- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Oviposition by lepidopteran herbivores on _Nicotiana attenuata_ primes plant defence responses that are induced by the feeding larvae. While oviposition by both the generalist
_Spodoptera exigua_ and the specialist _Manduca sexta_ primes the production of defensive phenylpropanoids, their larvae are differentially affected. We investigate here the impact of prior
oviposition on the transcriptome and phytohormone levels of plants that were later attacked by larvae to find regulatory signals of this priming. In a full-factorial design, we evaluated the
effects of oviposition and herbivory by both species. Oviposition alone had only subtle effects at the transcriptional level. Laval feeding alone induced species-specific plant responses.
Larvae of the generalist regulated phytohormones and gene expression stronger than larvae of the specialist. A day after larvae started to feed, we detected no significant alterations of the
plant’s response to larval feeding due to prior oviposition by conspecific moths. Yet, oviposition by each of the species profoundly influenced the plant’s transcriptional and phytohormonal
response to feeding larvae of the other species. Remarkably, the species-specific plant responses to larval feeding shifted towards the response normally elicited by larvae of the
ovipositing species. Thus, plants may already recognise an insect’s identity upon its oviposition. SIMILAR CONTENT BEING VIEWED BY OTHERS NYMPHAL FEEDING SUPPRESSES OVIPOSITION-INDUCED
INDIRECT PLANT DEFENSE IN RICE Article Open access 08 January 2025 SPIDER MITE EGG EXTRACT MODIFIES ARABIDOPSIS RESPONSE TO FUTURE INFESTATIONS Article Open access 06 September 2021
_AGASICLES HYGROPHILA_ ATTACK INCREASES NEROLIDOL SYNTHASE GENE EXPRESSION IN _ALTERNANTHERA PHILOXEROIDES_, FACILITATING HOST FINDING Article Open access 12 October 2020 INTRODUCTION Plants
evolved a multitude of adaptations to resist herbivorous insects. In turn, insects evolved adaptations to find and colonise suitable host plants. Plants possess diverse defence traits which
are often inducible upon herbivory1. Insects evolved traits to avoid, resist, or even manipulate a plant’s defence response2. The closer the evolutionary relationship between plants and
insect herbivores, the more sophisticated and specialised defence and counter-defence can be geared to each other. The degree of such coevolution is driven by the insect’s impact on plant
fitness and its host plant range. Specialised herbivores with narrow host plant ranges can often tolerate, detoxify or even co-opt plant defences for their own defence3. In several plants,
generalist and specialist insects elicit different responses4,5,6, which may be adaptive for the plant or the insect7. Plants may render their defence responses most efficient against
different insects that are differentially susceptible, and adapted insects may evolve traits to manipulate plant defence. Inducible plant defences allow plants to limit their investments of
resources into defence to circumstances in which these pay off, i.e. herbivores are present. Plants can even integrate environmental cues that predict upcoming herbivory to accelerate or
enhance their induced defences which is termed as priming8,9. For example volatiles of herbivore-attacked plants can prime herbivory-induced defence in adjacent plants10. The egg-deposition
of herbivorous insects on a host plant can also prime plant defence against the feeding larvae as has been shown for the wild tobacco _Nicotiana attenuata_11,12. Also in tomato, moth
oviposition primes the wound-induced expression of defence-related parameters13. Priming by insect oviposition is likely more common because several plants possess an increased resistance to
insect larvae if previously exposed to its eggs14,15,16,17. Yet, the larvae may also be affected by defence responses that are directly activated upon insect oviposition. Plant responses to
insect oviposition include the formation of neoplasms, necrosis, egg-crushing tissues, ovicidal substances or volatiles that attract egg predators and parasitoids18,19,20,21,22,23,24.
Several plant responses to oviposition as well as the effects on plant resistance to subsequent larval attack can be specific to the insect species. For example, _Brassica nigra_ plants are
more resistant to feeding larvae in response to a specialist’s but not a generalist’s moth oviposition25, suggesting a differential plant response to oviposition by both insects. Yet, _N.
attenuata_ plants are more resistant to larvae of only the generalist _Spodoptera exigua_ but not the specialist _Manduca sexta_ in response to oviposition by either of the two species12.
Oviposition by both moth species equally primes the feeding-induced plant production of phenylpropanoid-polyamine conjugates (PPCs), which reduces performance of _S. exigua_ larvae feeding
on oviposited plants11. This suggests a differential susceptibility of the larvae but no species-specific plant response to oviposition, though _N. attenuata_ may show other, still
undiscovered, responses that may differ for the two moth species. Plant responses to herbivory are multifaceted, which is reflected in a large reprogramming of a plant’s transcriptome and
various shifts in its primary and secondary metabolism26. This reprogramming is regulated by a network of signalling pathways in which the phytohormone jasmonic acid (JA) plays a central
role27. Differential activation of these signalling pathways in response to different elicitors, e.g. damage- and herbivore associated molecular patterns, allows species-specific responses
to different herbivores28,29,30. For example, larval feeding of _M. sexta_ and _S. exigua_ elicits distinct transcriptional profiles in _N. attenuata_4, likely due to differential
phytohormone induction patterns that result from different ratios of elicitors in the larval oral secretions of both species31. The phytohormonal and transcriptional plant responses to
insect oviposition are less examined than those to insect feeding but an involvement of salicylic acid (SA) and JA signalling pathways has been inferred32. _Arabidopsis thaliana_ leaves
oviposited by _Pieris brassicae_ accumulate high levels of SA33 and SA-responsive transcripts34. Consistent with the role of SA as a key regulator of plant responses to phytopathogens, the
transcriptional profile in response to _P. brassicae_ oviposition is more similar to that of plants harbouring a bacterial infection than to plants fed by _P. brassicae_ larvae34. The SA
signal is also detected in systemic leaves of oviposited plants that thereby even acquire resistance (SAR) to bacterial pathogens35. JA may also play a role in plant responses to oviposition
as indicated by an increased hatching rate of spider mite eggs on JA-deficient tomato mutants36. Moreover, JA-biosynthesis genes are induced upon oviposition that involves wounding of plant
tissue37,38 and plant responses to such oviposition can be mimicked with JA treatments39,40. Very few studies examined the influence of an earlier oviposition on plant signalling in
response to the feeding larvae. Transcriptome analyses revealed no significant effects of _P. brassicae_ oviposition on _A. thaliana_’s response to larval feeding41 or very few _B. nigra_
genes that were altered in larval attacked plants due to a pre-treatment with _P. brassicae_ egg extract42. In tomato, the increase of wound-induced expression of a defensive protease
inhibitor (PI) gene in response to a moth’s oviposition coincides with a stronger JA-burst13. Yet, in _N. attenuata_, the priming effect of moth oviposition on the induction of PI activity
and PPC production in response to larval feeding could not be explained by increased induction of JA11,43. Overall, our current knowledge on the signalling of oviposition-induced responses
is anecdotal, especially regarding the question how oviposition affects plant responses to subsequently feeding larvae. Here we explore _N. attenuata_’s signalling response to oviposition
and herbivory by two of its major herbivores. Specifically, we examine the plant for i) systemic imprints of oviposition by _M. sexta_ and _S. exigua_, ii) how the moth’s oviposition affects
the feeding-induced plant response to its larvae, and iii) whether these effects of oviposition on plant responses to feeding are specific for the insect species. In a full-factorial design
(Fig. 1), we explore the effects of larval feeding and prior oviposition by _S. exigua_ and _M. sexta_ on _N. attenuata_’s transcriptional and phytohormonal profiles in leaves that were
systemic to oviposition but local for the larval attack. RESULTS PLANT RESPONSES TO OVIPOSITION IN LEAVES SYSTEMIC TO THE OVIPOSITION SITE We evaluated the direct effects of oviposition on
plants without larval feeding. These leaves were harvested at the same time and from the same leaf position as the leaves of feeding-induced plants and were therefore systemic to the
oviposited leaf (see methods). We found no differences in the phytohormone profiles between oviposited and non-oviposited control plants. Levels of JA and JA-Ile generally varied around the
limit of quantification (ca. 7 and 3 ng/g FW, respectively) and are therefore not displayed. Levels of SA and ABA did not differ between plants oviposited by _S. exigua_ or _M. sexta_ and
control plants (see Supplementary Fig. S1). However, the transcriptome profiles of _M. sexta_-oviposited plants (EmsL0) separated from those of control plants (E0L0) in a principal component
analysis (PCA) along the second principal component (Fig. 2a). In contrast, transcriptome profiles of _S. exigua_-oviposited plants (EseL0) grouped within those of control plants.
