M1-like macrophages (Fig. 3c,d). We
subsequently examined whether increases in _Gdf15_ also occurred after acute treatment with adrenaline by isolating F4/80+ cells from the SVF of gWAT (Extended Data Fig. 5d). Consistent with
the scRNA-seq results, we found that _Gdf15_ expression was higher in F4/80+ cells than in other cells within the SVF and that adrenaline induced increases in _Gdf15_ in F4/80+ cells
without any change in F4/80− cells of the SVF (Fig. 3e). Taken together, these data demonstrate that macrophages are the primary source of GDF15 in response to β-adrenergic-induced
lipolysis. Fatty acids are the primary mediators of the transcriptional response to adipocyte lipolysis29. To determine whether fatty acids are responsible for GDF15 secretion from
macrophages, we turned to bone marrow-derived macrophages (BMDMs) polarized with LPS + interferon-γ (IFNγ) (M1-like activation) or interleukin-4 (IL-4) (M2-like) or left untreated (M0) (Fig.
3f)42. We then treated BMDMs with two of the most abundant fatty acid species that are increased in the circulation by β-adrenergic agonists43, namely oleate, an unsaturated fatty acid, and
palmitate, a saturated fatty acid. We also included tunicamycin, a compound that activates the unfolded protein response and is known to increase GDF15 (refs. 44,45). In line with our
scRNA-seq results, palmitate and tunicamycin stimulated the secretion of GDF15 only in M0 and M2-like macrophages but not in M1-like macrophages (Fig. 3g). Similar results were seen at the
transcriptional level, with _Gdf15_ expression being increased by palmitate only in M0 and M2-like macrophages (Fig. 3h). These results align with earlier work showing that adipocytes
preferentially secrete palmitate, which is a critical regulator of macrophage metabolism46. Macrophage polarization was confirmed by the expression levels of _Arg1_ and _iNos_, which were
elevated in M2- and M1-like macrophages, respectively (Extended Data Fig. 5e). Under basal conditions, GDF15 secretion into the medium was similar between M1-like and M2-like polarized
cells, both of which showed greater GDF15 levels than unpolarized M0 cells (Extended Data Fig. 5f). Importantly, treatment of macrophages with adrenaline did not affect GDF15 secretion
(Extended Data Fig. 5g), indicating that adrenergic signalling does not directly stimulate macrophage GDF15 secretion. We then used these scRNA-seq data to compare the M1- and M2-like
macrophage populations to identify possible mechanisms behind the M2-like-specific palmitate-induced GDF15 secretion. M2-like macrophages are known to use fatty acid metabolism to a greater
degree than M1-like macrophages; consistent with this, our analysis of the scRNA-seq data found that fatty acid metabolic processes (Fig. 3i) and fatty acid transporters (Extended Data Fig.
5h) were upregulated in M2- compared with M1-like macrophages. Many fatty acid transporters are upregulated by the transcription factor peroxisome proliferator-activated receptor-γ (PPARγ),
which is enriched in M2-like macrophages41 and was recently shown to increase the secretion of GDF15 from hepatocytes when activated47. To determine whether PPARγ is involved in controlling
the secretion of GDF15, we treated M2-like BMDMs with rosiglitazone, a PPARγ agonist, and T0070907, a PPARγ antagonist. We observed that rosiglitazone increased GDF15 levels in the medium
and that this effect was blocked by T0070907 (Fig. 3j). T0070907 also blocked palmitate-induced GDF15 secretion from M2-like macrophages (Fig. 3k). Together, these data show that palmitate
drives the secretion of GDF15 specifically from macrophages polarized to an alternative M2-like phenotype through a mechanism that probably involves PPARγ. Feeding mice a diet high in
saturated fatty acids can promote anxiety-like behaviour (reviewed in ref. 48). Exogenous delivery of fatty acids such as palm oil by acute oral gavage increases the expression of GDF15 in
the kidney and other tissues, and this leads to elevations in serum GDF15, an effect that peaks after 4 h (ref. 49). We tested whether an oral gavage of palm oil can produce anxiety-like
behaviour after 4 h and, if so, whether this effect is blunted in mice lacking the receptor for GDF15, GFRAL (_Gfral_−/− mice). In contrast to adrenaline injection or tube restraint,
treatment with palm oil did not increase anxiety-like behaviour in either genotype (Extended Data Fig. 6a). We next tested the effects of a high-fat diet predominantly composed of palm oil
for 4 weeks and found similarly increased anxiety-like behaviour in both WT and _Gfral_−/− mice (Extended Data Fig. 6b,c). These results suggest that GDF15–GFRAL signalling is not involved
in mediating anxiety-like behaviour in response to acute or chronic exogenous fatty acids. GDF15 PROMOTES ANXIETY-LIKE BEHAVIOUR THROUGH GFRAL GDF15 regulates food intake and energy
expenditure through its receptor, GFRAL15,18,26, the expression of which is isolated to the area postrema and nucleus tractus solitarius of the hindbrain17. To date, no other ligands besides
GDF15 have been shown to signal through GFRAL. To establish whether GFRAL signalling is important for mediating anxiety-like behaviour, we treated WT or GFRAL-knockout (_Gfral_−/−) mice
with IP injected adrenaline (as in Fig. 1a) 1 h before open-field tests. Adrenaline again induced anxiety-like behaviour in WT mice, but this response was lost in _Gfral_−/− mice (Fig. 4a)
without affecting total activity (Fig. 4b). Anxiety is also reflected by nonambulatory movements, such as grooming50. Further supporting that GFRAL is necessary for the behavioural responses
to acute stress, nonambulatory movements were increased in WT but not in _Gfral_−/− mice in response to IP injected adrenaline (Extended Data Fig. 7a); again, total physical activity was
not altered by adrenaline (Extended Data Fig. 7b). Other responses to adrenaline, including food intake, energy expenditure and substrate use, were similar between genotypes (Extended Data
Fig. 7c–e). We next tested whether GFRAL is important for the behavioural responses to psychological stress by applying acute physical restraint before the open-field test. As expected3,
restraint stress reduced the time spent in the centre, but, remarkably, this response was lost in _Gfral_−/− mice (Fig. 4c). Importantly, this effect was independent of genotypic differences
in the total activity levels during the open-field test, which were similarly reduced regardless of genotype (Fig. 4d). Anxiety-like behaviour was distinct from food intake or nausea, both
of which are altered by stress and can be controlled by GDF15 (ref. 19), given that the same restraint protocol did not affect chow or kaolin clay consumption in WT or _Gfral_−/− mice
(Extended Data Fig. 7f,g); stress-induced hypophagia and nausea have both been attributed to glucagon-like peptide 1 (refs. 51,52,53). In support of the anxiogenic effects of GDF15, a single
intraperitoneal injection of a pharmacological dose of GDF15 (ref. 26) produced anxiety-like behaviour in the open-field and light–dark box tests without affecting overall physical activity
(Extended Data Fig. 7h,i). Critically, the anxiogenic effects of GDF15 persisted following repeated daily exposure, suggesting that tachyphylaxis did not occur (Extended Data Fig. 7j–l),
similar to what we and others have shown for food intake and body weight loss with repeated GDF15 treatment26,54. Several brain regions are involved in the acute stress response2,3,55,56. To
determine the neurobiological substrates of GDF15-induced anxiogenesis, we measured _Fos_, an immediate early gene associated with neuronal activation, in several structures known to be
involved in stress-related behaviours. The locus coeruleus is the main source of noradrenaline in the mammalian brain and is one of the first structures recruited following stressful
stimuli3,4,57. Given that GDF15’s metabolic effects were recently shown to be dependent on β-adrenergic activity26, the locus coeruleus represented a particularly relevant target. However,
high-dose GDF15 did not increase c-Fos expression in locus coeruleus noradrenergic neurons (Extended Data Fig. 8a) nor in the medial prefrontal cortex and basolateral amygdala, both
downstream projections of the locus coeruleus known to drive anxiogenesis (Extended Data Fig. 8b)4,58. In contrast, GDF15 showed increased activity in the central amygdala and the bed
nucleus of the stria terminalis (Fig. 4e), each of which has well-established roles in anxiogenesis59,60,61,62,63. Consistent with other studies, we also observed activation within the
paraventricular nucleus of the hypothalamus (Fig. 4e)23,64. High levels of circulating GDF15 can activate corticotropin-releasing hormone (CRH) neurons within the paraventricular nucleus of
the hypothalamus and activate the HPA axis, leading to the secretion of corticosterone in mice23,24,64. We explored whether GDF15–GFRAL signalling is important for activating the HPA axis
under more physiological conditions by applying physical restraint stress to WT and _Gfral_−/− mice. Expectedly, restraint stress increased circulating corticosterone in both genotypes,
reflecting activation of the HPA axis, but significantly less so in _Gfral_−/− mice (Fig. 4f), despite circulating GDF15 levels being modestly elevated by restraint stress in both genotypes
(Extended Data Fig. 8c). CRH is critical in driving stress-induced behavioural responses, such as anxiety and aversion3,50. Intriguingly, exogenous CRH led to a marked increase in
circulating GDF15 levels (Fig. 4g), further demonstrating the involvement of GDF15 and GFRAL in the stress response. Importantly, exogenous CRH increased adrenocorticotropic hormone (ACTH)
and corticosterone levels, whereas dexamethasone decreased corticosterone levels similarly in WT and _Gfral_−/− mice, suggesting that there was no impairment in HPA axis signalling (Extended
Data Fig. 8d–f). Together, these data support the role of GDF15 in regulating acute stress-induced anxiety-like behaviour (Fig. 4h), possibly through the recruitment of multiple anxiogenic
brain regions56, and emphasize that GDF15–GFRAL signalling is transmitted broadly throughout the brain despite the brainstem-localized receptor expression. DISCUSSION Mammals have evolved
many mechanisms for detecting and avoiding noxious stimuli such as foodborne toxins, bacterial infections and allergens16,65,66,67,68. GDF15 has been linked to all of these by inducing
nausea, emesis (or pica) and aversive behaviour19,64,69. However, in addition to physical stimuli, psychological stress produces its own set of physiological and behavioural responses
intended to protect the organism. The fight-or-flight response is a classic example of psychological stress leading to metabolic responses that mobilize endogenous energy stores from adipose
tissue13,70 and elicit behavioural responses such as anxiety5,6,7,8,9. For decades, there has been evidence that adipose tissue lipolysis and peripheral adrenergic activity are associated
with feelings of anxiety10,11,12,13, but the link between these responses has remained elusive. Here, we identified a mechanism whereby adrenergic activation of adipocyte lipolysis promotes
the secretion of GDF15 from M2-like alternatively activated macrophages within adipose tissue, which, through its receptor, GFRAL, is critical for appropriate behavioural and physiological
responses to acute psychological stress. These results are consistent with previous studies in which chronically supraphysiological levels of GDF15 promoted anxiety-like behaviour whereas
lifelong deletion of GDF15 reduced anxiety21,22. We also identified various anxiogenic brain regions, including the central amygdala, bed nucleus of the stria terminalis and paraventricular
nucleus, that could be involved in mediating these responses. GDF15, at least at high circulating levels, has been shown to reduce physical activity71,72, produce anxiety-like
behaviour20,21,22 and activate the β-adrenergic25,26,68 and HPA axes23,24. Indeed, optogenetic activation of GFRAL-expressing neurons is sufficient to activate the HPA axis and increase the
circulating cortisol levels24. Clinical trials with GDF15 analogues did not report increases in anxiety73,74. However, while our paper was in review, a study was published in which plasma
proteomics conducted in individuals from the UK Biobank demonstrated that GDF15 is the circulating factor with the strongest association with anxiety in humans compared with more than 3,000
other measured proteins75. Herein, we show that GDF15–GFRAL signalling is a critical component of responses to acute psychological stress, including anxiety-like behaviour and HPA axis
activation. Some important limitations to the current data should be considered. First, the M1- and M2-like macrophage distinction is an oversimplification of the highly heterogeneous
macrophage populations. Other publications have identified distinct macrophage subpopulations that secrete GDF15, at least in skeletal muscle76, so it will be interesting to determine
whether a particular subpopulation is responsible for the fatty acid-dependent secretion of GDF15 demonstrated here. Moreover, it will need to be determined how palmitate drives GDF15
secretion from macrophages and whether endogenous adipocyte-derived palmitate is sufficient to elicit this response. It is appealing to speculate that fatty acid-sensing PPARs, which have
been shown to regulate GDF15 (ref. 47), could be involved, but this will need to be confirmed in vivo. Despite the rapid increase in the rates of anxiety, there is a dearth of new targets
for its treatment. The interactions between the brain and peripheral immune cells in the development of stress have been a growing area of interest77,78,79, but previous studies have focused
largely on classic inflammatory cytokines. Here, we provide additional insights into these interactions by identifying a new pathway for immune–brain crosstalk mediated by
adrenergic-induced ATGL-dependent lipolysis and M2-like macrophage-secreted GDF15. These results raise the possibility that blocking GDF15–GFRAL signalling could mitigate acute
stress-induced anxiety. METHODS MICE Germline GFRAL-knockout mice (_Gfral_−/−) were generated as previously described, with breeding pairs provided by R. Seeley26. Mice lacking β1, β2 and β3
adrenergic receptors (BR−/−) were generated as previously described26,80. Adipocyte-specific ATGL-knockout animals were generated by crossing _Adipoq_-Cre mice with _Atgl_/_Pnpla2_-floxed
mice81; _Atgl_/_Pnpla2_flox/flox littermates were used as controls. Animal studies were carried out at McMaster University (210104), the University of Toronto (21-467 and 24-0362H) or
Washington University in St. Louis (20-0139). All animals used in the study were housed and cared for in accordance with the Guidelines for Animal Use at McMaster University and were
approved by the McMaster University Animal Ethics Research Board or the ethics boards of their respective facilities. All mice were group-housed on a 12-h light–dark cycle with ad libitum
access to food and water. The animals were housed either in a HEPA-filtered unit at room temperature or in specific pathogen-free microisolators at 29 °C and 40–60% relative humidity.
Experiments were performed on mice at ages between 16 and 24 weeks. All mice were male unless stated otherwise, including for cell isolations. Experiments were performed, and mice were
killed in a fed state between 09:00 and 12:00. Terminal blood was collected by cardiac puncture, and blood from live animals (for example, time-course GDF15 levels) was collected from a tail
vein. Blood samples were centrifuged at 8,000_g_ for 10 min at 4 °C after clotting at room temperature for 30 min, and the supernatant was collected. Tissues were collected following
anaesthetization with ketamine (75 mg kg−1) and xylazine (10 mg kg−1). Tissues were frozen at −80 °C until future analyses. Male WT and _Gfral_−/− mice were placed on a high-fat,
high-fructose diet (40 kcal% fat (mostly palm oil), 20 kcal% fructose with 0.02% cholesterol; Research Diets, D19101102) starting at 12–20 weeks of age for 4 weeks. Palm oil (Sigma-Aldrich,
70905) was gavaged at ~09:00 as described previously49. MOUSE DRUG TREATMENTS Adrenaline hydrochloride (Sigma-Aldrich, E4642) was diluted in saline and injected IP (~09:00) at a dose of 0.5
mg per kg body weight based on previous studies82,83. CL (Sigma-Aldrich, C5976) was diluted in saline and injected IP (~09:00) at a dose of 1 mg per kg body weight84. Cilostamide
(Sigma-Aldrich, C7971; 10 mg per kg body weight, injected IP) was prepared in 10% DMSO and 5% Kolliphor85. All control mice received saline IP injected at the same volume. Recombinant human
GDF15 (5 nM kg−1; Novo Nordisk) or vehicle was injected IP at ~09:00 (ref. 26). To test for tachyphylaxis, we injected mice with either vehicle or GDF15 (5 nM kg−1) for 9 days at ~09:00.
Chow diet was measured daily. On the 10th day, mice were injected with either vehicle or GDF15 (5 nM kg−1), and open-field tests were performed 1 h later (described below). LPS
(Sigma-Aldrich, 0111:B4, L2630) was injected IP at a dose of 2 mg kg−1 starting at 09:00, and control mice were injected with saline. CRH (Sigma-Aldrich, C3042) was injected IP at ~09:00 at
a dose of 90 μg kg−1, and blood was collected from a tail vein at 0.5 and 3 h after injection86. Dexamethasone (Sigma-Aldrich, D4902) was injected IP at ~09:00 at a dose of 100 μg kg−1, and
blood was collected from a tail vein 6 h following injection87. HUMAN PAEDIATRIC ANXIETY SAMPLES Children with obesity who were enrolled in the Canadian Pediatric Weight Management Registry
at the McMaster site were included in this study. Study participants with an available fasting blood sample at their initial visit and a clinical diagnosis of anxiety but without
antipsychotic or antidepressant medications (_n_ = 23) were compared with those without any diagnosis of anxiety and also free from the use of medications (_n_ = 24). Control participants
and those with anxiety were matched for age (12.50 ± 2.97 and 12.44 ± 2.86 years), sex (12/11 and 12/12 male-to-female ratio), body weight (87 ± 31.88 and 88 ± 35.32 kg) and body mass index
(33.18 ± 6.41 and 34.08 ± 8.07 kg m−2). Serum GDF15 was assessed from previously frozen samples in duplicate, as described below. SEPARATION OF ADIPOSE TISSUE FRACTIONS Each gWAT or iWAT
sample was combined from two mice. WAT was collected, quickly rinsed in warm PBS, minced and incubated in collagenase (10 mM HEPES–Krebs–Ringer buffer, 4% BSA, 1.5 mg ml−1 collagenase type I
(Gibco, 17100017)) at 37 °C with gentle agitation for 30 min (gWAT) or 45 min (iWAT). Tissues were filtered (100 μm), and fractions were separated by repeated centrifugation at 500_g_ for 5
min. F4/80+ cells were isolated using a commercially available kit as per the manufacturer’s instructions (EasySep Mouse F4/80 Positive Selection Kit; STEMCELL Technologies, cat. no.
100-0659) along with an EasySep magnet (cat. no. 18000). ADIPOCYTE DIFFERENTIATION Immortalized mouse white adipocytes were generated as previously described88. Preadipocytes were grown to
confluence in DMEM supplemented with 1% GlutaMAX, 1% penicillin–streptomycin, 10% FBS, insulin (20 nM) and T3 (1 nM; differentiation medium). Confluent cells were incubated for 48 h in
differentiation medium further supplemented with isobutylmethylxanthine (0.5 mM), dexamethasone (0.5 μM) and indomethacin (0.125 mM; induction medium). All chemicals were from Sigma, and
experiments were performed within 20 passages following immortalization. Adipocytes were then treated with either CL (10 μM; Sigma-Aldrich, C5976), ATGListatin (40 μM; Sigma-Aldrich,
SML1075), neither of the compounds (control) or a combination of the two compounds for 24 h in serum-free DMEM supplemented with 1% BSA (Sigma-Aldrich, cat. no. A8806). Medium was collected,
and excess lipids and cellular debris were removed by centrifugation. Cells were washed with PBS and snap-frozen either in TRIzol for RNA extraction or in cell lysis buffer for protein
content quantification using BCA. Lipolysis was assessed by measuring glycerol levels in the medium using a commercially available kit (Cayman Chemical, cat. no. 10010755). BMDM ISOLATION,
DIFFERENTIATION AND POLARIZATION Femurs and tibias were collected from mice and cleaned of remaining muscle and soft tissues, and both ends of each bone were removed89. The bone marrow was
extracted by centrifugation at 1,900_g_ for 5 min. The marrow was resuspended in 1 ml DMEM (Wisent, cat. no. 319-005-CL, 25 mM glucose) and strained through a 40-μm cell strainer into a
50-ml Falcon tube. Cells were cultured for 4 h at 37 °C and 5% CO2. After incubation, nonadherent cells were collected and combined with M-CSF (20 ng ml−1; Peprotech, 315-02) to stimulate
macrophage differentiation and plated for 7 days in DMEM (Wisent, cat. no. 319-005-CL) supplemented with FBS (10%, Wisent, cat. no. 098150) and penicillin–streptomycin (1%, Invitrogen).
After differentiation, cells were washed, collected and plated onto 12-well plates (1 × 106 cells per ml) and left to adhere overnight. Fully differentiated BMDMs were then polarized either
with 100 ng ml−1 LPS (Sigma-Aldrich, L2630) + 20 ng ml−1 IFNγ (Peprotech, 315-05) to an M1-like phenotype or with 20 ng ml−1 IL-4 (Peprotech, 214-14) to an M2-like phenotype for 24 h (ref.
42). Following polarization, cells were treated with either sodium palmitate (500 μM, Sigma-Aldrich, P9767), sodium oleate (500 μM, Sigma-Aldrich, O7501), tunicamycin (5 ng ml−1, Cayman
Chemical, 11089-65-9), rosiglitazone (1 μM; Sigma-Aldrich, R2408) or T0070907 (1 μM; Sigma-Aldrich, T8703) for another 24 h in DMEM (Wisent, 319-005-CL) supplemented with 1% fatty acid and
endotoxin-free BSA (Sigma-Aldrich, cat. no. A8806). Medium was collected after 24 h and frozen at −80 °C until analysis. Cells were rinsed in PBS and collected either in TRIzol for RNA
extraction (see below) or in cell lysis buffer for protein quantification using BCA. RNA ISOLATION, CDNA SYNTHESIS AND QPCR Tissues were homogenized in TRIzol reagent. An RNeasy kit (Qiagen,
cat. no. 74106) was used for subsequent total RNA extraction and purification according to the manufacturer’s instructions. cDNA synthesis was performed using the SuperScript IV Reverse
Transcriptase kit (Invitrogen, cat. no. 18090010) following the manufacturer’s instructions. cDNA expression for specific genes was detected by qPCR using the AmpliTaq Gold DNA Polymerase
kit (Applied Biosystems, cat. no. N8080241). Relative mRNA levels were quantified with the Δ_C_T method using mouse _Ppia_ (Mm02342430_g1) as an endogenous control, except for adipocyte and
SVF comparisons for which _Polr2a_ was used (Mm00839502_m1). The gene-specific primers used were as follows: _Gdf15_ (Mm00442228_m1), _Atf4_ (Mm00515324_m1), _Chop_/_Ddit3_ (Mm01135937_g1),
_iNos_ (Mm00440502_m1), _Arg1_ (Mm00475988_m1), _Ppargc1a_ (Mm01208835_m1), _Emr1_/_F480_/_Adgre1_ (Mm00802529_m1), _Fgf21_ (Mm07297622_g1), _Atgl_/_Pnpla2_ (Mm00503040_m1) and _Lep_
(Mm00434759_m1). All probes were purchased from Thermo Fisher. RNA-SEQ AND ANALYSES For RNA-seq, mice were injected with adrenaline (0.5 mg kg−1 IP at 09:00) and gWAT was collected after 1
h. RNA was extracted as described above. Raw RNA-seq FASTQ data were imported into Galaxy for quality control and processing steps. The FastQC tool was used to check read quality, and the
Cutadapt tool was used to trim adaptor sequences and remove low-quality reads. The remaining reads were aligned to the mm10 _Mus musculus_ reference genome with the HISAT2 tool and
quantified with the featureCounts tool. These counts data were imported into R for differential expression analysis with the DESeq2 package to detect DEGs (adjusted _P_ value (_P_-adj) <
0.05). Principal component analysis was performed in R using variance stabilizing transformation (VST)-normalized data from the DESeq2 analysis. Pathway analysis was performed with the GSEA
software in combination with MSigDB gene sets. A list of human genes encoding secreted proteins (ligands) was obtained from the HUGO Gene Nomenclature Committee website. Mouse genes
orthologous to these human ligand-encoding genes were obtained using the g:Profiler tool. A heat map was created with DEGs belonging to this group of ligand-encoding genes in R with the
pheatmap package, using _z_-scored VST-normalized data. These genes were also highlighted in a volcano plot, which was created with the Enhanced Volcano package in R, using fold-change and
_P_-adj values from the DESeq2 analysis. SINGLE-CELL RNA-SEQ The scRNA-seq data used for the analyses described in this article were obtained from the National Center for Biotechnology
Information Sequence Read Archive under reference number SRP145475 (ref. 40). Cell Ranger was used to perform sample qualification, alignment, filtering, counting and aggregation using the
Linux system or R and RStudio software. Clustering and gene expression were visualized with the 10X Genomics Loupe Browser (v.6.5.0). 2SMR USING GWAS SUMMARY DATA 2SMR was performed using
the exposure and outcome from two nonoverlapping and independent datasets to conduct the summary-level instrument–exposure analysis and the instrument–outcome association analysis. 2SMR was
performed using the R package TwoSampleMR (v0.5.6)90. To verify the causal effect of GDF15 on nerves, anxiety, tension or depression in humans, we performed 2SMR using the exposure dataset
(GDF15, GWAS ID: ebi-a-GCST90011998, sample size: 21,758)91,92 and outcome dataset (nerves, anxiety, tension or depression, GWAS ID: ebi-a-GCST90013910, sample size: 407,746)93. We
identified genetic variants (single-nucleotide polymorphisms) associated with blood GDF15 protein levels in the GWAS catalogue dataset based on cis-protein quantitative trait loci (within
500 kb of the _Gdf15_ gene) and further selected proxy single-nucleotide polymorphisms by linkage disequilibrium clumping (_P_1 = 5 × 10−8, clump = TRUE, _P_2 = 1 × 10−7, _r_2 = 0.01, kb =
10,000). After dropping duplicate exposure–outcome summary sets, we further performed sensitivity analyses, including heterogeneity statistics, horizontal pleiotropy and leave-one-out
analysis. After confirming that there was no heterogeneity or horizontal pleiotropy, we next performed Mendelian randomization analysis and visualized the results using the scatter plot and
forest plot functions in the R package TwoSampleMR. The inverse-variance weighted method was used to assess the significance of the causal effect of the exposure on the outcome. 2SMR was
performed using R and RStudio. SERUM AND MEDIUM ANALYSES Serum and cell-based GDF15 levels were measured by ELISA (R&D Systems, DY6385) as per the manufacturer’s instructions18,45. Serum
corticosterone (Thermo Fisher, EIACORT), adrenocorticotropic hormone (Abcam, ab263880) and adrenaline (Abnova, KA1882) were measured following the manufacturer’s instructions. C-FOS
IMMUNOFLUORESCENCE AND QUANTIFICATION C57BL/6J mice were injected with GDF15 (5 nM kg−1) or vehicle; 90 min later, the mice were anaesthetized with a mixture of ketamine (69.57 mg ml−1),
xylazine (4.35 mg ml−1) and acepromazine (0.87 mg ml−1) IP and perfused with 10 ml of 0.1 M phosphate buffer (PB) followed by 25 ml of 4% paraformaldehyde (PFA) in 0.1 M PB. The brains were
removed, postfixed overnight (4% PFA in 0.1 M PB) and kept at 4 °C in 30% sucrose solution until cutting. Coronal sections (40 µm) were obtained using a microtome (Leica SM 2000R) and
serially collected in PBS. Sections were washed in PBS (3 × 10 min) and preincubated in PBS containing Triton X-100 (0.3%) and normal goat serum (5%) for 1 h. Sections were then incubated
overnight at room temperature in PBS containing Triton X-100 (0.3%), normal goat serum (1%) and rabbit anti-c-Fos (1:1,000, Cell Signaling Technology). Sections were then washed in PBS (3 ×
10 min), incubated with Alexa Fluor 488 goat anti-rabbit secondary antibody (1:400, Invitrogen) in PBS containing Triton X-100 (0.3%) and normal goat serum (1%) for 2 h, and washed in PBS (3
× 10 min). Sections were finally serially mounted in Vectashield medium with DAPI (Vector Laboratories). Image acquisition was performed using Zeiss Axio Scan 7. WESTERN BLOTTING Proteins
were extracted using lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 100 mM NaF, 10 sodium pyrophosphate, 5 mM EDTA, 250 mM sucrose, 1 mM DTT, 1 mM sodium orthovanadate, 1% Triton
X-100 and cOmplete Protease Inhibitor Cocktail (Roche). The total protein concentration in the samples was measured by the BCA protein assay. Protein concentrations were adjusted and
diluted in 4× SDS sample buffer. Proteins were separated using SDS–PAGE gels and transferred to poly(vinylidene difluoride) membranes. After blocking for 1 h with 5% BSA in TBST at room
temperature, membranes were incubated with the primary antibodies overnight at 4 °C (phosphorylated PKA substrates (1:1,000, Cell Signalling #9624) or tubulin (1:1,000, Abcam #ab4074)).
After washing, membranes were incubated with a secondary antibody at room temperature for 1 h. Protein bands were visualized with the Fusion FX7 system (MBI) and quantified using ImageJ
software. TUBE RESTRAINT AND STRESS TESTS Mice were put into 50-ml Falcon tubes (beginning at ~09:00) with holes at both ends so the tail was exposed and there was sufficient air flow3.
Control mice remained in their home cage with food and water removed at the initiation of tube restraint. Immediately following tube restraint, mice were either anaesthetized for tissue
collection or submitted to open-field test protocols (see below). Alternatively, social isolation was induced by individually housing previously group-housed mice for 3 days and comparing
them with their continually group-housed littermates31. In the cage-switch model, mice were removed from their home cage and placed in an identical cage with the dirty bedding of
nonlittermate males31. Nondisturbed socially housed littermates were applied as the controls. To assess stress-induced food intake and nausea, we provided mice with kaolin clay (Research
Diets, K50001) in addition to their regular diet for 3 days before testing. Mice were restrained (starting at ~09:00) for 4 h and returned to their cages (group-housed) with access to
standard chow and kaolin clay pellets, which were weighed before and after 18 h (~09:00 the following day). OPEN-FIELD AND LIGHT–DARK BOX TESTS Mice were moved to an isolated room the
evening before open-field testing to minimize stress on the day of testing. Immediately following 4-h tube restraint or 1 h following exogenous GDF15 injection (5 nM kg−1 IP) or 1 h
following exogenous adrenaline treatment (0.5 mg kg−1 IP), mice were placed in the centre of a box (40 × 40 × 40 cm, black walls and white plastic bottom). Mice remained in the box in the
same isolated room, and videos were recorded using a camera (GoPro, 1080p resolution and a sample rate of 3 frames per second) mounted to the ceiling. Videos were recorded for 20 min. The
two-dimensional mouse pose was analysed using DeepLabCut94 to extract the locations of arena boundaries and anatomical landmarks (nose, left ear, right ear, tail base). The DeepLabCut model
(ResNet50 architecture) was trained using 20 distinct frames (_k_-means clustering) from 20 input videos for a total of 200,000 epochs, resulting in a mean testing error of 3.74 pixels
across all tracked positional landmarks. Weighted spatial means of all four anatomical points taken to represent the location of the centre of the head were then analysed using custom MATLAB
(v2022b) scripts for the time spent in the centre (>8 cm from the arena boundaries), time spent in the periphery (<8 cm from one arena boundary), time spent in the corners (<8 cm
from two arena boundaries) and total distance moved during the test. Afterwards, the mice were returned to their home cages, and the boxes were thoroughly cleaned and left to dry before
subsequent rounds. The boxes in which groups (that is, WT/GFRAL, control/restraint, control/GDF15, etc.) were placed were systematically rotated to control for any possible differences in
box positioning during recording. Alternatively, mice were placed into an automated open-field test system (Opto-Varimex-5 Auto-Track, Columbus Instruments) for 20 min, and the time spent in
the centre and periphery and the total movement were analysed using equipment software. For light–dark box testing, mice were placed into the ‘light’ area and videos were recorded using a
camera (GoPro, 1080p resolution and a sample rate of 3 frames per second) for 10 min. The videos were analysed manually by a blinded assessor for the time spent by the mice in the light
area. BEHAVIOURAL AND METABOLIC ACTIVITY Metabolic and behavioural monitoring was conducted using the Promethion system (Sable Systems International). Data were collected following
acclimation to the system for 24 h. After acclimation, mice were injected with adrenaline (0.5 mg kg−1 IP) at 09:00, after which the mice remained in the system for another 24 h. Data on
food intake, physical activity and nonambulatory movements (beam breaks), oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (VCO2/VO2) and energy
expenditure (kcal h−1) were collected at 15-min intervals. STATISTICS Statistical analyses were performed using GraphPad Prism (version 10) or R software (RNA-seq). Data were analysed using
an unpaired _t_ test, a one-way analysis of variance (ANOVA) or a two-way ANOVA. When significant interactions were detected through ANOVA, subsequent post hoc analyses were reported with
appropriate corrections; where no post hoc correction is stated, there was no interaction detected. Correlational analyses were performed with Pearson’s correlation. No statistical methods
were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications18,26. Normality was tested using the Shapiro–Wilk test. Outliers were
defined as points outside 2 s.d. away from the group mean. All data are presented as mean ± s.e.m. with individual data points. Differences were considered significant when _P_ < 0.05.
REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Data that support the findings of
this study are available from the corresponding author on request. Bulk RNA-seq data have been deposited under accession code GSE267183. scRNA-seq data were obtained from the National Center
for Biotechnology Information Sequence Read Archive reference number SRP145475. Source data are provided with this paper. CODE AVAILABILITY The code for RNA-seq analysis and 2SMR is
available on request. REFERENCES * Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the
National Comorbidity Survey Replication. _Arch. Gen. Psychiatry_ 62, 617–627 (2005). Article PubMed PubMed Central Google Scholar * Ulrich-Lai, Y. M. & Herman, J. P. Neural
regulation of endocrine and autonomic stress responses. _Nat. Rev. Neurosci._ 10, 397–409 (2009). Article CAS PubMed PubMed Central Google Scholar * McCall, J. G. et al. CRH engagement
of the locus coeruleus noradrenergic system mediates stress-induced anxiety. _Neuron_ 87, 605–620 (2015). Article CAS PubMed PubMed Central Google Scholar * McCall, J. G. et al. Locus
coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. _eLife_ 6, e18247 (2017). Article PubMed PubMed Central Google Scholar * Burns, T. W., Mohs, J.
M., Langley, P. E., Yawn, R. & Chase, G. R. Regulation of human lipolysis. In vivo observations on the role of adrenergic receptors. _J. Clin. Invest._ 53, 338–341 (1974). Article CAS
PubMed PubMed Central Google Scholar * Elmadjian, F., Hope, J. M. & Lamson, E. T. Excretion of epinephrine and norepinephrine in various emotional states. _J. Clin. Endocrinol.
Metab._ 17, 608–620 (1957). Article CAS PubMed Google Scholar * Pollin, W. & Goldin, S. The physiological and psychological effects of intravenously administered epinephrine and its
metabolism in normal and schizophrenic men. II. Psychiatric observations. _J. Psychiatr. Res._ 1, 50–67 (1961). Article CAS PubMed Google Scholar * Clayton, E. C. & Williams, C. L.
Noradrenergic receptor blockade of the NTS attenuates the mnemonic effects of epinephrine in an appetitive light–dark discrimination learning task. _Neurobiol. Learn. Mem._ 74, 135–145
(2000). Article CAS PubMed Google Scholar * King, S. O. 2nd & Williams, C. L. Novelty-induced arousal enhances memory for cued classical fear conditioning: interactions between
peripheral adrenergic and brainstem glutamatergic systems. _Learn. Mem._ 16, 625–634 (2009). Article CAS PubMed Google Scholar * Gottschalk, L. A., Stone, W. N. & Gleser, G. C.
Peripheral versus central mechanisms accounting for antianxiety effects of propranolol. _Psychosom. Med._ 36, 47–56 (1974). Article CAS PubMed Google Scholar * Stone, W. N., Gleser, G.
C. & Gottschalk, L. A. Anxiety and β-adrenergic blockade. _Arch. Gen. Psychiatry_ 29, 620–622 (1973). Article CAS PubMed Google Scholar * Flemenbaum, A. Cyclic AMP, alpha and beta
receptors as a further explanation of the propranolol–FFA activity. _Psychosom. Med._ 37, 74 (1975). Article CAS PubMed Google Scholar * Gottschalk, L. A., Stone, W. N., Gleser, G. C.
& Iacono, J. M. Anxiety levels in dreams: relation to changes in plasma free fatty acids. _Science_ 153, 654–657 (1966). Article CAS PubMed Google Scholar * Bonn, J. A., Turner, P.
& Hicks, D. C. Beta-adrenergic-receptor blockade with practolol in treatment of anxiety. _Lancet_ 1, 814–815 (1972). Article CAS PubMed Google Scholar * Wang, D. et al. GDF15:
emerging biology and therapeutic applications for obesity and cardiometabolic disease. _Nat. Rev. Endocrinol._ 17, 592–607 (2021). Article CAS PubMed Google Scholar * Lockhart, S. M.,
Saudek, V. & O’Rahilly, S. GDF15: a hormone conveying somatic distress to the brain. _Endocr. Rev._ 41, bnaa007 (2020). Article PubMed PubMed Central Google Scholar * Yang, L. et al.
GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. _Nat. Med._ 23, 1158–1166 (2017). Article CAS PubMed Google Scholar * Day, E. A. et al.
Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. _Nat. Metab._ 1, 1202–1208 (2019). Article CAS PubMed Google Scholar * Borner, T.
et al. GDF15 induces an aversive visceral malaise state that drives anorexia and weight loss. _Cell Rep._ 31, 107543 (2020). Article CAS PubMed PubMed Central Google Scholar * Worth, A.
A. et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. _eLife_ 9, e55164 (2020). Article CAS PubMed PubMed
Central Google Scholar * Gil, C. I. et al. Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior. _Life Sci. Alliance_ 5, e202201495 (2022).
Article CAS Google Scholar * Low, J. K. et al. First behavioural characterisation of a knockout mouse model for the transforming growth factor (TGF)-β superfamily cytokine, MIC-1/GDF15.
_PLoS ONE_ 12, e0168416 (2017). Article PubMed PubMed Central Google Scholar * Cimino, I. et al. Activation of the hypothalamic–pituitary–adrenal axis by exogenous and endogenous GDF15.
_Proc. Natl Acad. Sci. USA_ 118, e2106868118 (2021). Article CAS PubMed PubMed Central Google Scholar * Ruud, L. E. et al. Activation of GFRAL+ neurons induces hypothermia and
glucoregulatory responses associated with nausea and torpor. _Cell Rep._ 43, 113960 (2024). Article PubMed Google Scholar * Sjøberg, K. A. et al. GDF15 increases insulin action in the
liver and adipose tissue via a β-adrenergic receptor-mediated mechanism. _Cell Metab._ 35, 1327–1340 (2023). Article PubMed Google Scholar * Wang, D. et al. GDF15 promotes weight loss by
enhancing energy expenditure in muscle. _Nature_ 619, 143–150 (2023). Article CAS PubMed PubMed Central Google Scholar * Stone, W. N., Gleser, G. C., Gottschalk, L. A. & Iacono, J.
M. Stimulus, affect, and plasma free fatty acid. _Psychosom. Med._ 31, 331–341 (1969). Article CAS PubMed Google Scholar * Williams, T. & Williams, C. L. Peripheral arousal-related
hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. _Behav. Brain Res._ 153, 87–95 (2004). Article PubMed Google Scholar * Markussen, L. K. et
al. Lipolysis regulates major transcriptional programs in brown adipocytes. _Nat. Commun._ 13, 3956 (2022). Article CAS PubMed PubMed Central Google Scholar * Bruford, E. A. et al.
Guidelines for human gene nomenclature. _Nat. Genet._ 52, 754–758 (2020). Article CAS PubMed PubMed Central Google Scholar * Qing, H. et al. Origin and function of stress-induced IL-6
in murine models. _Cell_ 182, 1660 (2020). Article CAS PubMed Google Scholar * Karusheva, Y. et al. The common H202D variant in GDF-15 does not affect its bioactivity but can
significantly interfere with measurement of its circulating levels. _J. Appl. Lab. Med._ 7, 1388–1400 (2022). Article PubMed Google Scholar * Jeong, K. H. et al. Impaired basal and
restraint-induced epinephrine secretion in corticotropin-releasing hormone-deficient mice. _Endocrinology_ 141, 1142–1150 (2000). Article CAS PubMed Google Scholar * Lafontan, M. &
Berlan, M. Fat cell adrenergic receptors and the control of white and brown fat cell function. _J. Lipid Res._ 34, 1057–1091 (1993). Article CAS PubMed Google Scholar * Patel, A. R. et
al. LPS induces rapid increase in GDF15 levels in mice, rats, and humans but is not required for anorexia in mice. _Am. J. Physiol. Gastrointest. Liver Physiol._ 322, G247–G255 (2022).
Article CAS PubMed Google Scholar * Haemmerle, G. et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. _Science_ 312, 734–737 (2006).
Article CAS PubMed Google Scholar * Zhou, C. et al. Sympathetic overdrive and unrestrained adipose lipolysis drive alcohol-induced hepatic steatosis in rodents. _Mol. Metab._ 78, 101813
(2023). Article CAS PubMed PubMed Central Google Scholar * Mayer, N. et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. _Nat. Chem. Biol._ 9, 785–787
(2013). Article CAS PubMed Google Scholar * L’Homme, L. et al. Adipose tissue macrophage infiltration and hepatocyte stress increase GDF-15 throughout development of obesity to MASH.
_Nat. Commun._ 15, 7173 (2024). Article PubMed PubMed Central Google Scholar * Burl, R. B. et al. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with
single-cell expression profiling. _Cell Metab._ 28, 300–309 (2018). Article CAS PubMed PubMed Central Google Scholar * Caputa, G., Castoldi, A. & Pearce, E. J. Metabolic adaptations
of tissue-resident immune cells. _Nat. Immunol._ 20, 793–801 (2019). Article CAS PubMed Google Scholar * Morgan, P. K. et al. Macrophage polarization state affects lipid composition and
the channeling of exogenous fatty acids into endogenous lipid pools. _J. Biol. Chem._ 297, 101341 (2021). Article CAS PubMed PubMed Central Google Scholar * Abe, I. et al.
Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation. _Dev. Cell_ 57, 2623–2637 (2022). Article CAS PubMed PubMed Central Google Scholar * Townsend, L. K. et
al. High-saturated-fat diet-induced obesity causes hepatic interleukin-6 resistance via endoplasmic reticulum stress. _J. Lipid Res._ 60, 1236–1249 (2019). Article CAS PubMed PubMed
Central Google Scholar * Townsend, L. K. et al. AMPK mediates energetic stress-induced liver GDF15. _FASEB J._ 35, e21218 (2021). Article CAS PubMed Google Scholar * Kratz, M. et al.
Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. _Cell Metab._ 20, 614–625 (2014). Article CAS PubMed PubMed Central
Google Scholar * Lu, J. F. et al. GDF15 is a major determinant of ketogenic diet-induced weight loss. _Cell Metab._ 35, 2165–2182 (2023). Article CAS PubMed Google Scholar * Fulton, S.,
Décarie-Spain, L., Fioramonti, X., Guiard, B. & Nakajima, S. The menace of obesity to depression and anxiety prevalence. _Trends Endocrinol. Metab._ 33, 18–35 (2022). Article CAS
PubMed Google Scholar * Wang, D. et al. Fatty acids increase GDF15 and reduce food intake through a GFRAL signaling axis. _Diabetes_ 73, 51–56 (2024). Article CAS PubMed Google Scholar
* Füzesi, T. et al. Hypothalamic CRH neurons orchestrate complex behaviours after stress. _Nat. Commun._ 7, 11937 (2016). Article PubMed PubMed Central Google Scholar * Bales, M. B. et
al. High fat diet blunts stress-induced hypophagia and activation of Glp1r dorsal lateral septum neurons in male but not in female mice. _Mol. Metab._ 64, 101571 (2022). Article CAS
PubMed PubMed Central Google Scholar * Holt, M. K. et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced
hypophagia, and limit unusually large intakes of food. _Diabetes_ 68, 21–33 (2019). Article CAS PubMed Google Scholar * Fortin, S. M. et al. The locus coeruleus contributes to the
anorectic, nausea, and autonomic physiological effects of glucagon-like peptide-1. _Sci. Adv._ 9, eadh0980 (2023). Article CAS PubMed PubMed Central Google Scholar * Hsu, J.-Y. et al.
Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. _Nature_ 550, 255–259 (2017). Article PubMed Google Scholar * Niu, M. et al. Claustrum mediates
bidirectional and reversible control of stress-induced anxiety responses. _Sci. Adv._ 8, eabi6375 (2022). Article CAS PubMed PubMed Central Google Scholar * Calhood, G. G. & Tye, K.
M. Resolving the neural circuits of anxiety. _Nat. Neurosci._ 18, 1394–1404 (2015). Article Google Scholar * Chandler, D. J. et al. Redefining noradrenergic neuromodulation of behavior:
impacts of a modular locus coeruleus architecture. _J. Neurosci._ 39, 8239–8249 (2019). Article CAS PubMed PubMed Central Google Scholar * Hirschberg, S., Li, Y., Randall, A., Kremer,
E. J. & Pickering, A. E. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in
rats. _eLife_ 6, e29808 (2017). Article PubMed PubMed Central Google Scholar * Kim, S.-Y. et al. Diverging neural pathways assemble a behavioural state from separable features in
anxiety. _Nature_ 496, 219–223 (2013). Article CAS PubMed PubMed Central Google Scholar * Cowley, N. A. et al. Dynorphin controls the gain of an amygdalar anxiety circuit. _Cell Rep._
14, 2774–2783 (2016). Article Google Scholar * Giplin, N. W., Herman, M. A. & Roberto, M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. _Biol.
Psychiatry_ 77, 859–869 (2015). Article Google Scholar * Borodovitsyna, O., Duffy, B. C., Pickering, A. E. & Chandler, D. J. Anatomically and functionally distinct locus coeruleus
efferents mediate opposing effects on anxiety-like behavior. _Neurobiol. Stress_ 13, 100284 (2020). Article PubMed PubMed Central Google Scholar * Sun, Y., Qian, L., Xu, L., Hunt, S.
& Sah, P. Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala. _Mol. Psychiatry_ 28, 4163–4174 (2023). Article
PubMed Google Scholar * Sabatini, P. V. et al. GFRAL-expressing neurons suppress food intake via aversive pathways. _Proc. Natl Acad. Sci. USA_ 118, e2021357118 (2021). Article CAS
PubMed PubMed Central Google Scholar * O’Rahilly, S. GDF15—from biomarker to allostatic hormone. _Cell Metab._ 26, 807–808 (2017). Article PubMed Google Scholar * Patel, S. et al.
GDF15 provides an endocrine signal of nutritional stress in mice and humans. _Cell Metab._ 29, 707–718 (2019). Article CAS PubMed PubMed Central Google Scholar * Florsheim, E. B. et al.
Immune sensing of food allergens promotes avoidance behaviour. _Nature_ 620, 643–650 (2023). Article CAS PubMed PubMed Central Google Scholar * Luan, H. H. et al. GDF15 is an
inflammation-induced central mediator of tissue tolerance. _Cell_ 178, 1231–1244 (2019). Article CAS PubMed PubMed Central Google Scholar * Borner, T. et al. GDF15 induces anorexia
through nausea and emesis. _Cell Metab._ 31, 351–362 (2020). Article CAS PubMed PubMed Central Google Scholar * Lönnqvist, F., Wennlund, A., Wahrenberg, H. & Arner, P. Effects of
mental stress on lipolysis in humans. _Metabolism_ 41, 622–630 (1992). Article PubMed Google Scholar * Klein, A. B. et al. Pharmacological but not physiological GDF15 suppresses feeding
and the motivation to exercise. _Nat. Commun._ 12, 1041 (2021). Article CAS PubMed PubMed Central Google Scholar * Macia, L. et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15)
decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. _PLoS ONE_ 7, e34868 (2012). Article CAS PubMed PubMed Central Google Scholar
* Benichou, O. et al. Discovery, development, and clinical proof of mechanism of LY3463251, a long-acting GDF15 receptor agonist. _Cell Metab._ 35, 274–286 (2023). Article CAS PubMed
Google Scholar * Zhang, Y. et al. Activity-balanced GLP-1/GDF15 dual agonist reduces body weight and metabolic disorder in mice and non-human primates. _Cell Metab._ 35, 287–298 (2023).
Article PubMed Google Scholar * Deng, Y.-T. et al. Atlas of the plasma proteome in health and disease in 53,026 adults. _Cell_ 188, 253–271 (2025). Article CAS PubMed Google Scholar *
Patsalos, A. et al. A growth factor-expressing macrophage subpopulation orchestrates regenerative inflammation via GDF-15. _J. Exp. Med._ 219, e20210420 (2022). Article CAS PubMed Google
Scholar * Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. _Nat. Rev. Immunol._ 11, 625–632 (2011). Article CAS PubMed PubMed Central
Google Scholar * Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. _Nat. Rev. Immunol._ 5, 243–251 (2005). Article CAS PubMed Google
Scholar * Le Thuc, O. & García-Cáceres, C. Obesity-induced inflammation: connecting the periphery to the brain. _Nat. Metab._ 6, 1237–1252 (2024). Article PubMed Google Scholar *
Bachman, E. S. et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. _Science_ 297, 843–845 (2002). Article CAS PubMed Google Scholar * Kaur, S. et al.
Adipose-specific ATGL ablation reduces burn injury-induced metabolic derangements in mice. _Clin. Transl. Med._ 11, e417 (2021). Article CAS PubMed PubMed Central Google Scholar * Dibe,
H. A., Townsend, L. K., McKie, G. L. & Wright, D. C. Epinephrine responsiveness is reduced in livers from trained mice. _Physiol. Rep._ 8, e14370 (2020). Article PubMed PubMed Central
Google Scholar * Pedersen, L. et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. _Cell Metab._ 23, 554–562
(2016). Article CAS PubMed Google Scholar * Townsend, L. K. et al. Loss of glucagon signaling alters white adipose tissue browning. _FASEB J._ 33, 4824–4835 (2019). Article CAS PubMed
Google Scholar * Medak, K. D., McKie, G. L., Shamshoumn, H., Seguin, I. & Wright, D. C. The glucose lowering effects of CL 316,243 dissipate with repeated use and are rescued
bycilostamide. _Physiol. Rep._ 10, e15187 (2022). Article CAS PubMed PubMed Central Google Scholar * Muglia, L. J. et al. Corticotropin-releasing hormone links pituitary
adrenocorticotropin gene expression and release during adrenal insufficiency. _J. Clin. Invest._ 105, 1269–1277 (2000). Article CAS PubMed PubMed Central Google Scholar * Laryea, G.,
Schütz, G. & Muglia, L. J. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. _Mol. Endocrinol._ 27, 1655–1665 (2013). Article CAS
PubMed PubMed Central Google Scholar * Mottillo, E. P. et al. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function.
_Cell Metab._ 24, 118–129 (2016). Article CAS PubMed PubMed Central Google Scholar * Galic, S. et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and
insulin resistance in obesity. _J. Clin. Invest._ 121, 4903–4915 (2011). Article CAS PubMed PubMed Central Google Scholar * Hemani, G. et al. The MR-Base platform supports systematic
causal inference across the human phenome. _eLife_ 7, e34408 (2018). Article PubMed PubMed Central Google Scholar * Taliun, S. A. G. & Evans, D. M. Ten simple rules for conducting a
Mendelian randomization study. _PLoS Comput. Biol._ 17, e1009238 (2021). Article Google Scholar * Sanderson, E. et al. Mendelian randomization. _Nat. Rev. Methods Primers_ 2, 6 (2022).
Article CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. _eLife_ 10, e65554 (2021).
Article CAS PubMed PubMed Central Google Scholar * Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. _Nat. Protoc._ 14, 2152–2176 (2019).
Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS G.R.S. acknowledges the support of a Diabetes Canada Investigator Award (OG-3-22-5645-GS), a Canadian Institutes of
Health Research (CIHR) Foundation Grant (201709FDN-CEBA-116200), a Tier 1 Canada Research Chair in Metabolic Diseases and a J. Bruce Duncan Endowed Chair in Metabolic Diseases. L.K.T.
acknowledges the support of a CIHR Post-doctoral Fellowship Award, a Michael DeGroote Fellowship Award in Basic Biomedical Science and a MITACS fellowship supported by Novo Nordisk. D.W.
acknowledges the support of fellowship grants from the McMaster Institute for Research on Aging (MIRA) at McMaster University. E.M.D. acknowledges the support of a Vanier Canada Graduate
Scholarship. A.M. was supported by the Natural Sciences and Engineering Research Council of Canada. D.P.B. acknowledges the support of the GSK Chair in Diabetes of Université de Sherbrooke
and an FRQS J1 salary award. A.C.C. acknowledges the support of a Tier 1 Canada Research Chair in Molecular Imaging of Diabetes and a CIHR Grant (no. 299962). J.G.M. acknowledges the support
of a National Institutes of Health award (R01NS117899). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre for Metabolism, Obesity and Diabetes Research, McMaster University, Hamilton,
Ontario, Canada Logan K. Townsend, Dongdong Wang, Russta Fayyazi, Evangelia E. Tsakiridis, Eric M. Desjardins, Zeel Patel, Celina M. Valvano, Junfeng Lu, Alice E. Payne, Ofure Itua, Daniel
M. Marko, Jonathan D. Schertzer, Katherine M. Morrison & Gregory R. Steinberg * Division of Endocrinology and Metabolism, Department of Medicine, McMaster University, Hamilton, Ontario,
Canada Logan K. Townsend, Dongdong Wang, Russta Fayyazi, Evangelia E. Tsakiridis, Eric M. Desjardins, Zeel Patel, Celina M. Valvano, Junfeng Lu, Alice E. Payne & Gregory R. Steinberg *
Sunnybrook Research Institute, University of Toronto, Toronto, Ontario, Canada Carly M. Knuth * Department of Health Science, Brock University, St. Catherines, Ontario, Canada Ahmad Mohammad
& Rebecca E. K. MacPherson * Department of Anesthesiology, Washington University in St. Louis, St. Louis, MO, USA Léa J. Becker & Jordan G. McCall * Department of Biochemistry and
Biomedical Sciences, McMaster University, Hamilton, Ontario, Canada Ofure Itua, Daniel M. Marko, Jonathan D. Schertzer & Gregory R. Steinberg * Human Health and Nutritional Science,
University of Guelph, Guelph, Ontario, Canada Kyle D. Medak * Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, Ontario, Canada Daniel M. Marko &
Jonathan D. Schertzer * School of Kinesiology, University of British Columbia, Vancouver, British Columbia, Canada David C. Wright * British Columbia Children’s Hospital Research Institute,
Vancouver, British Columbia, Canada David C. Wright * Faculty of Land and Food Systems, University of British Columbia, Vancouver, British Columbia, Canada David C. Wright * Department of
Kinesiology, Brock University, St. Catherines, Ontario, Canada Shawn M. Beaudette * Department of Pediatrics, McMaster University, Hamilton, Ontario, Canada Katherine M. Morrison *
Department of Medicine, Faculty of Medicine and Health Sciences, Université de Sherbrooke, Centre de recherche du Centre hospitalier universitaire de Sherbrooke, Sherbrooke, Quebec, Canada
André C. Carpentier & Denis P. Blondin * David Braley Cardiac, Vascular and Stroke Research Institute, Hamilton, Ontario, Canada Marc G. Jeschke * Hamilton General Hospital, Hamilton
Health Sciences, Hamilton, Ontario, Canada Marc G. Jeschke * Department of Surgery, McMaster University, Hamilton, Ontario, Canada Marc G. Jeschke Authors * Logan K. Townsend View author
publications You can also search for this author inPubMed Google Scholar * Dongdong Wang View author publications You can also search for this author inPubMed Google Scholar * Carly M. Knuth
View author publications You can also search for this author inPubMed Google Scholar * Russta Fayyazi View author publications You can also search for this author inPubMed Google Scholar *
Ahmad Mohammad View author publications You can also search for this author inPubMed Google Scholar * Léa J. Becker View author publications You can also search for this author inPubMed
Google Scholar * Evangelia E. Tsakiridis View author publications You can also search for this author inPubMed Google Scholar * Eric M. Desjardins View author publications You can also
search for this author inPubMed Google Scholar * Zeel Patel View author publications You can also search for this author inPubMed Google Scholar * Celina M. Valvano View author publications
You can also search for this author inPubMed Google Scholar * Junfeng Lu View author publications You can also search for this author inPubMed Google Scholar * Alice E. Payne View author
publications You can also search for this author inPubMed Google Scholar * Ofure Itua View author publications You can also search for this author inPubMed Google Scholar * Kyle D. Medak
View author publications You can also search for this author inPubMed Google Scholar * Daniel M. Marko View author publications You can also search for this author inPubMed Google Scholar *
Jonathan D. Schertzer View author publications You can also search for this author inPubMed Google Scholar * David C. Wright View author publications You can also search for this author
inPubMed Google Scholar * Shawn M. Beaudette View author publications You can also search for this author inPubMed Google Scholar * Katherine M. Morrison View author publications You can
also search for this author inPubMed Google Scholar * André C. Carpentier View author publications You can also search for this author inPubMed Google Scholar * Denis P. Blondin View author
publications You can also search for this author inPubMed Google Scholar * Rebecca E. K. MacPherson View author publications You can also search for this author inPubMed Google Scholar *
Jordan G. McCall View author publications You can also search for this author inPubMed Google Scholar * Marc G. Jeschke View author publications You can also search for this author inPubMed
Google Scholar * Gregory R. Steinberg View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The overall conceptualization of studies included in
this work was done by L.K.T. and G.R.S. Mouse experiments were designed, managed, performed and analysed by L.K.T., D.W., C.M.K., K.D.M., D.M.M., E.M.D., Z.P., E.E.T., J.L., A.E.P., O.I. and
C.M.V. RNA-seq analyses were performed by D.W. and R.F. Mouse behaviour studies were done with the assistance of A.M., S.M.B. and R.E.K.M. c-Fos staining experiments were designed, managed,
performed and analysed by L.J.B. and J.G.M. Adipocyte and macrophage experiments were completed by L.K.T., E.E.T., E.M.D., Z.P. and C.M.V. L.K.T. and G.R.S. wrote the paper, with the
assistance of J.G.M., M.G.J., A.C.C., D.P.B., K.M.M., J.D.S. and D.C.W. The paper was reviewed, edited and approved by all the authors. CORRESPONDING AUTHOR Correspondence to Gregory R.
Steinberg. ETHICS DECLARATIONS COMPETING INTERESTS G.R.S. is a cofounder and shareholder of Espervita Therapeutics, a company developing new medications for fibrosis and cancer. McMaster
University has received funding from Cambrian Biosciences, Catalym, Espervita Therapeutics, Esperion Therapeutics, Merck, Nestle, Novo Nordisk and Poxel Pharmaceuticals for research
conducted in the laboratory of G.R.S. G.R.S. has received consulting and speaking fees from AstraZeneca, CurieBio, Eli Lilly, Esperion Therapeutics, Korro Bio, Keros Therapeutics, Merck,
Novo Nordisk, Versant Ventures and Poxel Pharmaceuticals. A.C.C. received consulting and speaking fees from Eli Lilly, HLS Therapeutics, Janssen, Novartis Pharmaceuticals Canada and Novo
Nordisk Canada. The other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Metabolism_ thanks Mónica Moreira-Rodrigues and the other, anonymous,
reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the _Nature Metabolism_ team. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 SEX- AND HOUSING
TEMPERATURE-INDEPENDENT EFFECTS OF EXOGENOUS EPINEPHRINE ON GDF15. A) Pathway analysis from RNA-sequencing of gWAT following 1-hr epinephrine treatment. _n_ = _6_ per group. B) Heat map
showing ligand-encoded genes from RNA-sequencing of gWAT following 1-hr epinephrine treatment. _n_ = _6_ per group. C) Circulating GDF15 from age-matched male (saline _n_ = _9_, epinephrine
_n_ = _9_) and female mice (saline _n_ = _10_, epinephrine _n_ = _11_) following 1-hr epinephrine with fold-change relative to baseline levels (inset). Data presented as mean ± s.e.m. with
p-values calculated using 2-way ANOVA with post-hoc test and Tukey’s correction and unpaired two-tail t-test, respectively. D) Time-course of circulating GDF15 post-saline (RT _n_ = _5_, TN
_n_ = _4_) or epinephrine (RT _n_ = _5_, TN _n_ = _5_) in mice housed at room temperature (RT ~ 22 °C) or thermoneutrality (TN ~ 29 °C) for 4 weeks. n = 4-5/group. Data presented as mean ±
s.e.m. with p-values calculated using 2-way ANOVA at each time point. E) Serum epinephrine in control (_n_ = _9_ mice), 1-hr post IP epinephrine (_n_ = _7_), and 4-hr physical restraint (_n_
= _8_). Data presented as mean ± s.e.m. with p-values calculated using 1-way ANOVA with post-hoc test and Tukey’s correction. Source data EXTENDED DATA FIG. 2 CHRONIC PSYCHOLOGICAL STRESS
NOT ASSOCIATED WITH ELEVATED CIRCULATING GDF15 LEVELS IN HUMANS. A) Serum GDF15 levels in children with overweight and obesity with (_n_ = _23_) or without (_n_ = _24_) diagnosis of anxiety.
Data presented as mean ± s.e.m. with p-values calculated using unpaired two-tail t-test. B) Scatter plot of the SNP-effect on GDF15 and single nucleotide polymorphism (SNP)-effect on
nervous anxiety tension or depression in humans by using two sample Mendelian Randomization (2SMR). Data presented as mean ± error bars indicate 95% CI, n = 407,746 participants in UK
Biobank. MR analysis was performed by using Simple median method, MR weighted mode estimator, Weighted median method, MR Egger regression, Inverse variance weighted methods. C) Single SNP
analysis of GDF15 on anxiety and depression in humans, Data presented as mean ± error bars indicate 95% CI, n = 407,746 participants in UK Biobank. Source data EXTENDED DATA FIG. 3 EFFECTS
OF LPS IN BETA RECEPTOR KNOCKOUT MICE. A) Serum GDF15 from WT and β-adrenergic receptor knockout (BR-/-) mice following 2-hr LPS treatment (WT _n_ = _6_, BR-/- _n_ = _6_) or control (WT _n_
= _6_, BR-/- _n_ = _8_). Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. B) Blood glucose from WT and BR-/- mice following 2-hr LPS treatment (WT _n_ = _6_, BR-/-
_n_ = _6_) or control (WT _n_ = _6_, BR-/- _n_ = _8_). Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. Source data EXTENDED DATA FIG. 4 RESTRAINT STRESS IN
ADATGLFLOX/FLOX AND ADATGL-/- MICE. A) Blood glucose levels in AdATGLflox/flox mcie (control _n_ = _6_, restraint _n_ = _6_) and AdATGL-/- (control _n_ = _13_, restraint _n_ = _11_). Data
presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA with post-hoc test and Tukey’s correction. B) Total distance for AdATGLflox/flox mice (control _n_ = _6_, restraint _n_
= _6_) and AdATGL-/- (control _n_ = _13_, restraint _n_ = _11_) during open-field test. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA with post-hoc test and
Tukey’s correction. C) Time in the centre for AdATGLflox/flox mice (control _n_ = _6_, restraint _n_ = _6_) and AdATGL-/- (control _n_ = _13_, restraint _n_ = _11_) during open-field test.
Data presented as mean ± s.e.m. with no statistical test performed. Source data EXTENDED DATA FIG. 5 MACROPHAGES ARE THE MOST LIKELY SOURCE OF GDF15 WITHIN ADIPOSE TISSUE. A) _Fgf21_
expression in mouse adipocyte and SVF from gWAT post-saline (_n_ = _4_ per group) or epinephrine (_n_ = _5_ per group). Data presented as mean ± s.e.m. with p-values calculated using 2-way
ANOVA with post-hoc test and Tukey’s correction. B) _Leptin_ expression in mouse adipocyte and SVF from gWAT post-saline (_n_ = _3_ per group) or epinephrine (_n_ = _4_ per group). Data
presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. C) t-SNE plot of Lin- stromal vascular cells from gWAT of control mice and mice treated with CL for 3 days. Clustering
identified 6 major cell types/states. Clusters are highlighted in different colors. The data set was queried for cells expressing _Gdf15_. Heatmap shows expression of _Gdf15_ and other
cell-identifying factors in the various identified cell populations. D) _F480/Adgre1_ expression in mouse F480+ and F480- fractions of SVF from gWAT 1-hr post-epinephrine treatment (0.5
mg/kg). Data presented as mean ± s.e.m. with _n_ = _3_ per group. p-values calculated using 2-way ANOVA. E) _Arg1_ and _Nos2_ expression in bone marrow-derived macrophages (BMDMs) polarized
to either M1-like or M2-like. _n_ = _6_ per group. Individual data points represent duplicates from 3 independent experiments. Data presented as mean ± s.e.m. with p-values calculated using
1-way ANOVA with post-hoc test and Tukey’s correction. F) GDF15 levels in media from BMDMs polarized to either M1-like or M2-like for 24-hrs. _n_ = _3_ per group. Individual data points
represent triplicates from 3 independent experiments. Data presented as mean ± s.e.m. with p-values calculated using 1-way ANOVA with post-hoc test and Tukey’s correction. G) GDF15 levels in
media from M2-like BMDMs treated with epinephrine (1 μM) or tunicamycin (5 ng/mL) for 24-hrs. _n_ = _3_ per group. Individual data points represent triplicates from 3 independent
experiments. Data presented as mean ± s.e.m. with p-values calculated using 1-way ANOVA with post-hoc test and Tukey’s correction. H) Volcano plot showing fatty acid transporters identified
between M1- and M2-like macrophage populations from scRNA-seq data. Source data EXTENDED DATA FIG. 6 LIPIDS AND ANXIETY. A) Time in the centre during open-field test (% total) following 4-hr
palm oil gavage (10 mL/kg) gavage in WT and _Gfral_ -/- mice. _n_ = _8_ per group. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. B) Time in the centre during
open-field test for chow (WT _n_ = _6_ mice, _Gfral_-/- _n_ = _6) or_ 4-week high-fat diet fed mice (WT _n_ = _7_ mice, _Gfral_-/- _n_ = _6)_. Data presented as mean ± s.e.m. with p-values
calculated using 2-way ANOVA. C) Total distance traveled during open-field test for chow (WT _n_ = _6_ mice, _Gfral_-/- _n_ = _6) or_ 4-week high-fat diet fed mice (WT _n_ = _7_ mice,
_Gfral_-/- _n_ = _6)_. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. Source data EXTENDED DATA FIG. 7 GDF15-GFRAL SIGNALLING IS IMPORTANT FOR BEHAVIOURAL
RESPONSES TO EPINEPHRINE. A) Non-ambulatory movements, B) Total physical activity, C) Total food intake, D) respiratory exchange ratio (RER), E) Energy expenditure in WT (saline _n_ = _4_,
epinephrine _n_ = _4_) and _Gfral_-/- mice (saline _n_ = _4_, epinephrine _n_ = _4_). Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA with post-hoc test and
Tukey’s correction. F) Chow intake (18-hrs) following tube restraint in WT (control _n_ = _7_, restraint _n_ = _7_) and _Gfral_ -/- mice (control _n_ = _10_, restraint _n_ = _9_). Data
presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. G) Kaolin clay intake (18-hrs) following tube restraint in WT (control _n_ = _7_, restraint _n_ = _7_) and _Gfral_ -/-
mice (control _n_ = _10_, restraint _n_ = _9_). Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. H) Time in center and total distance during open-field test
following GDF15 treatment with representative images showing movement of individual mice. _n_ = _9_ per group. Data presented as mean ± s.e.m. with p-values calculated using unpaired
two-tail t-test. I) Time in light during light-dark box test following vehicle (_n_ = _8_) or GDF15 treatment (_n_ = _9_). Data presented as mean ± s.e.m. with p-values calculated using
unpaired two-tail t-test. J) Daily chow food intake throughout repeated GDF15 (5 nM/kg IP). _n_ = _12_ per group. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA
with post-hoc test and Tukey’s correction. K) Time in the center during open-field test following GDF15 treatment (5 nM/kg IP) with representative images of movement of individual mice. _n_
= _6_ per group. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. Chr. GDF15: Chronic GDF15. L) Total distance traveled during open-field test following GDF15
treatment (5 nM/kg IP) with representative images showing representative movement of individual mice. _n_ = _6_ per group. Data presented as mean ± s.e.m. with p-values calculated using
2-way ANOVA. Chr. GDF15: Chronic GDF15. Source data EXTENDED DATA FIG. 8 NEITHER THE LOCUS COERULEUS, NOR ITS ANGIOGENIC DOWNSTREAM BRAIN REGIONS, ARE ACTIVATED BY GDF15. A) Quantification
of c-Fos positive cells in the locus coeruleus 90-min following GDF15 treatment (5 nm/kg IP), with representative pictures of the staining for TH and c-Fos. _n_ = _4_ per group. Data
presented as mean ± s.e.m. with p-values calculated using unpaired two-tail t-test. Scale bar=200 µm. 4 V: 4th ventricle, LC: locus coeruleus, TH: tyrosine hydroxylase. B) Quantification of
c-Fos positive cells in the PFC and BLA 90-min following GDF15 treatment (5 nm/kg IP), with representative pictures of the staining. _n_ = _4_ per group. Data presented as mean ± s.e.m. with
p-values calculated using unpaired two-tail t-test. Scale bar=200 µm. BLA: basolateral amygdala, cc: corpus callosum, mPFC: medial prefrontal cortex. C) Serum GDF15 in WT (control _n_ =
_9_, restraint _n_ = _9_) and _Gfral_ -/- mice (control _n_ = _8_, restraint _n_ = _8_). Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA with Tukey’s post-hot
correction. D) Serum corticosterone in WT (control _n_ = 4, CRH _n_ = _6_) and _Gfral_ -/- mice (control _n_ = _4_, CRH _n_ = _6_). Data presented as mean ± s.e.m. with p-values calculated
using 2-way ANOVA at each timepoint. E) Serum adrenocorticotropic hormone (ACTH) in WT (control _n_ = 4, CRH _n_ = _6_) and _Gfral_ -/- mice (control _n_ = _4_, CRH _n_ = _6_). Data
presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA at each timepoint. F) Serum corticosterone 6-hr post treatment with dexamethasone (Dexa, 100 µg/kg IP). _n_ = _4_ per
group. Data presented as mean ± s.e.m. with p-values calculated using 2-way ANOVA. Source data SUPPLEMENTARY INFORMATION REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Source data. SOURCE
DATA FIG. 2 Source data. SOURCE DATA FIG. 2 Unprocessed western blots and gels. SOURCE DATA FIG. 3 Source data. SOURCE DATA FIG. 4 Source data. SOURCE DATA EXTENDED DATA FIG. 1 Source data.
SOURCE DATA EXTENDED DATA FIG. 2 Source data. SOURCE DATA EXTENDED DATA FIG. 3 Source data. SOURCE DATA EXTENDED DATA FIG. 4 Source data. SOURCE DATA EXTENDED DATA FIG. 5 Source data. SOURCE
DATA EXTENDED DATA FIG. 6 Source data. SOURCE DATA EXTENDED DATA FIG. 7 Source data. SOURCE DATA EXTENDED DATA FIG. 8 Source data. RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed
material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are
included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Townsend, L.K., Wang, D., Knuth, C.M. _et al._ GDF15 links
adipose tissue lipolysis with anxiety. _Nat Metab_ 7, 1004–1017 (2025). https://doi.org/10.1038/s42255-025-01264-3 Download citation * Received: 27 August 2024 * Accepted: 06 March 2025 *
Published: 15 April 2025 * Issue Date: May 2025 * DOI: https://doi.org/10.1038/s42255-025-01264-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)