- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The metabolism of polyunsaturated fatty acids (PUFAs) plays an important role in male reproduction. Linoleic and alpha-linolenic acids need to be provided in the diet and they are
converted into long chain polyunsaturated fatty acids by steps of elongation and desaturation, exerted by elongases 2 (ELOVL2) and 5 (ELOVL5) and Δ5- (FADS1) and Δ6-desaturase (FADS2). This
study aims to assess the gene expression and localization of enzymes involved in the synthesis of n-3 and n-6 long-chain PUFAs in control rabbits and those fed diets containing 10% extruded
flaxseed. Enzyme and PUFA localization were assessed in the testes and epididymis by immunofluorescence. Testes showed high gene expression of FADS2, ELOVL2 and ELOVL5 and low expression of
FADS1. Intermediate metabolites, enzymes and final products were differently found in Leydig, Sertoli and germinal cells. FADS2 was localized in interstitial cells and elongated spermatids;
ELOVL5 in meiotic cells; FADS1 was evident in interstitial tissue, Sertoli cells and elongated spermatids; ELOVL2 in interstitial cells. Epididymal vesicles were positive for FADS1, ELOVL2
and ELOVL5 as well as docosahexaenoic, eicosapentaenoic, and arachidonic acids. This knowledge of fatty acids (FA) metabolism in spermatogenesis and the influence of diet on FA profile could
help identify causes of male infertility, suggesting new personalized therapy. SIMILAR CONTENT BEING VIEWED BY OTHERS OMEGA-6 HIGHLY UNSATURATED FATTY ACIDS IN LEYDIG CELLS FACILITATE MALE
SEX HORMONE PRODUCTION Article Open access 21 September 2022 MOLECULAR CHARACTERISTICS AND REGULATORY ROLE OF INSULIN-LIKE GROWTH FACTOR 1 GENE IN TESTICULAR LEYDIG CELLS OF TIBETAN SHEEP
Article Open access 22 October 2024 FLUTAMIDE TREATMENT REVEALS A RELATIONSHIP BETWEEN STEROIDOGENIC ACTIVITY OF LEYDIG CELLS AND ULTRASTRUCTURE OF THEIR MITOCHONDRIA Article Open access 02
July 2021 INTRODUCTION Lipids are essential for spermatogenesis as they are crucial for the membrane remodelling of developing germ cells. The testes have a characteristic lipid composition
with an amount of long-chain polyunsaturated fatty acids (LCP), predominantly docosapentaenoic acid (DPA, 22:5n-6) in rodents and docosahexaenoic acid (DHA, 22:6n-3) in rodents1 and other
mammals2,3. Human and animals cannot synthesize n-6 or n-3 PUFA due to a lack of appropriate fatty acid desaturase and elongase enzymes (i.e. plants employ oleic acid to obtain linoleic
acid, LA and alpha-linolenic acid, ALA using Δ12 and Δ15 desaturases) thus, they need dietary supply of LA (C18:2 n-6) and ALA (C18:3 n-3). LA and ALA are essential fatty acids, which need
to be provided in the diet and they are converted into vital fatty acids (FAs, e.g. arachidonic [ARA C20:4n-6], eicosapentaenoic [EPA, C20:5n-3], n-3 DPA and DHA) by alternating steps of
elongation and desaturation, exerted by elongases 2 (ELOVL2) and 5 (ELOVL5) and Δ5- (FADS1) and Δ6-desaturase (FADS2)4. LA and ALA and their metabolites, ARA, n-6 DPA, EPA, n-3 DPA and DHA,
in reproductive tissues strongly influence the reproductive function5,6,7,8. The mRNA levels of these key enzymes involved in FA metabolism have been investigated in the testis9,10,11,12,13;
high mRNA levels of desaturase and elongase were detected in semen, indicating that alterations in FA synthesis may lead to male infertility. Besides germ cells, different somatic cell
types are present in the testis, including Leydig cells, myoid cells and Sertoli cells that constitute the microenvironment or the niche of the testis, which is essential for regulating
normal spermatogenesis. Two distinct processes allow the accumulation of FAs in these cells, a passive diffusion through the lipid bilayer and/or protein-facilitated transport mediated by
the glycoprotein CD36, which is widely expressed in Sertoli cells. At the same time, it is reported that Sertoli cells are more active in LCP metabolism than germ cells, which in turn are
richer in polyunsaturated fatty acids (PUFAs)14. This correlates well with the high expression of Δ5- and Δ6-desaturase in Sertoli cells and with the low expression in germ cells15.