Oviposition by _M. sexta_, but not by _S. exigua_, significantly altered the expression of 59 genes, several of which are related to ethylene and auxin signalling as well as regulators of
transcription (Supplementary Table S1). _N. ATTENUATA_ RESPONDS SPECIES-SPECIFIC TO LARVAL FEEDING Altogether, transcriptomes of plants attacked by larvae clearly separated from the
non-attacked plants along the first principal component in a PCA (Fig. 2a). Along the second principal component, explaining about 10% of the variation, _S. exigua_-fed plants (E0Lse; filled
squares) clearly separated from _M. sexta-_fed plants (E0Lms; filled triangles). Herbivory by any of the two species significantly altered the expression of 4749 genes (11% of all genes on
the array) relative to control plants (E0L0). Whereas feeding by _S. exigua_ altered the expression of almost 92% (4361) of these genes, _M. sexta_ feeding regulated only about half (2302)
of them (Fig. 2b). As a consequence, the feeding-induced plant responses to both species overlapped just for 40% (1914). Of all feeding-inducible transcripts, only about 8% (388) were
specific to _M. sexta_ but almost 52% (2447) were unique for feeding _S. exigua_. In a direct comparison between _S. exigua_-fed (E0Lse) and _M. sexta_-fed (E0Lms) plants, 20% of the
feeding-induced genes differed significantly. The transcriptional changes (log2-fold changes; log2-FC) relative to untreated controls correlated with an R2 of 0.72 between plants fed by
larvae of the two different species (Fig. 2c), which is about 20% lower than correlations between plants fed by larvae of the same species (either or not previously oviposited by
conspecifics; see below and Fig. 2d). The difference in the transcriptional response to the two herbivore species was accompanied by a different induction of phytohormones. Feeding by both
species increased levels of JA, JA-Ile, SA and ABA. In _S. exigua_-fed plants, levels of SA and the ratio of JA-Ile to JA were about twofold higher compared to _M. sexta_-fed plants (see
outer bars in Fig. 3), while ABA was altered similarly by both species (Supplementary Fig. S1). EFFECTS OF OVIPOSITION ON _N. ATTENUATA’S_ RESPONSE TO HERBIVORY BY CONSPECIFIC LARVAE
Oviposition by neither _M. sexta_ nor _S. exigua_ altered any of the assessed phytohormone accumulations in response to herbivory by conspecific larvae (Fig. 3, see Supplementary Fig. S1 and
Supplementary Table S2). For each of the two species, non-oviposited and previously oviposited plants shared about 75% of the transcriptional regulation in response to feeding by
conspecific larvae (relative to control plants; see Supplementary Fig. S2). To test whether the previous oviposition changed the plant’s transcriptional response to the subsequent feeding by
conspecific larvae, we evaluated the effects of previous oviposition and the larval feeding in two-factorial analyses (including E0L0/EmsL0/E0Lms/EmsLms in case of _M. sexta_ and
E0L0/EseL0/E0Lse/EseLse plants in case of _S. exigua_), but we found no interactions between oviposition and larval feeding for neither of the two species. Similarly, we found no significant
differences in direct comparisons of gene expression in plants only exposed to larval feeding and plants that were oviposited prior to larval herbivory by the same species (E0Lms versus
EmsLms and E0Lse versus EseLse). In accordance with that, the feeding-induced transcriptional changes (log2-FC) relative to control plants correlated very strongly for previously oviposited
and non-oviposited plants (Fig. 2d). Also in the PCA, _M. sexta-_fed plants with a prior oviposition by conspecifics (EmsLms) cluster closely together with plants only fed by _M. sexta_
(E0Lms) and similarly do _S. exigua-_fed plants with a prior oviposition by conspecifics (EseLse) and plants only fed by _S. exigua_ (E0Lse) cluster together (Fig. 2a). OVIPOSITION SHAPES
THE SPECIES-SPECIFIC RESPONSE OF _N. ATTENUATA_ TO LARVAL FEEDING We investigated furthermore, whether the plant response to feeding larvae is altered in plants previously oviposited by
heterospecific moths. In the PCA, transcript profiles of the EseLms cross-species treatment (blue stars in Fig. 2a) clearly separated from the other two _M. sexta_-fed plant treatments (blue
filled triangles) and aggregated with the _S. exigua_-fed treatments (red filled squares). Similarly, transcript profiles of the other EmsLse cross-species treatment (red stars) shifted
towards plants fed by _M. sexta_. This pattern was further supported by a clustering analysis of the six feeding induced treatments, in which EseLms plants clustered together with _S.
exigua-_fed plants that were either oviposited by conspecific moths or not (Fig. 2e). _Vice versa_, EmsLse clustered with _M. sexta-_fed plants. The numbers of genes regulated by the
cross-species treatments fall in-between those regulated in plants that only experienced one of the species (Fig. 4a). For example, the number of genes regulated in EseLms plants increased
by more than 1000 compared to the other two _M. sexta_-fed plant treatments (E0Lms, EmsLms). As a consequence, EseLms plants shared only 51% of its regulated genes with E0Lms plants, while
these overlapped with 82% of the genes regulated in EmsLms (Fig. 4b). Instead, genes regulated in EseLms plants overlap with 62% of the genes regulated in E0Lse plants, which is considerably
more than the 44–48% that the other _M. sexta_-fed plants overlapped with the E0Lse plants. Similarly, plants of the _S. exigua_-fed cross-species treatment (EmsLse) had about 500 genes
less regulated than the other two _S. exigua_-fed plant treatments. While, E0Lse and EseLse plants overlapped in 85–88% of their regulated genes, EmsLse plants overlapped only with 68% of
the genes regulated in E0Lse plants. Conversely, the proportion of _M. sexta_-responsive genes (E0Lms) that are also altered in response to _S. exigua_ feeding increased from 83% (E0Lse) to
91% (EmsLse) when _M. sexta_ oviposition preceded _S. exigua_ feeding. Thus, the comparisons of the regulated gene sets support that the plant response in cross-species treatments is
intermediate to the species-specific responses to larval feeding. Finally, the shift in the species-specific response to feeding was also reflected in pairwise regression analyses of the
log2-FC relative to untreated controls (Fig. 4c, see Supplementary Fig. S3). The strong correlations between E0Lse and EseLse as well as between E0Lms and EmsLms with an R2 around 0.9 were
not found between the cross-species treatments (EmsLse and EseLms) and the E0Lms and E0Lse treatments. Instead, the expression pattern of EmsLse plants correlated similarly with E0Lms and
E0Lse (R2 of 0.82 and 0.83) and EseLms plants correlated even slightly stronger with E0Lse than with E0Lms plants. Lastly, the cross-species treatment for which phytohormones were analysed
(EmsLse) showed a phytohormonal induction pattern in-between of E0Lms and E0Lse plants (Fig. 3a,b). The intermediate levels of SA and JA-Ile/JA in EmsLse plants did not significantly differ
from both treatments with _M. sexta_ feeding and JA-Ile/JA levels in EmsLse plants even differed significantly from E0Lse plants. VALIDATION OF MICROARRAY ANALYSIS BY REAL-TIME QPCR OF
CANDIDATE GENES We quantified transcripts in 9-10 biological replicates for a set of genes that we selected based on their regulation in the microarray analysis or that are known to be key
regulators in different plant signalling pathways. We selected (i) genes that were significantly regulated by the oviposition alone (Supplementary Table S3) or that indicated an altered
regulation after larval feeding due to prior oviposition by the same species (Fig. 5), (ii) signalling-related genes that may reflect the species-specific response to the larval feeding of
both species (Fig. 6), and (iii) genes that signify the shift of the species-specific feeding-induced response by the other species’ oviposition (Fig. 7). Overall, we validated the
expression patterns determined by the microarray analysis for all 17 genes by real-time qPCR (Supplementary Figs S4 and S5). Our analyses verified the up-regulation of the oligo peptide
transporter _PTR1_-like in response to the oviposition by both species whereas up-regulation of a _defensin_-like protein by _M. sexta_ oviposition was only indicated by trend (Fig. 5).
Plants with _M. sexta_ oviposition had significantly reduced transcript levels of a predicted _WRKY71_ transcription factor and an L-aspartate oxidase gene (_AO_). While plants with _S.
exigua_ oviposition had increased transcript levels of the trypsin protease inhibitor gene _NaPI_ and the geranylgeranyl diphosphate synthase gene _NaGGPPS2_. Transcripts of _PTR1_ and
_defensin_ also increased in response to larval feeding by both species, which tended to be stronger when the plants were previously oviposited by conspecifics. Though not statistically
significant, this could point towards additive effects of oviposition and feeding. The increased transcript accumulation of the _NaPI_ gene in response to _S. exigua_ oviposition did also
not result in increased _NaPI_ transcripts after larval feeding compared to only feeding-damaged plants (Fig. 5e). In contrast, dual comparisons between feeding-induced plants revealed
significantly increased transcripts of _NaGGPPS2_ in _S. exigua_-oviposited compared to only feeding-induced plants (Fig. 5f). Transcript levels of the _WRKY71_-like transcription factor and
the _AO_ gene were similar in feeding-induced plants whether the plants were oviposited or not. But since these genes were down-regulated in only-oviposited plants, they show a significant
interaction between oviposition and larval feeding by _S. exigua_ and _M. sexta_ respectively (Fig. 5b,d). In summary, 24 hours after onset of larval feeding, we could still determine some
genes affected by the previous oviposition _per se_ but we could not pin down the effect of prior oviposition by conspecific moths on feeding-induced gene expression. We additionally
analysed a set of well characterised _N. attenuata_ genes that play a role in different plant signalling pathways related to herbivory. These genes are involved in the regulation of the JA
pathway (_NaLOX3_, _NaTD_, _NaWRKY6_), biosynthesis of ethylene (_NaACO2_) and of SA signalling (_NaNPR1_). All of these genes were significantly induced by larval feeding but not by
oviposition and oviposition did not affect the level of induction after feeding (Fig. 6). They all showed a higher transcript accumulation in response to _S. exigua_ than to _M. sexta_,
except for _NaLOX3_, which was not differently regulated between the two species (Supplementary Fig. S4). As oviposition shifted the species-specific transcriptomic imprint of the larval
feeding, we also verified such a transcriptional shift for a selected set of genes. Three of them, the _mitogen-activated protein kinase kinase NaMKK1_, the _SA methyl transferase SAMT_ and
the _1-aminocyclopropane-1-carboxylic acid oxidase NaACO2_ were stronger upregulated in response to _S. exigua_ feeding than in response to _M. sexta_ feeding but showed an intermediate
expression to feeding by both larvae when they were previously oviposited by the other species (Fig. 7, Supplementary Fig. S5, Supplementary Table S4). Three other genes, the circadian clock
gene _timing of CAB expression NaTOC1_, an _early flowering-like_ gene and a gene homologous to _Jumonji C-domain-containing proteins, JmjC30-like_ showed a higher expression to _M. sexta_
than to _S. exigua_ feeding, which was switched when the plant was previously oviposited by the other species. DISCUSSION We examined whether the oviposition _per se_ left imprints in the
plant’s phytohomonal and transcriptional regulation in a systemic leaf. In line with previous studies11,12, we found no differences in phytohormone levels between oviposited and control
plants. The transcript profile in plants oviposited by _S. exigua_ did also not differ from that of control plants, but our microarray analysis revealed an imprint of _M. sexta_ oviposition
on _N. attenuata_’s transcriptome. Even a day after the end of the egg incubation time, expression of some genes was significantly altered and many of them were annotated to signalling and
regulatory functions (e.g. transcription factors, hormone biosynthesis, see Supplementary Table S1). Moreover, about half of these genes were also altered in response to _M. sexta_ feeding
(see Supplementary Fig. S2). This is consistent with the idea that also subtle changes in gene regulation can be biologically relevant44. We identified several genes responsive to
oviposition by both _M. sexta_ and _S. exigua_ in our better replicated qPCR analysis (Fig. 5). Among them are genes involved in plant defence, for example _NaGGPPS_ and _NaPI_45,46. These
were induced by _S. exigua_- but not by _M. sexta_-oviposition. Interestingly, PI activity in response to larval feeding was also increased in _S. exigua_- but not in _M. sexta_-oviposited
plants11,12. However, compared to the effect of larval feeding, oviposition only slightly induced _NaPI_ expression and whether that could explain the priming of feeding-induced PI activity
remains an open question. Generally, it is not surprising that one day after egg-removal, systemic _N. attenuata_ leaves did not display the large extent of changes in gene expression as
described for the local leaves of _A. thaliana_ 24–72 hours after oviposition by _Pieris_ moths34. Even in local _A. thaliana_ leaves, no transcriptional imprint was detected anymore, one
day after egg-removal41. Yet, both plant species differ in their physiological response to insect oviposition. Other than _N. attenuata_, _A. thaliana_ responds to oviposition with HR-like
symptoms34 which can result in SAR against phytopathogenic bacteria33,35. Whereas oviposition by the specialist _P. brassicae_ induces HR-like necrosis in many brassicaceous plants17,21,
oviposition by the generalist _Mamestra brassicae_ does not, nor does the latter affect larval performance25. However, in _N. attenuata_, oviposition by both a specialist and a generalist
moth species prime feeding-induced defences and lower the performance of _S. exigua_ larvae11,12. In line with that, all defence-related candidate genes that were affected by oviposition in
our qPCR analysis showed similar tendencies for the oviposition by both moth species even if the effect was only significant for one of the two species. As expected, _N. attenuata_ exhibited
diverging responses to the feeding larvae of both species. Larvae of _S. exigua_ induced more SA than those of _M. sexta_, just as had been previously described31. Contrasting this earlier
study31, _M. sexta_ feeding also induced some SA and _S. exigua_-fed plants showed no lower JA induction but a greater JA-Ile/JA ratio than _M. sexta_-fed plants, which may even suggest a
higher conversion of JA to the biologically active conjugate. However, in line with our results, a recent study47 also describes SA induction after _M. sexta_ feeding and an equal
JA-elicitation for _N. attenuata_’s response to _M. sexta_ and _Spodoptera littoralis_. Consistent with our phytohormone analysis, transcripts of the Ile-biosynthesis gene _NaTD_ as well as
the SA-receptor _NaNPR1_ were differentially activated by larval feeding of both species, whereas that of the JA-biosynthesis gene _NaLOX3_ was not (Fig. 6, Supplementary Fig. S4). Thus, our
results support that SA signalling plays a role in tuning _N. attenuata_’s species-specific response to larval feeding, but this tuning is not necessarily linked to JA-SA antagonism as had
been suggested earlier31. The distinguished species-specific response to the feeding larvae was reflected in the number of regulated genes as well as in PCA, regression and cluster analyses
of the plant’s transcriptome (Figs 2–4). Our results match well with those of a study that pioneered _N. attenuata_’s species-specific transcriptional response to different herbivores4 using
cDNA microarrays displaying only 240 genes with a bias for herbivory-related genes. Like this earlier study4, our unbiased transcriptome microarray, showed that a large fraction of
feeding-responsive genes was specifically regulated by feeding of the generalist _S. exigua_ and this fraction was greater among down- than among up-regulated genes. In line with this, the
shared transcriptional response to two generalist herbivores _Heliothis virescens_ and _S. exigua_ differentiating from the response to _M. sexta_ is also dominated by down-regulated genes4.
Differential plant responses to herbivores of the same feeding guild but different diet breadth are also apparent in other plant-insect interactions, which could be either adaptive for the
plant or the result of host manipulation by insects5,6,7,26. Presumably, generalist herbivores are more likely to evolve mechanisms to suppress plant defences than specialists as they have
to cope with diverse plant defences7. However, _S. exigua_ feeding altered more than 2000 genes more than _M. sexta_ feeding. Although, this was more pronounced for down-regulated genes,
defence-related genes and signalling pathways were often stronger induced in response to the generalist (Figs 2–7) and the majority of genes down-regulated after herbivory are usually
related to primary plant metabolism48. _N. attenuata_ effectively defends itself against _S. exigua_11,46,49, whereas _M. sexta_ is relatively tolerant to many of its direct defences47,50.
Thus, it is more likely that the differential plant response is driven either by the specialist herbivore or the plant itself. The distinct response of _N. attenuata_ to both herbivores is
likely due to the divergent composition of elicitors in their oral secretions31. The receptors recognizing these elicitors have not been identified so far but they presumably activate
mitogen-activated protein (MAP) kinases, usually three in a cascade, that mediate the transduction of the signal. We found that one of the MAP kinases (_NaMKK1_) that are involved in the
regulation of _N. attenuata_’s defence response to herbivory51, reflected the species-specific expression pattern (Fig. 7). Even though _NaMKK1_ is not required for feeding-induced JA and
ethylene production, it fine-tunes _N. attenuata_’s defence response52 and thus it may also be involved in adjusting the plant’s response to the attacking herbivore species. To examine plant
responses that may be associated with _N. attenuata_’s oviposition-primed defence response to larval feeding11, we compared only feeding-induced plants with plants that had been
additionally oviposited by conspecific moths. Yet, we could not determine how insect oviposition on _N. attenuata_ alters feeding-induced plant defence metabolites and the performance or
immune state of _S. exigua_ and _M. sexta_ larvae, respectively11,12. The transcriptional responses between only feeding-induced plants and plants with prior oviposition by conspecifics
correlated to 90%, which only emphasizes the high reproducibility of the herbivory treatments within our microarray analysis. However, despite their biological relevance, subtle changes in
only few genes can be easily overlooked due to the large number of variables that are analysed with a very limited number of replicates44. For example, pre-treatments with phytopathogenic
bacteria or egg extract that both have a negative impact on larval performance of _P. brassicae_, show just very subtle effects on the transcriptional response of _B. nigra_ to larval
feeding42. That we could not determine signalling components which could explain these effects may be due to one or a combination of the following reasons: The regulation of the
oviposition-priming of plant defence may (i) occur at different levels, e.g. post-transcriptionally, (ii) involve only few key components at an effect-size that is too small to be revealed
in a low-replicated multi-variate approach, and (iii) occur at other time points after the onset of larval feeding. Priming of feeding-induced responses may result in an earlier, faster,
stronger, more sensitive or even qualitatively different response on the molecular level9. In order to minimize variation between plant-replicates, that may be driven by random differences
in the feeding behaviour of the larvae on a shorter time scale, we examined a time point at which the feeding-induced response on the transcriptional and phytohormonal level is well settled.
However, thereby we may have missed the time point at which the plant defence priming by oviposition is regulated, for instance if it would cause an earlier or faster plant response just
upon the larvae started feeding. Opposite to prior oviposition by conspecifics, our results revealed that oviposition by one species altered the species-specific feeding response to larvae
of the other species. In both cross-species treatments, the plant’s response to feeding larvae became intermediate in those characteristics that were specific for the larval feeding of both
species. This was consistently apparent in all analyses of _N. attenuata_’s transcriptional response (PCA, cluster and regression analyses as well as the numbers and overlaps of
significantly regulated genes) as well as in the phytohormone data for SA and JA-Ile/JA (Figs 2–4). The oviposition-dependent shift of the species-specific gene expression in response to
larval feeding was further confirmed by qPCR on six selected genes. Half of them were higher expressed in response to _S. exigua_ than to _M. sexta_ and showed an intermediate expression in
the cross-species treatments (Fig. 7, Supplementary Fig. S5). These genes (_ACO_, _SAMT_, _NaMKK1_) are related to signalling pathways that mediate plant defence. Again, as _NaMKK1_ is
fine-tuning _N. attenuata_’s defence response52, it may also play a role in the oviposition-mediated shift of the plant response to larval feeding. Three circadian clock genes were stronger
expressed after _M. sexta_- than after _S. exigua_-feeding but showed the reversed pattern in the cross-species treatments. Two of them were also induced by _M. sexta_ oviposition at levels
comparable to feeding (Supplementary Table S1). Previous studies suggested that diurnal regulation plays a role in orchestrating plant anti-herbivore defences53,54 and this may be the case
for the oviposition-mediated responses we found in _N. attenuata_ as well. Attack by multiple herbivores modifies plant responses in a non-additive manner and consequently can strongly
affect the outcome of plant-insect interactions55. Our study shows that such interactions between different insects can begin before actual herbivory occurs. That insect oviposition alters
plant resistance to the ovipositing species is well-established today56 while the notion that also other biotic stressors can be affected is just emerging12,35. Our study revealed that
insect oviposition can shape the species-specificity of a plant’s response to feeding larvae. What are possible explanations of this surprising phenomenon? On the one hand, _N. attenuata_
may prepare its feeding-induced response to defend most effectively against the herbivore that announced itself by its oviposition. On the other hand, herbivores may start to manipulate
their host plant right upon oviposition. The latter scenario may be more likely for the specialist, yet the shift of the species-specific plant response was more pronounced in response to
the generalist’s oviposition. Despite its wide host plant range, _S. exigua_ is a frequent herbivore in the native habitat of _N. attenuata_49 and as such it may exert considerable selection
pressure on the plant. If the plant would benefit from rendering its response specific to the insect species, the effect that oviposition can determine this species-specificity connotes a
novel mechanism of defence priming. METHODS PLANTS AND INSECTS Seeds of a _Nicotiana attenuata_ Torr. ex Watson inbred line (15x) from a field collection were sterilized and smoke-germinated
on agar plates and seedlings were transferred to potting soil and grown in 1.5 L pots in a greenhouse (24 °C (±10):15 °C; 16:8 h L:D) as described earlier11. _Manduca sexta_ L. and
_Spodoptera exigua_ Hübner were cultured in a climate chamber (24 °C; 16:8 L:D). Insect cultures and the wheat germ based artificial diets of the larvae were described earlier57.
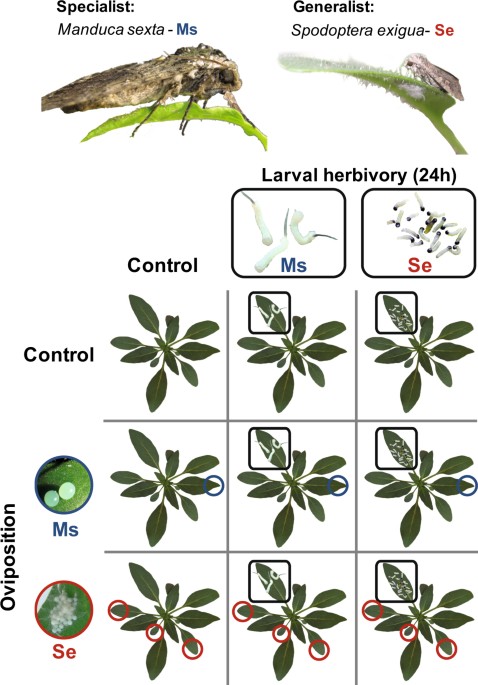
EXPERIMENTAL PROCEDURE We assigned 5-week-old early elongated experimental plants of equal size and elongation state to one of nine treatments for each of 12 biological replicate blocks
(Fig. 1). In a 3 × 3 full-factorial experimental design, we examined the plant responses to oviposition and larval feeding by _M. sexta_ and _S. exigua_ and the interactive effects between
them. The setup of the oviposition and larval feeding treatments were previously described in detail58. In brief, we exposed the second youngest source leaf (2 positions below the
sink-source transition leaf) through slots in the side of a flight cage to _M. sexta_ oviposition. As _S. exigua_ does not accept this setup for oviposition, we exposed whole plants to
oviposition by _S. exigua_ for 12 h. _M. sexta_-oviposited plants received 2.2 ± 0.4 (mean ± SEM) and _S. exigua_-oviposited 37.7 ± 5.6 eggs (dispersed on one to four elder leaves). Within
the range of the natural egg incubation time, 3 and 4 days after oviposition by _S. exigua_ and _M. sexta_ respectively, we removed the eggs of all plants without damaging the leaf surface
either with a soft brush or a featherweight forceps. Within 12 h after egg-removal, we applied either three _M. sexta_ or 20 _S. exigua_ neonates per plant to a standardised leaf position
relative to the sink-source transition leaf. It was the next younger leaf above the _M. sexta_-oviposited leaf and the corresponding leaf position for control or _S. exigua_-oviposited
plants. Due to the size difference of both species, these numbers of larvae inflict comparable amounts of damage. Larvae were kept on the leaf in vented clip cages and plants without
herbivory treatments received empty clip cages at corresponding leaf positions. After 24 hours of larval feeding, the standardised leaf (either exposed to larvae or empty clip cages) was
harvested from all plants, flash-frozen in liquid nitrogen and stored at −80 °C. Aliquots of the leaf material powdered using mortar and pestle under liquid nitrogen were weighed into
separate tubes. EXTRACTION AND ANALYSIS OF PHYTOHORMONES As previously described in more detail59, we extracted about 150 mg of the samples in 1 mL ethyl acetate spiked with deuterated
internal standards of JA, JA-Ile, SA, ABA and re-eluted the dried extracts in 400 µL 70% methanol with 0.1% formic acid before analysis by UPLC-ESI-MS/MS (Synapt G2-S HDMS; Waters®, Milford,
Massachusetts, USA). After separation on a C18-column (Acquinity UPLC BEH-C18) with water and methanol (both containing 0.1% formic acid) as eluents in gradient mode (250 μL min−1),
phytohormones were quantified (negative ionization mode, parent/daughter ion selections for JA: 209/59, JA-Ile: 322/130, SA: 137/93, ABA: 263/153, D6-JA: 215/59, D6-JA-Ile: 328/130, D4-SA:
141/97, D6-ABA: 269/159) according to the peak areas of the respective fragment ions relative to that of the internal standards using MassLynxTM (4.1, Waters). RNA EXTRACTION We extracted
total RNA from 100 mg leaf powder of all individual plants (including those of the EseLms treatment) with TRIzol® Reagent before the precipitated RNA (using 1.2 M sodium chloride and 0.8 M
sodium citrate) was DNase digested using TURBO DNA-free™ (both AmbionTM, Thermo Fisher Scientific, Waltham, MA USA; http://www.thermofisher.com) according to the manufacturer’s instructions.
After a clean-up of the RNA using NucleoSpin® RNA (Macherey-Nagel, Düren, Germany; http://www.mn-net.com/), we adjusted the RNA to 200 ng/µL based on photometric concentration measurements
(μDrop™ Plate; Multiskan™ GO Microplate Spectrophotometer, Thermo Scientific). MICROARRAY ANALYSIS RNA of 3-4 plant replicates per treatment was pooled at equal proportions resulting in 3
biological replicates for each treatment on the microarray (performed by Oaklabs, http://www.oak-labs.com/). All samples had a RNA integrity index between 7.7 and 8.0 as determined with a
2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA, http://www.agilent.com). Fluorescent cRNA was generated using the Low Input QuickAmp Labeling Kit (Agilent Technologies)
and oligo-dT primers following the manufacturer’s protocol. Of the cyanine 3-CTP-labelled cRNA, 600 ng were hybridised using the Agilent Gene Expression Hybridisation Kit (Agilent
Technologies) following the manufacturer’s protocol on the microarray at 65 °C for 17 h. After the microarray was washed twice, the fluorescence signals on microarrays were detected by the
SureScan Microarray Scanner (Agilent Technologies) at a resolution of 3 micron per pixel. The 8 × 60 K microarray harboured the same 60-mer oligonucleotide probes as the Agilent 4 × 44 K
microarray with the GEO accession no. GPL1352760 that was designed based on the _N. attenuata_ transcriptome (BioProject PRJNA223344 at http://www.ncbi.nlm.nih.gov/). Although all RNA
samples were processed at once, a first hybridisation on three arrays (24 samples) included only one cross-species treatment. The EmsLse was chosen because oviposition by _M. sexta_ affects
larval performance of _S. exigua_ but not _vice versa_12. The analysis revealed that, opposite to the effect of prior oviposition of the same species that later fed on the plants, prior
exposure to _M. sexta_ oviposition shifted the response to larval feeding by _S. exigua_. Therefore, a second analysis including the EseLms treatment was performed. REAL-TIME QPCR ANALYSIS
To verify microarray results and to test the effects of oviposition and larval feeding on potentially regulated genes with a higher statistical power, we performed real-time qPCR on 9-10
individual plants of each treatment. The verification of the qPCR data was initially performed for 11 genes on the same 8 treatments used in the first microarray hybridisations and was then
extended for 6 genes to all feeding-induced treatments including both cross-species treatments and control plants. We synthesized cDNA from the total RNA with the Reverse Transcriptase Core
kit (Eurogentec, Seraing, Belgium, http://www.eurogentec.com) according to the manufacturer’s instructions. For each gene, 1 µL cDNA was used in 10 µL reactions of SYBR®Green I-based
real-time PCR using a qPCR kit without ROX (Eurogentec) on a StratageneTM Mx3005P® instrument (Agilent Technologies). Real-time PCR was performed with gene-specific primers (see
Supplementary Table S5) in triplicates with all treatments of a replicate on one 96 well plate. We inspected melting curves and used LinRegPCR (http://LinRegPCR.HFRC.nl) to determine
amplification efficiencies and the relative quantities (RQ) of each gene61. The RQ of the genes of interest was normalised to the averaged RQ of two reference genes _β-actin_ and _EF1α_
(NRQ). Transcript accumulations were analysed on the log2-normalized NRQs and depicted relative to the mean of the control treatment. DATA ANALYSIS All statistics were performed in R version
3.2.3 with the packages _lme4, multcomp_, _psych_ and _stats_ (http://www.r-project.org). Phytohormone and qPCR data were checked for normality and variance homogeneity (_QQ_-plots and
Bartlett’s test) before their statistical evaluation. To test for differences in phytohormone levels between oviposited and control plants and for differences among the five groups of plants
exposed to larval feeding, we used either ANOVA followed by paired _t_-tests or Kruskal Wallis test followed by pairwise Wilcoxon rank sum tests. To evaluate the effect of the factors
oviposition and larval feeding as well as their interaction within the 2 × 2 full-factorial priming experiments for each species separately we used linear mixed-effects models (LMMs) and
included the replicate blocks as random factor. We also analysed transcript accumulations determined by qPCR with LMMs to evaluate differences between control and oviposited plants as well
as those between plants of the different larval feeding treatments. We used the _limma_ package62 to analyse the microarray data. To reduce background noise, a detection limit was set at the
10% quantile of fluorescence signal intensity of each array. All structural spots, as well as oligos that were not above this detection limit in any of the arrays, were removed from further
analysis. Subsequently, the expression levels were background-corrected using the _normexp_ method63 and normalized between the arrays using quantile normalisation64. Principal component
analysis was performed on normalised fluorescence signals using _prcomp_ and visualised using _ggplot2_65. We performed hierarchical clustering using _hcust_ and analysed gene expression
using _lmFit_. Genes were assumed to be significantly regulated when they differed between treatments by a log2-fold change of at least 1 and at _P_-values of below 0.05 after correction for
false discovery rate according to Benjamini-Hochberg. Genes of interest were functionally annotated according to NCBI nucleotide blast and blastx of the corresponding p454 sequences
(BioProject PRJNA223344). ACCESSION CODES The microarray data are available at NCBI Gene Expression Omnibus under accession GSE116273. REFERENCES * Schaller, A. _Induced plant resistance to
herbivory_ (Springer, 2008). * Karban, R. & Agrawal, A. A. Herbivore offense. _Annu. Rev. Ecol. Syst._ 33, 641–664, https://doi.org/10.1146/annurev.ecolsys.33.010802.150443 (2002).
Article Google Scholar * Agrawal, A. A., Petschenka, G., Bingham, R. A., Weber, M. G. & Rasmann, S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant-herbivore
interactions. _New Phytol._ 194, 28–45, https://doi.org/10.1111/j.1469-8137.2011.04049.x. (2012). Article PubMed CAS Google Scholar * Voelckel, C. & Baldwin, I. T. Generalist and
specialist lepidopteran larvae elicit different transcriptional responses in _Nicotiana attenuata_, which correlate with larval FAC profiles. _Ecol. Lett._ 7, 770–775,
https://doi.org/10.1111/j.1461-0248.2004.00633.x (2004). Article Google Scholar * Vogel, Hea Different transcript patterns in response to specialist and generalist herbivores in the wild
_Arabidopsis_ relative _Boechera divaricarpa_. _PLoS. ONE_ 2, e1081, https://doi.org/10.1371/journal.pone.0001081 (2007). Article ADS PubMed PubMed Central CAS Google Scholar * Zong,
N. & Wang, C.-Z. Larval feeding induced defensive responses in tobacco: comparison of two sibling species of _Helicoverpa_ with different diet breadths. _Planta_ 226, 215–224,
https://doi.org/10.1007/s00425-006-0459-x (2007). Article PubMed CAS Google Scholar * Ali, J. G. & Agrawal, A. A. Specialist versus generalist insect herbivores and plant defense.
_Trends Plant Sci._ 17, 1360–1385, https://doi.org/10.1016/j.tplants.2012.02.006 (2012). Article CAS Google Scholar * Conrath, U., Beckers, G. J. M., Langenbach, C. J. G. &
Jaskiewicz, M. R. Priming for enhanced defense. _Annu. Rev. Phytopath._ 53, 97–119, https://doi.org/10.1146/annurev-phyto-080614-120132 (2015). Article CAS Google Scholar * Hilker, M. _et
al_. Priming and memory of stress responses in organisms lacking a nervous system. _Biol. Rev._ 91, 1118–1133, https://doi.org/10.1111/brv.12215 (2016). Article PubMed Google Scholar *
Heil, M. & Kost, C. Priming of indirect defences. _Ecol. Lett._ 9, 813–817, https://doi.org/10.1111/j.1461-0248.2006.00932.x (2006). Article PubMed Google Scholar * Bandoly, M.,
Hilker, M. & Steppuhn, A. Oviposition by _Spodoptera exigua_ on _Nicotiana attenuata_ primes induced plant defense against larval herbivory. _Plant J._ 83, 661–672,
https://doi.org/10.1111/tpj.12918 (2015). Article PubMed CAS Google Scholar * Bandoly, M., Grichnik, R., Hilker, M. & Steppuhn, A. Priming of anti-herbivore defence in _Nicotiana
attenuata_ by insect oviposition: Herbivore specific effects. _Plant Cell Environ._ 39, 848–859, https://doi.org/10.1111/pce.12677 (2016). Article PubMed CAS Google Scholar * Kim, J.,
Tooker, J. F., Luthe, D. S., De Moraes, C. M. & Felton, G. W. Insect eggs can enhance wound response in plants: A study system of tomato _Solanum lycopersicum_ L. and _Helicoverpa zea_
Boddie. _PLoS. ONE_ 7, e37420, https://doi.org/10.1371/journal.pone.0037420 (2012). Article ADS PubMed PubMed Central CAS Google Scholar * Austel, N., Eilers, E. J., Meiners, T. &
Hilker, M. Elm leaves “warned” by insect egg deposition reduce survival of hatching larvae by a shift in their quantitative leaf metabolite pattern. _Plant Cell Environ._ 39, 366–376,
https://doi.org/10.1111/pce.12619 (2016). Article PubMed CAS Google Scholar * Beyaert, I. _et al_. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous
larvae? _Proc. R. Soc. B_ 279, 101–108, https://doi.org/10.1098/rspb.2011.0468 (2012). Article PubMed Google Scholar * Geiselhardt, S. _et al_. Egg laying of cabbage white butterfly
(_Pieris brassicae_) on _Arabidopsis thaliana_ affects subsequent performance of the larvae. _PLoS. ONE_ 8, e59661, https://doi.org/10.1371/journal.pone.0059661 (2013). Article ADS PubMed
PubMed Central CAS Google Scholar * Pashalidou, F. G., Fatouros, N. E., van Loon, J. J. A., Dicke, M. & Gols, R. Plant-mediated effects of butterfly egg deposition on subsequent
caterpillar and pupal development, across different species of wild Brassicaceae. _Ecol. Entomol._ 40, 444–450, https://doi.org/10.1111/een.12208 (2015). Article Google Scholar * Bittner,
N., Trauer-Kizilelma, U. & Hilker, M. Early plant defence against insect attack: involvement of reactive oxygen species in plant responses to insect egg deposition. _Planta_ 245,
993–1007, https://doi.org/10.1007/s00425-017-2654-3 (2017). Article PubMed CAS Google Scholar * Desurmont, G. A. & Weston, P. A. Aggregative oviposition of a phytophagous beetle
overcomes egg-crushing plant defences. _Ecol. Entomol._ 36, 335–343, https://doi.org/10.1111/j.1365-2311.2011.01277.x (2011). Article Google Scholar * Doss, R. P. _et al_. Bruchins:
Insect-derived plant regulators that stimulate neoplasm formation. _Proc. Natl. Acad. Sci. USA_ 97, 6218–6223 (2000). Article ADS PubMed CAS PubMed Central Google Scholar * Fatouros,
N. E. _et al_. Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. _Proc. R. Soc. B_ 281, https://doi.org/10.1098/rspb.2014.1254 (2014). *
Geuss, D., Stelzer, S., Lortzing, T. & Steppuhn, A. _Solanum dulcamara_’s response to eggs of an insect herbivore comprises ovicidal hydrogen peroxide production. _Plant Cell Environ._
40, 2663–2677, https://doi.org/10.1111/pce.13015 (2017). Article PubMed CAS Google Scholar * Petzold-Maxwell, J., Wong, S., Arellano, C. & Gould, F. Host plant direct defence against
eggs of its specialist herbivore. _Heliothis subflexa. Ecol. Entomol._ 36, 700–708, https://doi.org/10.1111/j.1365-2311.2011.01315.x (2011). Article Google Scholar * Seino, Y., Suzuki, Y.
& Sogawa, K. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, _Sogatella furcifera_ (HORVATH) (Homoptera: Delphacidae). _Appl.
Entomol. Zool._ 31, 467–473, https://doi.org/10.1303/aez.31.467 (1996). Article CAS Google Scholar * Pashalidou, F. G., Lucas-Barbosa, D., van Loon, J. J. A., Dicke, M. & Fatouros, N.
E. Phenotypic plasticity of plant response to herbivore eggs: effects on resistance to caterpillars and plant development. _Ecology_ 94, 702–713, https://doi.org/10.1890/12-1561.1 (2013).
Article PubMed Google Scholar * Appel, H. M. _et al_. Transcriptional responses of _Arabidopsis thaliana_ to chewing and sucking insect herbivores. _Frontiers in Plant Science_ 5, 565,
https://doi.org/10.3389/fpls.2014.00565 (2014). Article PubMed PubMed Central Google Scholar * Pieterse, C. M. J., Van der Does, D., Zamioudis, C., Leon-Reyes, A. & Van Wees, S. C.
M. Hormonal modulation of plant immunity. _Annu. Rev. Cell Develop. Biol._ 28, 489–521, https://doi.org/10.1146/annurev-cellbio-092910-154055 (2012). Article CAS Google Scholar *
Bonaventure, G. Perception of insect feeding by plants. _Plant Biol._ 14, 872–880, https://doi.org/10.1111/j.1438-8677.2012.00650.x (2012). Article PubMed CAS Google Scholar * Heil, M.
Damaged-self recognition in plant herbivore defence. _Trends Plant Sci._ 14, 356–363, https://doi.org/10.1016/j.tplants.2009.04.002 (2009). Article PubMed CAS Google Scholar * Schmelz,
E. A., Engelberth, J., Alborn, H. T., Tumlinson, J. H. III & Teal, P. E. A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. _Proc. Natl. Acad. Sci. USA_ 106,
653–657, https://doi.org/10.1073/pnas.0811861106 (2009). Article ADS PubMed PubMed Central Google Scholar * Diezel, C., von Dahl, C. C., Gaquerel, E. & Baldwin, I. T. Different
lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. _Plant Physiol._ 150, 1576–1586, https://doi.org/10.1104/pp.109.139550 (2009). Article PubMed
PubMed Central CAS Google Scholar * Reymond, P. Perception, signaling and molecular basis of oviposition-mediated plant responses. _Planta_ 238, 247–258,
https://doi.org/10.1007/s00425-013-1908-y (2013). Article PubMed PubMed Central CAS Google Scholar * Bruessow, F., Gouhier-Darimont, C., Buchala, A., Metraux, J. P. & Reymond, P.
Insect eggs suppress plant defence against chewing herbivores. _Plant J._ 62, 876–885, https://doi.org/10.1111/j.1365-313X.2010.04200.x (2010). Article PubMed CAS Google Scholar *
Little, D., Gouhier-Darimont, C., Bruessow, F. & Reymond, P. Oviposition by pierid butterflies triggers defense responses in. _Arabidopsis. Plant Physiol._ 143, 784–800,
https://doi.org/10.1104/pp.106.090837 (2007). Article PubMed CAS Google Scholar * Hilfiker, O. _et al_. Insect eggs induce a systemic acquired resistance in _Arabidopsis_. _Plant J._ 80,
1085–1094, https://doi.org/10.1111/tpj.12707 (2014). Article PubMed CAS Google Scholar * Ament, K., Kant, M. R., Sabelis, M. W., Haring, M. A. & Schuurink, R. C. Jasmonic acid is a
key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. _Plant Physiol._ 135, 2025–2037, https://doi.org/10.1104/pp.104.048694 (2004). Article
PubMed PubMed Central CAS Google Scholar * Buchel, K. _et al_. An elm EST database for identifying leaf beetle egg-induced defense genes. _BMC Genomics_ 13,
https://doi.org/10.1186/1471-2164-13-242 (2012). * De Puysseleyr, V., Hofte, M. & De Clercq, P. Ovipositing _Orius laevigatus_ increase tomato resistance against _Frankliniella
occidentalis_ feeding by inducing the wound response. _Arthropod Plant Interact._ 5, 71–80, https://doi.org/10.1007/s11829-010-9117-0 (2011). Article Google Scholar * Hilker, M., Kobs, C.,
Varama, M. & Schrank, K. Insect egg deposition induces _Pinus sylvestris_ to attract egg parasitoids. _J. Exp. Biol._ 205, 455–461 (2002). PubMed Google Scholar * Meiners, T.,
Westerhaus, C. & Hilker, M. Specificity of chemical cues used by a specialist egg parasitoid during host location. _Entomol. Exp. Appl._ 95, 151–159,
https://doi.org/10.1046/j.1570-7458.2000.00653.x (2000). Article Google Scholar * Firtzlaff, V., Oberländer, J., Geiselhardt, S., Hilker, M. & Kunze, R. Pre-exposure of _Arabidopsis_
to the abiotic or biotic environmental stimuli ‘chilling’ or ‘insect eggs’ exhibits different transcriptomic responses to herbivory. _Sci. Rep._ 6, 28544, https://doi.org/10.1038/srep28544
(2016). Article ADS PubMed PubMed Central CAS Google Scholar * Bonnet, C. _et al_. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against
a chewing herbivore in _Brassica nigra_. _BMC Plant Biol._ 17, 127, https://doi.org/10.1186/s12870-017-1074-7 (2017). Article PubMed PubMed Central Google Scholar * Kaur, H., Heinzel,
N., Schottner, M., Baldwin, I. T. & Galis, I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against
insect herbivores in _Nicotiana attenuata_. _Plant Physiol._ 152, 1731–1747, https://doi.org/10.1104/pp.109.151738 (2010). Article PubMed PubMed Central CAS Google Scholar * Sanchez, D.
H., Szymanski, J., Erban, A., Udvardi, M. K. & Kopka, J. Mining for robust transcriptional and metabolic responses to long-term salt stress: a case study on the model legume _Lotus
japonicus_. _Plant Cell Environ._ 33, 468–480, https://doi.org/10.1111/j.1365-3040.2009.02047.x (2010). Article PubMed CAS Google Scholar * Jassbi, A. R., Gase, K., Hettenhausen, C.,
Schmidt, A. & Baldwin, I. T. Silencing geranylgeranyl diphosphate synthase in _Nicotiana attenuata_ dramatically impairs resistance to tobacco hornworm. _Plant Physiol._ 146, 974–986,
https://doi.org/10.1104/pp.107.108811 (2008). Article PubMed PubMed Central CAS Google Scholar * Steppuhn, A. & Baldwin, I. T. Resistance management in a native plant: nicotine
prevents herbivores from compensating for plant protease inhibitors. _Ecol. Lett._ 10, 499–511, https://doi.org/10.1111/j.1461-0248.2007.01045.x (2007). Article PubMed Google Scholar *
Xu, S., Zhou, W., Pottinger, S. & Baldwin, I. T. Herbivore associated elicitor-induced defences are highly specific among closely related _Nicotiana_ species. _BMC Plant Biol._ 15, 2,
https://doi.org/10.1186/s12870-014-0406-0 (2015). Article PubMed PubMed Central CAS Google Scholar * Lortzing, T. _et al_. Transcriptomic responses of _Solanum dulcamara_ to natural and
simulated herbivory. _Mol. Ecol. Resour._ 17, e196–e211, https://doi.org/10.1111/1755-0998.12687 (2017). Article PubMed CAS Google Scholar * Steppuhn, A., Gase, K., Krock, B.,
Halitschke, R. & Baldwin, I. T. Nicotine’s defensive function in nature. _PLoS. Biol._ 2, 1074–1080, https://doi.org/10.1371/journal.pbio.0020217 (2004). Article CAS Google Scholar *
Wink, M. & Theile, V. Alkaloid tolerance in _Manduca sexta_ and phylogenetically related sphingids (Lepidoptera: Sphingidae). _Chemoecology_ 12, 29–46,
https://doi.org/10.1007/s00049-002-8324-2 (2002). Article CAS Google Scholar * Hettenhausen, C., Schuman, M. C. & Wu, J. Q. MAPK signaling: A key element in plant defense response to
insects. _Insect Sci._ 22, 157–164, https://doi.org/10.1111/1744-7917.12128 (2015). Article PubMed CAS Google Scholar * Heinrich, M., Baldwin, I. T. & Wu, J. Q. Two mitogen-activated
protein kinase kinases, MKK1 and MEK2, are involved in wounding- and specialist lepidopteran herbivore _Manduca sexta_-induced responses in _Nicotiana attenuata_. _J. Exp. Bot._ 62,
4355–4365, https://doi.org/10.1093/jxb/err162 (2011). Article PubMed PubMed Central CAS Google Scholar * Goodspeed, D., Chehab, E. W., Min-Venditti, A., Braam, J. & Covington, M. F.
_Arabidopsis_ synchronizes jasmonate-mediated defense with insect circadian behavior. _Proc. Natl. Acad. Sci. USA_ 109, 4674–4677, https://doi.org/10.1073/pnas.1116368109 (2012). Article
ADS PubMed PubMed Central Google Scholar * Kim, S. G., Yon, F., Gaquerel, E., Gulati, J. & Baldwin, I. T. Tissue specific diurnal rhythms of metabolites and their regulation during
herbivore attack in a native tobacco. _Nicotiana attenuata. PLoS. ONE_ 6, e26214, https://doi.org/10.1371/journal.pone.0026214 (2011). Article ADS PubMed CAS Google Scholar * Stam, J.
M. _et al_. Plant Interactions with Multiple Insect Herbivores: From Community to Genes. _Annu. Rev. Plant Biol._ 65, 689–713, https://doi.org/10.1146/annurev-arplant-050213-035937 (2014).
Article PubMed CAS Google Scholar * Hilker, M. & Fatouros, N. E. Plant responses to insect egg deposition. _Annu. Rev. Entomol._ 60, 493–515,
https://doi.org/10.1146/annurev-ento-010814-020620 (2015). Article PubMed CAS Google Scholar * Trauer, U. & Hilker, M. Parental legacy in insects: variation of transgenerational
immune priming during offspring development. _PLoS. ONE_ 8, e63392, https://doi.org/10.1371/journal.pone.0063392 (2013). Article ADS PubMed PubMed Central CAS Google Scholar * Bandoly,
M. & Steppuhn, A. Bioassays to investigate the effects of insect oviposition on a plant’s resistance to herbivores. _BioProtocol_ 6, e1823, https://doi.org/10.21769/BioProtoc.1823
(2016). Article Google Scholar * Nguyen, D. _et al_. Drought and flooding have distinct effects on herbivore-induced responses and resistance in _Solanum dulcamara_. _Plant, Cell &
Environ._ 39, 1485–1499, https://doi.org/10.1111/pce.12708 (2016). Article CAS Google Scholar * Gulati, J., Kim, S.-G., Baldwin, I. T. & Gaquerel, E. Deciphering herbivory-induced
gene-to-metabolite dynamics in _Nicotiana attenuata_ tissues using a multifactorial approach. _Plant Physiol._ 162, 1042–1059, https://doi.org/10.1104/pp.113.217588 (2013). Article PubMed
PubMed Central CAS Google Scholar * Ruijter, J. M. _et al_. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. _Nucleic Acids Research_ 37,
https://doi.org/10.1093/nar/gkp045 (2009). * Ritchie, M. E. _et al_. Limma powers differential expression analyses for RNA-sequencing and microarray studies. _Nucleic Acids Research_ 43,
e47, https://doi.org/10.1093/nar/gkv007 (2015). Article PubMed PubMed Central CAS Google Scholar * Ritchie, M. E. _et al_. A comparison of background correction methods for two-colour
microarrays. _Bioinformatics_ 23, 2700–2707, https://doi.org/10.1093/bioinformatics/btm412 (2007). Article PubMed CAS Google Scholar * Bolstad, B. M., Irizarry, R. A., Astrand, M. &
Speed, T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. _Bioinformatics_ 19, 185–193,
https://doi.org/10.1093/bioinformatics/19.2.185 (2003). Article PubMed CAS Google Scholar * Wickham, H. _ggplot2 Elegant graphics for data analysis_ (Springer, 2009). Download references
ACKNOWLEDGEMENTS We thank Lara Friebe and Julia Felderhoff for help with the experiments and the plant and insect cultures, Vivien Firtzlaff for help with the initial microarray analysis,
Martina Schad and Jim Kallarackal from Oaklabs for great support on the microarray hybridisations and Andreas Springer for excellent service with the UPLC-ESI-MS/MS. We are grateful for the
financial support of the German Research Foundation (DFG; project B2 within the Collaborative Research Centre ‘SFB973’) and the stipend of the German Federal Environmental Foundation (DBU)
for Michele Bandoly. AUTHOR INFORMATION Author notes * Sylvia Drok and Michele Bandoly contributed equally to this work. AUTHORS AND AFFILIATIONS * Freie Universität of Berlin/Institute of
Biology/Dahlem Centre of Plant Sciences, Laboratory of Molecular Ecology, Albrecht-Thaer Weg 6, Berlin, 14195, Germany Sylvia Drok, Michele Bandoly, Sandra Stelzer, Tobias Lortzing &
Anke Steppuhn Authors * Sylvia Drok View author publications You can also search for this author inPubMed Google Scholar * Michele Bandoly View author publications You can also search for
this author inPubMed Google Scholar * Sandra Stelzer View author publications You can also search for this author inPubMed Google Scholar * Tobias Lortzing View author publications You can
also search for this author inPubMed Google Scholar * Anke Steppuhn View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.B., S.D. and A.S.
designed this study. M.B., S.D. and S.S. conducted the experimental and laboratory work. S.D., M.B. and T.L. analysed the results. S.D., M.B. and A.S. wrote the first draft of the manuscript
that was edited and revised by all authors. CORRESPONDING AUTHOR Correspondence to Anke Steppuhn. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Drok, S., Bandoly, M., Stelzer, S. _et al._ Moth oviposition shapes the species-specific transcriptional and phytohormonal response of _Nicotiana attenuata_ to
larval feeding. _Sci Rep_ 8, 10249 (2018). https://doi.org/10.1038/s41598-018-28233-z Download citation * Received: 19 January 2018 * Accepted: 13 June 2018 * Published: 06 July 2018 * DOI:
https://doi.org/10.1038/s41598-018-28233-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative