- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
We investigated the neural correlates for chronic cancer pain conditions by retrospectively analyzing whole brain regions on 18F-fluoro-2-deoxyglucose-positron emission tomography images
acquired from 80 patients with head and neck squamous cell carcinoma and esophageal cancer. The patients were divided into three groups according to perceived pain severity and type of
analgesic treatment, namely patients not under analgesic treatment because of no or minor pain, patients with good pain control under analgesic treatment, and patients with poor pain control
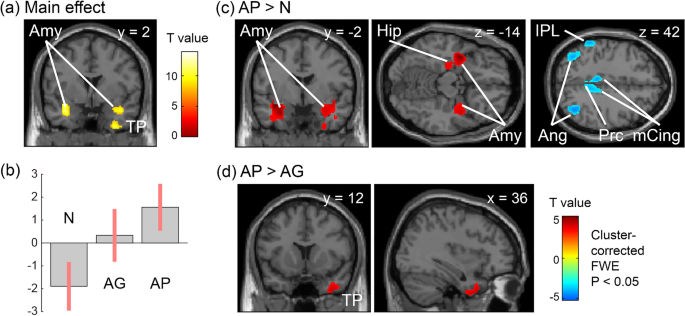
despite analgesic treatment. Uncontrollable cancer pain enhanced the activity of the hippocampus, amygdala, inferior temporal gyrus, and temporal pole. Metabolic connectivity analysis
further showed that amygdala co-activation with the hippocampus was reduced in the group with poor pain control and preserved in the groups with no or minor pain and good pain control. The
increased although imbalanced activity of the medial temporal regions may represent poor pain control in patients with cancer. The number of patients who used anxiolytics was higher in the
group with poor pain control, whereas the usage rates were comparable between the other two groups. Therefore, further studies should investigate the relationship between psychological
conditions and pain in patients with cancer and analyze the resultant brain activity.
Trial registration: This study was registered at clinicaltrials.gov on 9/3/20 (NCT04537845).
Pain is one of the most prevalent symptoms in patients with cancer. Overall, 55% of patients with cancer experience pain during treatment, and the prevalence increases up to 80% depending on
the stages or sites of the cancer1,2,3. The actual etiology of cancer pain remains poorly understood owing to the various potential direct and indirect causes4, which has resulted in
approximately one-fourth to half of the patients with cancer reporting cancer-pain undertreatment5,6,7.
Evidence suggests a strong interaction between pain and emotion, that is, negative emotions can worsen pain experiences8, and chronic pain also frequently coexists with anxiety and
depression9. The limbic system integrates information from the sensory and affective components of pain and anxiety, which include the limbic lobe, hippocampal formation, amygdala, septal
area, and hypothalamus10,11. The altered response of the amygdala to chronic pain may further affect the functional brain networks related to interoception, emotion, and cognitive status,
all of which may result in maladaptive pain perception12,13. Elucidating the cortical representation of cancer pain perception and the differences in cortical responses between well-managed
and poorly-managed chronic cancer pain could further improve our understanding of the central mechanism of chronic cancer pain and lead to potential treatments.
Therefore, we investigated the resting-state brain activity of patients with cancer reporting various intensities of perceived pain by analyzing 18F-fluoro-2-deoxyglucose (FDG)-positron
emission tomography/computed tomography (PET/CT) images acquired for routine clinical examination purposes. FDG-PET images can be used for quantitative evaluations in clinical oncology14 and
serve as a functional neuroimaging tool to investigate brain areas and metabolic connectivity in various pain conditions15,16,17,18. Our previous animal study on cancer-induced bone pain
confirmed the reliability of FDG-PET for cancer pain investigations19. However, the utilization of FDG-PET in patients with cancer pain had been limited to a small-group study20 with results
that were difficult to interpret because of the various types of cancers and other clinical conditions included in the sample.
We investigated signature cortical responses reflecting good or poor pain control by comparing the cortical activity of 80 patients with cancer across three groups of perceived pain
intensities and on pain control statuses based on analgesic use. We hypothesized that cancer pain activates the amygdala and limbic system compontents involved in pain-emotion interactions
and that this activity is inhibited in patients who are appropriately treated with analgesics. We also investigated differences in the cortical metabolic network emerging from these medial
temporal regions within other resting-state networks to determine the functional organization in conditions of good and poor control of chronic cancer pain. The working hypothesis is that
the pain-related changes in the metabolic network may also be ameliorated under the good pain control conditions.
Patients who underwent FDG-PET/CT between March 2015 and July 2020 at the National Taiwan University Hospital were retrospectively enrolled. This study was approved by the institutional
ethics committees of the National Taiwan University Hospital and conducted in accordance with the Declaration of Helsinki. The requirement for informed patient consent was waived owing to
the retrospective nature of the study. We included patients diagnosed with head and neck squamous cell carcinoma or esophageal cancer who completed a PET/CT study for the clinical indication
of cancer staging. These cancer types were selected because they share a cancer cell origin, clinical risk factors, and genetic alterations in tumors21,22,23,24. Patients with brain
metastases based on brain magnetic resonance imaging or CT and patients without a pain rating on the day of the PET scan were excluded. Data collected for analysis included age, sex, cancer
diagnosis, pain condition including location, pain score, medication, and FDG-PET/CT findings of the patients.
We divided the patients into three groups: patients treated with analgesics with good pain control (AG group, numerical rating scale [NRS] scores ranging from 0 [no pain] to 10 [worst pain
imaginable];