Consecutively, the epididymis modulates several sperm surface remodelling events and, in this regard, the role of PUFA metabolism may represent an interesting issue. During epididymal
maturation, PUFAs remain almost stable16; however, the content of DHA is higher in testicular than in epididymal mouse sperm17. Recently, Gautier and co-workers described an active PUFA
metabolism during spermatogenesis and epididymal sperm maturation in stallions18. Moreover, the elongation/desaturation rate of PUFAs differs between species, and it is affected by sex,
hormonal status and feed8,19. Because n-6 and n-3 FAs compete for the same enzyme pathways, their metabolism is largely affected by the availability of the ALA and LA substrates and by the
affinity of these FAs for the different enzymes20,21. Accordingly, in some studies it was observed that dietary enrichment in n-3 and n-6 PUFAs increased the quality of fresh or post-thawing
sperm of different animal species22,23,24,25. In the present paper, characterization of the gene and enzyme expression involved in the synthesis of n-3 and n-6 LCP was done in rabbits. LCP
biosynthetic metabolic pathway and PUFA localization were assessed by immunofluorescence in the testes and epididymis in rabbit bucks fed control or enriched (10% extruded flaxseed) diets,
to better underline the role of ALA enrichment in the metabolism of the enzymes during spermatogenesis. MATERIALS AND METHODS ANIMALS AND EXPERIMENTAL DESIGN Ten New Zealand White rabbit
bucks, 140 days old, were selected and divided into two experimental groups (n = 5 per group) (Table 1). * Control (CNT) group was fed a standard diet ad libitum. * Flax group (FLAX) was fed
a standard diet supplemented with 10% extruded flaxseed. The experimental protocol involved 110 days of feeding. All methods are reported in accordance with ARRIVE guidelines for the
reporting of animal experiments. This study was conducted in accordance with the Guiding Principles in the Use of Animals and approved by the Animal Ethics Monitoring Committee of the
University of Siena (CEL AOUS; authorization no. 265/2018-PR, ISOPRO 7DF19.23). SAMPLING OF RABBIT ORGANS At the end of the experiment, the rabbits were killed in the university facility
after overdose of barbiturates as approved by Animal Ethics Monitoring Committee of the University of Siena. The testes and epididymis (both sides) were accurately removed, and portions were
placed in sterile tubes, immediately snap-frozen using liquid nitrogen and stored at − 80 °C for evaluation of the gene (RT-PCR) and enzyme (immunohistochemistry) expression and FA profile
by GC-FID. Five samples per organ were collected and analysed. ANALYTICAL DETERMINATIONS DETERMINATION OF GENE EXPRESSION IN RABBIT TESTIS AND EPIDIDYMIS Total RNA from the testes was
extracted from around 30 mg of frozen tissue using NucleoSpin RNA (Macherey‐Nagel, Germany) following the specific manufacturer protocol. RNA integrity was checked through electrophoresis in
formaldehyde gel and the RNA concentration was determined with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). An amount of 1 µg of total RNA was used to synthesize the
cDNA using Superscript ii and Random Hexamers (Thermo Fisher Scientific) according to the manufacturer’s instructions. Primer-BLAST was used as a tool for primer design for the genes of
interest: fatty acid desaturases (FADS1 and FADS2) and fatty acid elongases (ELOVL2 and ELOVL5) (Table S1)27. The relative gene expression levels were normalized to β2-microglobulin (β2-MG)
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as the housekeeping genes (Table S1). The real-time PCR was conducted in triplicate for each biological sample, in a CFX96 real-time PCR
Detection System (Bio-Rad, Hercules, CA, USA) using EvaGreen dye (Bio-Rad, Hercules, CA, USA). The optimized RT-PCR mixture consisted of total reaction volumes of 20 μL that contained 0.01
ng of cDNA, 10 μL of SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA), 0.4 μM of each primer and sterile distilled water to reach the final volume. The PCR programme consisted of: 98
°C for 2 min, 40 cycles at 98 °C for 3 s and 60 °C for 10 s, 95 °C for 1 min, cooling at 70 °C for 1 min, and finally an increase to 95 °C at a 0.2 °C increase every 10 s, with measurement
of fluorescence. Threshold cycles (Ct) were used to quantify the relative gene expression and normalized to the two above-mentioned housekeeping genes according to the ΔΔCt method28.
DETERMINATION OF ENZYME LOCALIZATION IN RABBIT TESTIS AND EPIDIDYMIS The testes and epididymis of rabbit bucks fed control and n-3-enriched FLAX diets were cut into small blocks, treated
with 10% buffered formalin for 24 h at 4 °C and then washed in water for 1 h. After fixation, the tissues were dehydrated in a graded ethanol series (50%, 75%, 95%, 100%) and cleared with
xylene. The specimens were treated with three infiltrations of molten paraffin at 60 °C for 1 h and then they were allowed to solidify at room temperature. The obtained blocks were sectioned
using a Leica RM2125 RTS microtome (Leica Biosystems, Germany); Sects. (4 µm) were collected on glass slides and stained by the haematoxylin–eosin method for routine histology. The paraffin
sections from the testicular tissue of control and treated rabbits were deparaffinized with xylene, and then treated in a graded ethanol series (100%, 90%, 80%, 70%) for 5 min and, finally,
in water to rehydrate the tissue. For antigen retrieval, the sections were washed and treated with heat-induced epitope retrieval 1 (HIER 1) buffer (10 mM sodium citrate) at pH 6 for 20 min
at 95 °C. Specimens were treated overnight at 4 °C with the primary antibodies anti-FADS1 (Δ5-desaturase; Sigma-Aldrich, St. Louis, MO, USA) diluted 1 : 20, anti-FADS2 (Δ6-desaturase;
Sigma-Aldrich, St. Louis, MO, USA) diluted 1 : 20, anti-ELOVL5 (elongase 5; Sigma-Aldrich, St. Louis, MO, USA) diluted 1 : 100, anti-ELOVL2 (elongase 2; Sigma-Aldrich, St. Louis, MO, USA)
diluted 1 : 70, anti-DHA, anti-FITC-linked EPA and anti-PE-linked ARA (MyBioSource Inc., San Diego, CA, USA) diluted 1 : 50. After three washes for 10 min in phosphate-buffered saline (PBS),
the slides (excluding those treated with conjugated primary antibody) were incubated with goat anti-rabbit antibody Alexa Fluor® 488 conjugate (Invitrogen, Thermo Fisher Scientific,
Carlsbad, CA, USA), diluted at 1 : 100, for 1 h at room temperature. The slides were washed three times with PBS and treated with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, Milan,
Italy) for 10 min, followed by washing with PBS for 10 min. Finally, the slides were mounted with 1,4-diazabicyclo[2.2.2]octane (DABCO, Sigma-Aldrich, Milan, Italy). DETERMINATION OF FA
PROFILE IN RABBIT DIETS, TESTIS AND EPIDIDYMIS Lipids were extracted from the feed and different tissues according to Mattioli et al.29. To obtain fatty acid methyl esters, the lipid extract
was dried with a rotary evaporator (Strike 10 Steroglass, Italy), and 1 ml of n-hexane was added. Finally, the trans-methylation procedure was performed with 0.5 ml of 2 M KOH–methanol
solution at 60 °C for 15 min. To calculate the amount of each FA, heneicosanoic acid was used as the internal standard (C21:0, Sigma-Aldrich analytical standard). The recovery rate of the
internal standard in the testis was 83% ± 3%. The FA composition was determined using a Varian gas chromatograph (CP-3800) equipped with a flame ionization detector and a capillary column
100 m long × 0.25 mm × 0.2 μm film (WAX-10; Supelco, Bellefonte, PA, USA). Helium was used as the carrier gas with a flow of 0.6 mL/min. The split ratio was 1 : 20. The oven temperature was
programmed as reported by Mattioli et al.29. Individual FA methyl esters (FAME) were identified by comparing the relative retention times of peaks in the sample with those of a standard
mixture (FAME Mix Supelco, Sigma-Aldrich). STATISTICAL ANALYSIS All the numerical results (gene expression and FA profile) were analysed with a linear model analysing the effect of diet
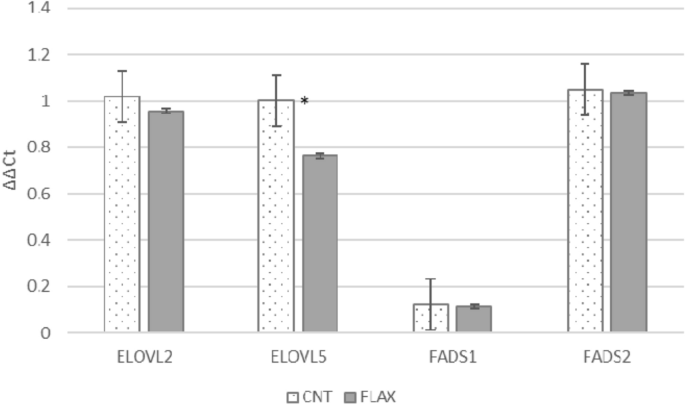
(control and flax)30. Results were expressed as LS means and differences were considered significant when _p_ ≤ 0.05. RESULTS The testes, independently of the diet administered, showed high
expression of FADS2, ELOVL2 and ELOVL5 and lower expression of FADS1 (Fig. 1). FLAX administration partially affected the gene expression, testes showing significantly lower values only for
the ELOVL5 gene. To study the localization of the different enzymes, it is essential to understand their activity; we used immunofluorescence performed in rabbit testis and cauda epididymis
tissues. In the testis of control rabbits, FADS2, acting on both LA and ALA, was clearly localized in interstitial cells (Fig. 2a); in the FLAX group, the fluorescence was also highlighted
in the elongated spermatids (Fig. 2b). In the cauda epididymis of both control and FLAX groups, FADS2 seemed to be present in connective epididymal tissue but it was not so evident in
epididymal vesicles or in principal epididymal cells (Fig. 2c,d). In the epididymis of the FLAX group, the FADS2 signal increased in interstitial connective tissue. ELOVL5 was mainly
localized in meiotic cells (spermatocytes and round spermatids) and it was not present in spermatogonia, elongated spermatids, Sertoli cells or interstitial tissue (Fig. 2e,f). In the cauda
epididymis, ELOVL5 was expressed in principal epididymal cells as well as in the epididymal vesicles (Fig. 2g,h). In addition, connective interstitial tissue showed the presence of the
enzyme. Δ5-Desaturase (FADS1) localization was evident in interstitial tissue, Sertoli cells and in spermatogonia (Fig. 2i); in the testis of rabbits fed FLAX diet, the signal was more
evident and clearer also in the elongated spermatids (Fig. 2j). In the cauda epididymis, both control and FLAX, FADS1 was localized in interstitial connective tissue, in basal and principal
epididymal cells, and in a conspicuous number of epididymal vesicles (Fig. 2k,l). ELOVL2 was localized in the interstitial cells of the testis (Fig. 2m,n), in interstitial epididymal
connective tissue and in the principal and basal cells of the epididymis (Fig. 2o,p). The number of marked epididymal vesicles was higher in rabbits fed FLAX diet (Fig. 2p). To better
understand the role of these enzymes, we also reported the localization of DHA, as well as EPA and ARA, in the testis, cauda epididymis and epididymal vesicles in rabbits fed control and
FLAX diets (Fig. 3). In the testis, DHA localization appeared in interstitial tissue, Sertoli cells and spermatogonia in control rabbits (Fig. 3a); after consumption of FLAX diet, it was
also evident in elongated spermatids (Fig. 3d). The EPA label appeared in the germ cells (Fig. 3b) and it was intense after consumption of FLAX diet (Fig. 3e). The ARA signal was evident in
interstitial tissue as well as in Sertoli cells and elongated spermatids of seminiferous tubules in both control (Fig. 3c) and FLAX diet groups (Fig. 3f). In the epididymis from control and
FLAX-fed rabbits, localization of the DHA label was detected (Fig. 3g,j) in interstitial connective tissue and principal cells as well as in a few vesicles. The signal appeared more evident
in FLAX-fed rabbits (Fig. 3j). A reduced number of epididymal vesicles were also labelled using anti-ARA antibodies in the epididymis from control and FLAX-fed rabbits (Fig. 3i,l). In these
last, the ARA localization appeared increased in interstitial connective tissue. Otherwise, many vesicles positive for EPA were detected (Fig. 3h,k) in epididymis from both control and
FLAX-fed rabbits. Figure 4 reports the PUFA profile of whole testis of control and FLAX groups. The PUFA profile showed significant differences in LA, ALA, 22:5n-6, ARA, and DHA, mainly in
the group supplemented with flaxseed. LA, ALA and DHA increased in the FLAX group; ARA and 22:5n-6 were increased in the control group. Other intermediate PUFAs (18:3 n-6, 18:4 n-3 and 20:4
n-3) are not evident in the PUFA profile, probably because they represented only metabolic steps, which will be easily converted into metabolites that were more relevant or were under the
detection limits. DISCUSSION Apart from the liver, brain and adipocytes, the reproductive apparatus of mammals is widely involved in PUFA metabolism25,31,32. Indeed, mature sperm have a high
level of LCP in their membranes, which assures fluidity and the movement of sperm with great speed6,33. This level of LCP is mainly guaranteed by liver metabolism21,34 which shows higher
activity and expression of critical enzymes (i.e. FADS2, ELOVL2). However, several reproductive structures like the testes, epididymis and epididymosomes also exert a specific role18,32. In
the present research, the gene expression of testis enzymes was slightly affected by dietary PUFAs (Fig. 5). Only the expression of ELOVL5 was downregulated when additional ALA was
administered, probably since ELOVL5 is involved in the first steps of FA elongation, and FLAX diets provided a large amount of precursor (ALA)35. Meanwhile, no differences were found in the
gene expression of ELOVL2, FADS2 or FADS1. Similarly, in rat testis, no gene expression of any of the desaturase enzymes (stearoyl-CoA desaturase—SCD1, SCD2, FADS1 and FADS2) was induced by
an increase in n-3 PUFA content36. However, in both the dietary regimes, gonads maintained a certain level of gene expression, confirming the role of reproductive tissues in LCP
metabolism29,32,37. In the liver, it is reported that many factors interfere with the gene expression of these enzymes, i.e. diet, body tissue and genetic variability38; nevertheless,
supplementary dietary n-3 PUFAs reduce gene expression and the activity of SCD1, FADS1, FADS2 and ELOVL239,40, whereas n-3 deficiency determines an increase of these enzymes41. To our
knowledge, a detailed analysis of the metabolic pathway of LCP in mammalian testis is shown here for the first time. The data show that the whole testis seems to be involved in LCP
generation. Both n-6 and n-3 precursors (LA and ALA) were expressed, whereas intermediate metabolites and final products were differently found in the cells present in the testis (Leydig,
Sertoli, germinal cells). A certain amount of essential FAs reaches the testis25 through blood vessels, and different dietary administration of substrates (LA, ALA, EPA, DHA) may activate
and modify this metabolism, inducing de novo synthesis. Our study suggests that the testis is the preferential site of LA and ALA metabolism in the reproductive apparatus. Epididymal
vesicles contain only a minimal amount of key enzymes of FA metabolism, e.g. FADS2. FADS2 is considered the rate-limiting step in LCP synthesis because it acts twice in this pathway,
introducing a double bond to ALA and LA and to 22:5 both from n-6 and n-3 series, respectively42,43,44. This enzyme is widely evident in different cell lines of the testis (i.e. Leydig
cells, elongated spermatids) and its products may be used at different developing cell stages. A PUFA-enriched diet may influence Leydig cells and spermatogenesis45; our data seem to also
suggest an intriguing role of the interstitial tissue. In fact, the increase of FADS2 in spermatids after consumption of FLAX diet could indicate that interstitial cells are able to support
spermatogenesis in the production of metabolites, increasing at this stage also the production of EPA, ARA and DHA. On the other hand, the fluorescent signal of FADS2 after dietary intake of
FLAX increased in connective tissue but not in epididymal cells and vesicles, excluding their involvement in FADS2 activity. This study also confirmed that ELOVL2 plays a crucial role in
the lipid metabolic pathway, being required for the generation of very long-chain FAs (≥ 22 carbon atoms). ELOVL2 appears strongly localized in the testicular interstitial tissue and FLAX
diet increases its quantity in both testis and epididymis. In the epididymis, the labelling is amplified in both connective and principal cells, determining the secretion of vesicles rich in
this enzyme. FLAX diet also increases the presence of FADS1 and ELOVL5 in epididymal vesicles. Accordingly, the body of literature reports that both FADS2 and ELOVL2 knockout male mice are
infertile since they cannot sustain sufficient levels of DHA, n-6 DPA and other PUFAs in the testis35,46. Fish testis exhibits high expression of FADS1, FADS2 and ELOVL2 genes, which encode
key enzymes to produce DHA. Recently, Bogevik et al.7 described phospholipid and LCP metabolism in Atlantic salmon (_Salmo salar_) testis during sexual maturation. ELOVL5, ELOVL2 and ELOVL4
mRNAs have also been detected in rat testis5 and were correlated with different maturation stages of sperm cells. ELOVL5 metabolism takes place in the seminiferous tubules, in meiotic stages
(spermatocytes and round spermatids), and probably uses as substrates the metabolites previously produced in Sertoli cells by FADS2; the resulting metabolic derivatives (20:3n-6 and
20:4n-3) represent substrates for FADS1 that was found in the stage of elongated spermatids. At the same time, the presence of ELOVL5 in epididymal vesicles suggests that vesicles may carry
some preformed LCP (22:4n-6 and 22:5n-3) to the sperm membrane (see Fig. 5). Sertoli cells have a fundamental role in normal and altered spermatogenesis. The numerous junctional complexes
and membrane specializations made by Sertoli cells provide a scaffold and a peculiar environment for germ cell development47. The energy source of germinal cells originates from lactate
produced by Sertoli cells, which in turn is provided mainly by mitochondrial oxidation of FAs. The metabolites of FADS1 activity (ARA and EPA) seem to have a role in Sertoli and Leydig cells
and are present during spermatogenesis until the elongated spermatid stage. Production of the enzymes involved in the specific metabolic pathway increased in the presence of a diet enriched
in ALA and became more evident in some stages of the cell germinal line, indicating that normal sperm maturation is dependent on these metabolites (Fig. 6). Luo et al.48 reported that a
high-fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. Furthermore, the involvement of Leydig cells in PUFA synthesis could be also linked to the
effect of LCP on sterol regulatory element binding protein (SREBP)49). However, the mechanisms by which these FAs regulate SREBP are not completely clear; probably EPA, DHA and ARA have far
more capacity to inhibit SREBP processing than do the shorter-chain PUFAs (e.g. C18:1 n-9, LA and ALA)49. The immunolocalization of FADS1, FADS2 and ELOVL2 underlines the active metabolism
of Leydig cells, where ARA and its metabolites influence cholesterol transport from the outer to the inner mitochondrial membrane to regulate steroidogenesis. In rats, ARA is secreted by
Sertoli cells in an LH-dependent manner: LH recognizes the LHR of Leydig cells, which activates cAMP through G protein-coupled receptor (GPCR) signalling50. ARA can induce the release of
Ca2+ from internal stores in round spermatids and pachytene spermatocytes; therefore, in the seminiferous tubule, unsaturated free FAs probably act as novel regulatory components of
spermatogenesis51. FADS1, FADS2 and ELOVL2 as well as ARA, EPA and DHA appeared widely localized in interstitial tissue. Matsuzaka et al.52 suggested that FADS1 and FADS2 expression is both
regulated by transcription factors for FA metabolism and could be involved in lipogenic gene regulation by producing PUFAs. In general, interstitial cells take part in complex signalling
interactions with both interstitial and tubular cell populations, influencing Sertoli cell function, spermatogenesis and immune regulation53. In accordance with our data, in the stallion,
Gautier et al.18, detected the presence of FADS1 in elongated spermatids and in epididymal cells even though, in their research, the localization of other enzymes was different (e.g. ELOVL5
was found in the interstitial compartment). Probably, each animal species has variability in enzyme expression, as demonstrated for the gene expression and enzyme activity54. Moreover, the
presence of enzymes in the epididymal vesicles suggests that vesicles can produce and add FAs to the sperm membrane with their fusion, as confirmed by the presence of DHA, EPA and ARA (Fig.
5). It is known that epididymal vesicles, containing hundreds of proteins from different epididymal regions, have a role in establishing sperm competency in the complex process of
reproduction55. In this study, we clarify the main metabolic pathway to produce EPA, DHA and n-3 DPA that represent the most important PUFAs in rabbit testis and epididymis. A great support
to understanding the steps of this process was comparison of the testis from control rabbits with that from those fed an ALA-enriched diet. In this group, the increased n-3 PUFA metabolism
allowed us to better differentiate the enzyme localization and the different LCP during spermatogenesis. The PUFA profile of the whole testis and the labelling of ARA, EPA and DHA are
concordant with the enzymatic localization highlighted in this study. Testis showed a significantly higher proportion of n-6 PUFAs in control rabbits than in the FLAX group, where the most
abundant FAs were ALA and DHA (_p_ < 0.05). Moreover, the presence of these FAs in the epididymal vesicles suggests that they may be carriers able to modify the FA composition of sperm
membrane. This study carried out in rabbit testis may also represent a model for understanding the LCP mechanisms in humans. Different amounts of FAs have been reported in the sperm of
fertile and infertile men56,57; in particular, ALA, EPA and DHA are reduced in oligoasthenoteratozoospermic patients56,57 and LA and ARA are low in fertile men. Indeed, it is known that the
FA profile affects not only live cells and sperm motility but also capacitation, the acrosomal reaction and sperm–oocyte fusion, influencing male fertility8. A deep understanding of
physiological enzyme function could also help in characterizing some diseases, by emphasizing its role in the regulation of lipid storage and lipid oxidation in Sertoli cells58 and of
testosterone in Leydig cells59. Knowledge of the role of FA metabolism in sperm and spermatogenesis, and the influence of dietary FAs on the sperm FA profile, could help in identifying
potential causes of male infertility, suggesting new personalized therapy. REFERENCES * Retterstøl, K., Tran, T. N., Haugen, T. B. & Christophersen, B. O. Metabolism of very long chain
polyunsaturated fatty acids in isolated rat germ cells. _Lipids_ 36, 601–606. https://doi.org/10.1007/s11745-001-0763-z (2001). Article PubMed Google Scholar * Van Tran, L., Malla, B. A.,
Kumar, S. & Tyagi, A. K. Polyunsaturated fatty acids in male ruminant reproduction: A review. _Asian Australas. J. Anim. Sci._ 30, 622–637. https://doi.org/10.5713/ajas.15.1034 (2017).
Article CAS PubMed Google Scholar * Wathes, D. C., Abayasekara, D. R. & Aitken, R. J. Polyunsaturated fatty acids in male and female reproduction. _Biol. Reprod._ 77, 190–201.
https://doi.org/10.1095/biolreprod.107.060558 (2007). Article CAS PubMed Google Scholar * Rustan, A. C. & Drevon, C. A. Fatty acids: structures and properties in _Encyclopedia of
Life Sciences_; www.els.net; https://doi.org/10.1038/npg.els.000389 (John Wiley & Sons, 2005). * Santiago Valtierra, F. X. _et al._ Elovl4 and Fa2h expression during rat spermatogenesis:
A link to the very-long-chain PUFAs typical of germ cell sphingolipids. _J. Lipid Res._ 59, 1175–1189. https://doi.org/10.1194/jlr.M081885 (2018). Article CAS PubMed PubMed Central
Google Scholar * Rodríguez, M. G., Rebollar, P., Mattioli, S. & Castellini, C. n-3 PUFA sources (precursor/products): A review of current knowledge on rabbit. _Animals (Basel)_ 9, 806.
https://doi.org/10.3390/ani9100806 (2019). Article Google Scholar * Bogevik, A. S. _et al._ Phospholipid and LC-PUFA metabolism in Atlantic salmon (_Salmo salar_) testes during sexual
maturation. _PLoS One_ 15, e0233322. https://doi.org/10.1371/journal.pone.0233322 (2020). Article CAS PubMed PubMed Central Google Scholar * Collodel, G. _et al._ Relevance of fatty
acids to sperm maturation and quality. _Oxid. Med. Cell. Longev._ 2020, 7038124. https://doi.org/10.1155/2020/7038124 (2020). Article CAS PubMed PubMed Central Google Scholar * Cho, H.
P., Nakamura, M. & Clarke, S. D. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. _J. Biol. Chem._ 274, 37335–37339.
https://doi.org/10.1074/jbc.274.52.37335 (1999). Article CAS PubMed Google Scholar * Leonard, A. E. _et al._ Cloning of a human cDNA encoding a novel enzyme involved in the elongation of
long-chain polyunsaturated fatty acids. _Biochem. J._ 350, 765–770 (2000). Article CAS PubMed PubMed Central Google Scholar * Stoffel, W. _et al._ Delta6-desaturase (FADS2) deficiency
unveils the role of omega3- and omega6-polyunsaturated fatty acids. _EMBO J._ 27, 2281–2292. https://doi.org/10.1038/emboj.2008.156 (2008). Article CAS PubMed PubMed Central Google
Scholar * Stroud, C. K. _et al._ Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. _J. Lipid Res._ 50, 1870–1880.
https://doi.org/10.1194/jlr.M900039-JLR200 (2009). Article CAS PubMed PubMed Central Google Scholar * Casado, M. E. _et al._ Hormone-sensitive lipase deficiency disturbs the fatty acid
composition of mouse testis. _Prostaglandins Leukot. Essent. Fatty Acids_ 88, 227–233. https://doi.org/10.1016/j.plefa.2012.12.005 (2013). Article CAS PubMed Google Scholar * Rato, L.,
Alves, M. G., Cavaco, J. E. & Oliveira, P. F. High-energy diets: A threat for male fertility?. _Obes. Rev._ 15, 996–1007. https://doi.org/10.1111/obr.12226 (2014). Article CAS PubMed
Google Scholar * Saether, T., Tran, T. N., Rootwelt, H., Christophersen, B. O. & Haugen, T. B. Expression and regulation of Δ5-desaturase, Δ6-desaturase, stearoyl-coenzyme A (CoA)
desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. _Biol. Reprod._ 69, 117–124. https://doi.org/10.1095/biolreprod.102.014035 (2003). Article CAS PubMed Google Scholar * Fasel,
N. J. _et al._ Modification of sperm fatty acid composition during epididymal maturation in bats. _Reproduction_ 157, 77–85. https://doi.org/10.1530/REP-18-0463 (2019). Article CAS PubMed
Google Scholar * Ollero, M., Powers, R. D. & Alvarez, J. G. Variation of docosahexaenoic acid content in subsets of human spermatozoa at different stages of maturation: Implications
for sperm lipoperoxidative damage. _Mol. Reprod. Develop._ 55, 326–334. https://doi.org/10.1002/(SICI)1098-2795(200003)55:3%3c326 (2000). Article CAS Google Scholar * Gautier, C. _et al._
Expression of enzymes involved in polyunsaturated fatty acid synthesis in the stallion testis and epididymis. _Reprod. Fertil. Dev._ 32, 851–861. https://doi.org/10.1071/RD19342 (2020).
Article CAS PubMed Google Scholar * de Catalfo, H. G. E. & de Dumm, G. I. N. Polyunsaturated fatty acid biosynthesis from [1–14C]20:3 n-6 acid in rat cultured Sertoli cells. Linoleic
acid effect. _Int. J. Biochem Cell Biol._ 34, 525–532. https://doi.org/10.1016/s1357-2725(01)00152-2 (2002). Article Google Scholar * Jones, P. J. H. & Kubow, S. Lipids, Sterols, and
Their Metabolites. In _Modern Nutrition in Health and Disease_ 9th edn (eds Shils, M. E. _et al._) 71 (Lippincott/Williams & Wilkins, 1999). Google Scholar * Castellini, C. _et al._
Activity, expression, and substrate preference of the Δ(6)-desaturase in slow- or fast-growing rabbit genotypes. _Agric. Food Chem._ 64, 792–800. https://doi.org/10.1021/acs.jafc.5b0542
(2016). Article CAS Google Scholar * Rooke, J. A. _et al._ Dietary carbohydrates and amino acids influence oocyte quality in dairy heifers. _Reprod. Fertil. Dev._ 21, 419–427.
https://doi.org/10.1071/rd08193 (2009). Article CAS PubMed Google Scholar * Tran, L. V. _et al._ Effect of omega-3 and omega-6 polyunsaturated fatty acid enriched diet on plasma IGF-1
and testosterone concentration, puberty and semen quality in male buffalo. _Anim. Reprod. Sci._ 173, 63–72. https://doi.org/10.1016/j.anireprosci.2016.08.012 (2016). Article CAS PubMed
Google Scholar * Mussa, N. J. _et al._ Lipid profile of sperm cells in Thai native and commercial roosters and its impact on cryopreserved semen quality. _Trop Anim. Health Prod._ 53, 1–9.
https://doi.org/10.1016/j.anireprosci.2016.08.012 (2021). Article MathSciNet CAS Google Scholar * Castellini, C. _et al._ Effect of dietary n-3 source on rabbit male reproduction. _Oxid.
Med. Cell. Long._ 2019, 3279670. https://doi.org/10.1155/2019/3279670 (2019). Article CAS Google Scholar * De Blas, C. & Mateos, G. G. _Feed Formulation. Nutrition of the Rabbit
222–232_ (CABI Publisher, 2010). Google Scholar * Ye, J. _et al._ Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. _BMC Bioinform._ 13, 1–11.
https://doi.org/10.1186/1471-2105-13-134 (2012). Article CAS Google Scholar * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative
PCR and the 2−ΔΔCT method. _Methods_ 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001). Article CAS PubMed Google Scholar * Mattioli, S. _et al._ Dietary fish oil and flaxseed
for rabbit does: Fatty acids distribution and Δ6-desaturase enzyme expression of different tissues. _Animal_ 13, 1934–1942. https://doi.org/10.1017/S175173111900020X (2019). Article CAS
PubMed Google Scholar * Software, S. S. S. _Release 14_ (StataCorp LP, 2015). Google Scholar * Mattioli, S. _et al._ Performance and egg quality of laying hens fed flaxseed: Highlights on
n-3 fatty acids, cholesterol, lignans and isoflavones. _Animal_ 11, 705–712. https://doi.org/10.1017/S175173111600207X (2017). Article CAS PubMed Google Scholar * Mattioli, S. _et al._
Tissue antioxidant status and lipid peroxidation are related to dietary intake of n-3 polyunsaturated acids: A rabbit model. _Antioxidants (Basel)_ 10, 681.
https://doi.org/10.3390/antiox10050681 (2021). Article CAS Google Scholar * Mourvaki, E., Cardinali, R., Roberti, R., Dal Bosco, A. & Castellini, C. Desmosterol, the main sterol in
rabbit semen: Distribution among semen subfractions and its role in the in vitro spermatozoa acrosome reaction and motility. _Asian J. Androl._ 12, 862–870.
https://doi.org/10.1038/aja.2010.25 (2010). Article CAS PubMed PubMed Central Google Scholar * Crespo-Piazuelo, D. _et al._ Identification of strong candidate genes for backfat and
intramuscular fatty acid composition in three crosses based on the Iberian pig. _Sci. Rep._ 10, 1–17. https://doi.org/10.1038/s41598-020-70894-2 (2020). Article CAS Google Scholar *
Zadravec, D. _et al._ ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. _J. Lipid Res._ 52, 245–255.
https://doi.org/10.1194/jlr.M011346 (2011). Article CAS PubMed PubMed Central Google Scholar * Saether, T. _et al._ Essential fatty acid deficiency induces fatty acid desaturase
expression in rat epididymis, but not in testis. _Reproduction_ 133, 467–477. https://doi.org/10.1530/REP-06-00294 (2007). Article CAS PubMed Google Scholar * Weems, C. W., Weems, Y. S.
& Randel, R. D. Prostaglandins and reproduction in female farm animals. _Vet. J._ 171, 206–228. https://doi.org/10.1016/j.tvjl.2004.11.014 (2006). Article CAS PubMed Google Scholar *
Portolesi, R., Powell, B. C. & Gibson, R. A. Competition between 24:5n–3 and ALA for delta 6 desaturase may limit the accumulation of DHA in HepG2 cell membranes. _J. Lipid Res._ 48,
1592–1598. https://doi.org/10.1194/jlr.M700081-JLR200 (2007). Article CAS PubMed Google Scholar * Guillou, H., Zadravec, D., Martin, P. G. & Jacobsson, A. The key roles of elongases
and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. _Prog. Lipid Res._ 49, 186–199. https://doi.org/10.1016/j.plipres.2009.12.002 (2010). Article CAS PubMed
Google Scholar * Nakamura, M. T. & Nara, T. Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. _Ann. Rev. Nutr._ 24, 345–376.
https://doi.org/10.1146/annurev.nutr.24.121803.063211 (2004). Article CAS Google Scholar * Igarashi, M. _et al._ Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic
acid in rats on a 15 week n-3 PUFA-deficient diet. _J. Lipid Res._ 48, 152–164. https://doi.org/10.1194/jlr.M600396-JLR200 (2007). Article CAS PubMed Google Scholar * Sanz, M., Flores,
A. & Lopez-Bote, C. J. Effect of fatty acid saturation in broiler diets on abdominal fat and breast muscle fatty acid composition and susceptibility to lipid oxidation. _Poult. Sci._ 78,
378–382. https://doi.org/10.1093/ps/78.3.378 (1999). Article CAS PubMed Google Scholar * Crawford, M. A. Fatty-acid ratios in free-living and domestic animals possible implications
atheroma. _Lancet_ 1, 1329–1333. https://doi.org/10.1016/s0140-6736(68)92034-5 (1968). Article CAS PubMed Google Scholar * Davidson, B., Maciver, J., Lessard, E. & Connors, K. Meat
lipid profiles: A comparison of meat from domesticated and wild Southern African animals. _In Vivo_ 25, 197–202 (2011). CAS PubMed Google Scholar * Arisha, S. M., Saber, A. S. &
Abd-Elhaseeb, F. R. Cinnamomum zeylanicum alleviate testicular damage induced by high fat diet in albino rats; histological and ultrastructural studies. _Heliyon_ 6, e05584.
https://doi.org/10.1016/j.heliyon.2020.e05584 (2020). Article PubMed PubMed Central Google Scholar * Roqueta-Rivera, M. _et al._ Docosahexaenoic acid supplementation fully restores
fertility and spermatogenesis in male delta-6 desaturase-null mice. _J. Lipid Res._ 51, 51360–51367. https://doi.org/10.1194/jlr.M001180 (2011). Article CAS Google Scholar * Griswold, M.
D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. _Biol. Reprod._ 99, 87–100. https://doi.org/10.1093/biolre/ioy027 (2018). Article PubMed PubMed
Central Google Scholar * Luo, D. _et al._ High fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. _Life Sci._ 257, 118028.
https://doi.org/10.1016/j.lfs.2020.118028 (2020). Article CAS PubMed Google Scholar * Deckelbaum, R. J., Worgall, T. S. & Seo, T. n-3 fatty acids and gene expression. _Am. J. Clin.
Nutr._ 83, 1520S-1525S. https://doi.org/10.1093/ajcn/83.6.1520S (2006). Article CAS PubMed Google Scholar * Zhou, R. _et al._ The roles and mechanisms of Leydig cells and myoid cells in
regulating spermatogenesis. _Cell. Mol. Life Sci._ 76, 2681–2695. https://doi.org/10.1007/s00018-019-03101-9 (2019). Article CAS PubMed Google Scholar * Paillamanque, J. _et al._ Effects
of fatty acids on intracellular [Ca2+], mitochondrial uncoupling and apoptosis in rat pachytene spermatocytes and round spermatids. _PLoS One_ 11, e0158518.
https://doi.org/10.1371/journal.pone.0158518 (2016). Article CAS PubMed PubMed Central Google Scholar * Matsuzaka, T. _et al._ Dual regulation of mouse delta(5)- and delta(6)-desaturase
gene expression by SREBP-1 and PPARalpha. _J. Lipid Res._ 43, 107–114. https://doi.org/10.1016/S0022-2275(20)30193-0 (2002). Article CAS PubMed Google Scholar * Heinrich, A. &
DeFalco, T. Essential roles of interstitial cells in testicular development and function. _Andrology_ 8, 903–914. https://doi.org/10.1111/andr.12703 (2020). Article CAS PubMed Google
Scholar * Twining, C. W. _et al._ The evolutionary ecology of fatty-acid variation: Implications for consumer adaptation and diversification. _Ecol. Lett._ 24, 1709–1731.
https://doi.org/10.1111/ele.13771 (2021). Article PubMed Google Scholar * James, E. R. _et al._ The role of the epididymis and the contribution of epididymosomes to mammalian
reproduction. _Int. J. Mol. Sci._ 21, 5377. https://doi.org/10.3390/ijms21155377 (2020). Article CAS PubMed Central Google Scholar * Safarinejad, M. R. Effect of omega-3 polyunsaturated
fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind,
placebo-controlled, randomised study. _Andrologia_ 43, 38–47. https://doi.org/10.1111/j.1439-0272.2009.01013.x (2011). Article CAS PubMed Google Scholar * Aksoy, Y., Aksoy, H.,
Altinkaynak, K., Aydin, H. R. & Ozkan, A. Sperm fatty acid composition in subfertile men. _Prostaglandins Leukot. Essent. Fat. Acids_ 75, 75–79.
https://doi.org/10.1016/j.plefa.2006.06.002 (2006). Article CAS Google Scholar * Regueira, M. _et al._ Apoptotic germ cells regulate Sertoli cell lipid storage and fatty acid oxidation.
_Reproduction_ 156, 515–525. https://doi.org/10.1530/REP-18-0181 (2018). Article CAS PubMed Google Scholar * Xu, A. _et al._ Linoleic acid promotes testosterone production by activating
leydig cell GPR120/ ERK pathway and restores BPA-impaired testicular toxicity. _Steroids_ 163, 108677. https://doi.org/10.1016/j.steroids.2020.108677 (2020). Article CAS PubMed Google
Scholar Download references FUNDING The work was supported by the Plan of Research 2020 (Department of Molecular and Developmental Medicine, University of Siena). AUTHOR INFORMATION AUTHORS
AND AFFILIATIONS * Department of Agricultural, Environmental, and Food Science, University of Perugia, Borgo XX Giugno 74, 06123, Perugia, Italy Cesare Castellini, Simona Mattioli, Elisa
Cotozzolo, Francesco Perini, Alessandro Dal Bosco & Emiliano Lasagna * Department of Molecular and Developmental Medicine, Policlinico Santa Maria alle Scotte, University of Siena, Viale
Bracci 14, 53100, Siena, Italy Elena Moretti, Cinzia Signorini, Daria Noto, Giuseppe Belmonte & Giulia Collodel * Department of Veterinary Medicine, University of Milano, Via
dell’Università, 6, 26900, Lodi, Italy Gabriele Brecchia Authors * Cesare Castellini View author publications You can also search for this author inPubMed Google Scholar * Simona Mattioli
View author publications You can also search for this author inPubMed Google Scholar * Elena Moretti View author publications You can also search for this author inPubMed Google Scholar *
Elisa Cotozzolo View author publications You can also search for this author inPubMed Google Scholar * Francesco Perini View author publications You can also search for this author inPubMed
Google Scholar * Alessandro Dal Bosco View author publications You can also search for this author inPubMed Google Scholar * Cinzia Signorini View author publications You can also search for
this author inPubMed Google Scholar * Daria Noto View author publications You can also search for this author inPubMed Google Scholar * Giuseppe Belmonte View author publications You can
also search for this author inPubMed Google Scholar * Emiliano Lasagna View author publications You can also search for this author inPubMed Google Scholar * Gabriele Brecchia View author
publications You can also search for this author inPubMed Google Scholar * Giulia Collodel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
C.C. wrote the original paper; S.M. performed the PUFA profiles (Fig. 4), wrote the original paper; E.M. performed data interpretation and immunochemistry analysis, Figs. 2 and 3; E.C.
performed animal care; F.P. performed the gene expression experiments, prepared Fig. 1; A.D.B. performed statistical analysis; C.S. performed the immunochemistry analysis Figs. 2 and 3; D.N.
performed animal care and immunochemistry analysis (Figs. 2 and 3); G. Belmonte performed testis immunocytochemistry experiments and image acquisition (Figs. 2 and 3); E.L. performed gene
expression experiments (Fig. 1); G. Brecchia was responsible for animals and sacrifice; G.C. performed immunocytochemical analysis (Figs. 2 and 3), conceptualization, project design and
administration, and wrote the original paper. All authors have read and agreed to the published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Elena Moretti. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Castellini, C., Mattioli, S., Moretti, E. _et al._ Expression of genes and
localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. _Sci Rep_ 12, 2637 (2022). https://doi.org/10.1038/s41598-022-06700-y Download
citation * Received: 16 October 2021 * Accepted: 27 January 2022 * Published: 16 February 2022 * DOI: https://doi.org/10.1038/s41598-022-06700-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative