- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Various functional magnetic resonance imaging studies addressed the effects of antidepressant drugs on brain functioning in healthy subjects; however, none specifically investigated
positive mood changes to antidepressant drug. Sixteen subjects with no personal or family history of psychiatric disorders were selected from an ongoing 4-week open trial of small doses of
clomipramine. Follow-up interviews documented clear positive treatment effects in six subjects, with reduced irritability and tension in social interactions, improved decision making, higher
self-confidence and brighter mood. These subjects were then included in a placebo-controlled confirmatory trial and were scanned immediately after 4 weeks of clomipramine use and again 4
weeks after the last dose of clomipramine. The functional magnetic resonance imaging (fMRI) scans were run during emotion-eliciting stimuli. Repeated-measures analysis of variance of brain
activity patterns showed significant interactions between group and treatment status during induced irritability (_P_<0.005 cluster-based) but not during happiness. Individuals displaying
a positive subjective response do clomipramine had higher frontoparietal cortex activity during irritability than during happiness and neutral emotion, and higher temporo–parieto–occipital
cortex activity during irritability than during happiness. We conclude that antidepressants not only induce positive mood responses but also act upon autobiographical recall of negative
emotions. SIMILAR CONTENT BEING VIEWED BY OTHERS ACUTE NEURAL EFFECTS OF THE MOOD STABILISER LAMOTRIGINE ON EMOTIONAL PROCESSING IN HEALTHY VOLUNTEERS: A RANDOMISED CONTROL TRIAL Article
Open access 27 May 2024 NEURAL PROCESSING OF SAD AND HAPPY AUTOBIOGRAPHICAL MEMORIES IN WOMEN WITH DEPRESSION AND BORDERLINE PERSONALITY DISORDER Article Open access 28 December 2024
PREFRONTAL, PARIETAL, AND LIMBIC CONDITION-DEPENDENT DIFFERENCES IN BIPOLAR DISORDER: A LARGE-SCALE META-ANALYSIS OF FUNCTIONAL NEUROIMAGING STUDIES Article Open access 13 February 2023
INTRODUCTION The specific neural mechanisms whereby selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors exert their therapeutic effects are not fully
established. Recent functional magnetic resonance imaging (fMRI) studies conducted under controlled conditions assessed brain activation in healthy individuals receiving citalopram,1, 2, 3,
4 fluvoxamine,5 reboxetine6, 7, 8, 9, 10 or escitalopram2,11, 12, 13 administered in single or multiple doses for 1–3 weeks. Using a variety of emXotion-eliciting experimental paradigms,
these studies provided evidence that selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors modulate the changes in cortical and subcortical limbic activity
that are typically elicited during the presentation of emotionally salient stimuli.14 It has been postulated that the efficacy of antidepressants is related to the reversal of automatic
responses to emotional information and of mood-dependent negative emotional biases.15, 16, 17, 18 Conversely, other studies reported decreased negative affects, improved cognition and
increased social behaviors after use of low doses of antidepressants in a subset of healthy individuals.19,20 However, the aforementioned fMRI studies in normals did not specify the features
characterizing subgroups displaying clear improvement of mood, self-perception and performance changes in response to antidepressants. To date, fMRI studies of this nature have been
restricted to patients who respond positively to antidepressant treatment. They have changes in a wide range of brain regions believed to be implicated in the evaluation of emotionally
salient stimuli and generation of emotional states, including the following: amygdala, ventral striatum, orbitofrontal and visual cortical areas; the ventral anterior cingulate cortex and
insula (involved in the central mapping of autonomic and visceral reactions associated with emotions); or areas implicated in attention, memory and regulation of emotion-triggered behaviors,
including the hippocampus, dorsal anterior cingulate, and parietal and dorsolateral prefrontal cortices.15,21, 22, 23, 24, 25, 26, 27, 28, 29 However, recent fMRI studies argue that such
neural correlates of changes may be confounded by disease and treatment-related variables, as well as brain activity changes due to the remission of insomnia and other features of
depression.15, 16, 17, 18,24 In this study, we investigated brain activity differences between a group of healthy subjects without personal or family history of psychiatric disorders who
showed positive mood and behavior changes in response to continued use of low doses of clomipramine, and a control group of healthy individuals who displayed no mood changes under
clomipramine treatment. fMRI data during presentation of emotion-provoking stimuli under clomipramine use and after clomipramine washout were acquired for both groups, in order to check for
distinct activity patterns in the brain regions relevant to emotional processing. MATERIALS AND METHODS SUBJECTS Sixteen healthy individuals (21–50 years of age), right-handed according to
the Edinburgh Handedness Inventory30 were selected from a larger sample included in a controlled drug trial of low doses of clomipramine in healthy subjects.19 Participants were recruited
through newspaper and radio advertisements, and were screened by psychiatrists using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders.31 Subjects
had no personal or first-degree family history of psychiatric disorders such as psychosis, mood disorders or drug addiction, as assessed by the Family History Screen32 and no personal
history of neurological or general medical conditions, as assessed by a clinical interview, physical examination, electrocardiography, and blood and urine tests. They were not using drugs
with psychotropic effects. Pregnant or lactating females in the past 6 months were excluded. The study was approved by the local ethics committees, and all participants gave written informed
consent. TREATMENT TRIAL Clomipramine was chosen for the present study because of its potency in panic disorder/agoraphobia, allowing reports of subtle mood changes that might otherwise be
masked by the side-effects of higher doses of this and other antidepressant drugs.33 Accordingly, pharmacological studies using animal tissue or human cloned receptors have shown that
clomipramine displays strong, dual action both as serotonin and noradrenalin reuptake inhibitor, fulfilling the criteria of an serotonin–norepinephrine reuptake inhibitor antidepressant even
in greater conformity than the more recently marketed agents venlafaxine and duloxetine.34 Low doses of clomipramine, such as 10 mg, have been shown to occupy 80% of 5-HT transporters,
which is similar to the occupation pattern obtained with clinical doses of more selective 5-HT reuptake inhibitors, such as fluoxetine.35 The design of the treatment trial consisted of a
first phase in which clomipramine or an active placebo was administered under single-blind conditions. The clomipramine doses were gradually increased over 2 weeks, according to tolerability
up to a maximum of 40 mg per day and maintained thereafter for an additional period of 2 weeks. Subjects underwent a weekly semi-structured interview by two experienced psychiatrists who
inquired about subjective changes in emotional responses to everyday stimuli. At the end of week 4, subjects were classified as responders or non-responders on the basis of whether or not
they met at least three of the following criteria: increased interpersonal tolerance (less irritability and tension in social interactions); increased mental efficiency (better decision
making, improved ability to prioritize tasks and increased self-confidence); increased well-being (brighter mood); and awareness of a substantial change from their usual subjective state.
These four criteria were selected on the basis of the mood and performance effects observed in healthy volunteers during a previous clomipramine trial carried out by our group.19 The
semi-structured interview employed probe questions with standard wording to elucidate the changes experienced by individuals and a glossary defining each type of change. Two psychiatrists
classified individuals as responders or non-responders by consensus. All subjects completing the 4 weeks of single-blind clomipramine use (both responders and non-responders) were invited to
take part in the present fMRI study. The mean dose of clomipramine was 37 mg per day (s.d. 6.8 mg per day) in the non-responder group and 36.7 mg per day (s.d. 5.2 mg per day) in the
responder group. Only the responders were then included in a confirmatory double-blind cross-over trial in which they were randomized to first receive clomipramine during 4 weeks (at the
same respective final doses) or placebo (propanteline, 30 mg per day). All responders displayed a similar pattern of mood and perceived behavioral changes in the first (single-blind) and
second (double-blind) phases of the clinical trial. To decrease the risk of dropouts of clomipramine responders in this fMRI study, a fixed MRI scanning order was established: responders and
non-responders underwent a first scanning session at the end of the 4-week single-blind (first phase) of clomipramine (medicated state), and a second fMRI session after 4 weeks of
clomipramine washout. The second scanning session was carried out 4 weeks after the end of the single-blind phase of clomipramine in the group of non-responders (washout). The time of the
second fMRI scanning session of the responder group varied depending on whether the subject received clomipramine or propanteline as the first treatment during the confirmatory double-blind
trial. Those on clomipramine first had a washout of 4 weeks, then immediately underwent the second fMRI session and subsequently entered the final phase of propanteline use. Those subjects
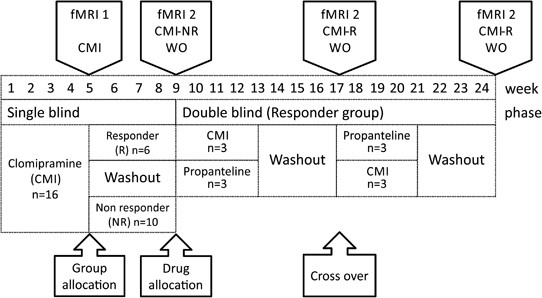
who started the confirmatory trial using propanteline were switched after 4 weeks to clomipramine and underwent their second fMRI session after 4 weeks of clomipramine washout. Figure 1
displays the timeline of group allocation and treatment protocols. Therefore, all individuals underwent a first scanning session immediately after 4 weeks of clomipramine treatment, and a
second scanning after clomipramine washout. Table 1 shows the demographic characteristics of the two groups. All subjects in the responder group were females. The non-responder group had
three males and seven females. There were no other significant differences between groups (Table 1). EXPERIMENTAL PARADIGM A previously validated interview was applied 2 weeks before the
first fMRI session to select emotionally salient autobiographical experiences occurring over the past 6 months.25,26 Subjects completed the Vividness of Visual Imagery Questionnaire36 during
this interview. There was no between-group difference in the Vividness of Visual Imagery Questionnaire scores (Table 1). Within 5 days before the first fMRI session, subjects were trained
and adapted to the fMRI procedure in a sham session, in a mock scanner reproducing the MRI environment and the sounds emitted during image acquisition. During the fMRI session, subjects
listened to recordings through non-magnetized headsets (Commander-XG; Resonance Technology, Los Angeles, CA, USA). Three separate runs were performed, each consisting of an initial baseline
period of rest (80 s) followed by three emotion-eliciting trials (happiness, neutral emotion and irritability). Each trial consisted of the presentation of one non-personal text (20 s) and
one personal script of specific emotional state induction (60 s). In view of the possible confounding effects, the order of the happiness and irritability trials was alternated, both within
and between subjects, although the neutral emotion trial was always performed between the other two. Immediately after the presentation of each personal script or baseline rest period, a
self-report assessment was performed using four-point visual scales of happiness, irritability or anxiety (applied in pseudo-randomized order). The visual scales were presented via a mirror
mounted on the head coil of the fMRI scanner. To determine the levels of anxiety across the overall scanning procedure, the state form of the State-Trait Anxiety Inventory (STAI-S)37 was
applied immediately before and after image acquisition. BEHAVIORAL DATA ANALYSIS For each of the three visually rated emotions (irritability, happiness and anxiety), repeated-measures
analyses of variance (ANOVAs) checked for significant interactions between group (responders versus non-responders) and treatment status (medicated versus unmedicated); this was used to
verify whether the responder group displayed lesser negative emotional reactions during fMRI scanning (as assessed by lower irritability and anxiety ratings) and greater positive responses
(as assessed by higher happiness rating scores) compared with non-responders. For these ANOVAs, the scores for each visual rating scale of emotions (happiness, irritability and anxiety)
during the irritability, and happiness-provoking trials were subtracted from the corresponding scores during the neutral scripts’ trial. Therefore, the average ratings for each of those
three visually rated emotions were expressed as the difference between the scores obtained during the emotion-provoking situations and the neutral situation, that is, irritability minus
neutral (I−N) and happiness minus neutral (H−N) visual rating scores. Such normalization of scores to the neutral condition was intended to decrease intersubject variability of emotional
ratings. _t_-tests were performed for the _post hoc_ evaluation of any statistically significant interactions. ANOVA was also used to compare pre- and post-fMRI scanning STAI-S scores. IMAGE
ACQUISITION For each fMRI run, 220 gradient-echo T2*-weighted echo-planar imaging sets were obtained using a GE LX-MR 1.5T scanner (General Electric, Milwaukee, WI, USA). Each set consisted
of 15 interleaved non-contiguous 7.0-mm-thick transaxial slices, with 0.7-mm gap, parallel to the intercommisural line. Imaging parameters were as follows: echo time=40 ms; repetition
time=2 s; matrix 64 × 64; interslice gap=0.3 mm; field-of-view=200 × 200 mm; and flip angle=90°. Stimulus presentation was synchronized with image acquisition via an optical relay, triggered
by the radiofrequency pulse. A purpose-written software was used for synchronizing the presentation of stimuli and visual analog scales, as well as the capture of subject responses and
image acquisition. FMRI DATA ANALYSIS Image processing involved, first, data realignment and spin history correction to minimize motion-related artifacts38 and spatial Gaussian smoothing
(full width at half-maximum=7.2 mm). The modeling of the blood oxygen level-dependent (BOLD) response curve was carried out by using a linear combination of two Poisson functions with peaks
at 4 and 8 s after the onset. The goodness of fit statistic was computed at each voxel35 by the residual sum of squares ratio between the constrained (null) model (assuming the respective
beta coefficients as zero) and the full model. The sum of square ratio distribution under the null hypothesis was obtained by permutations of the time-series using wavelet-based re-sampling
as previously described.39 This permutation method has been shown to provide good type I error control with minimal distributional assumptions. The sum of square ratio maps were registered
into standard space by rigid body transformation of the fMRI data into structural images obtained for the same subjects, followed by affine transformations onto a template.40 In the
individual analysis within each fMRI run, the sum of square ratio map for the irritability provoking trial was subtracted from the map for the neutral trial and happiness-provoking trial.
Likewise, the map for the happiness trial was subtracted from the map for neutral trial. Therefore, the average maps of the three runs were expressed as irritability minus neutral (I−N),
irritability minus happiness (I−H) and happiness minus neutral (H−N) contrasts. The average contrast maps across the three runs were subsequently used in the group comparisons. For each
contrast (I−N, I−H and H−N), in order to identify voxel clusters showing significant BOLD response differences between groups, a two-way ANOVA was carried out searching for significant
interactions between group (responders versus non-responders) and treatment status (medicated versus unmedicated). Statistical significance was assessed non-parametrically by permutations,
considering voxel and cluster type I errors of 0.05 and 0.005, respectively. Finally, with the aim of facilitating the interpretation of the direction of brain activity differences detected
by the above ANOVA interactions, we also conducted within-group analyses investigating BOLD signal differences between the unmedicated and medicated states in each of the two groups
separately, using one-way ANOVA (see the Supplementary Material). In these analyses, statistical significance was assessed considering a flexible threshold of 0.05 for both voxel and cluster
type I errors. RESULTS BEHAVIORAL DATA ANXIETY DURING SCANNING SESSIONS There was no significant difference between the pre- and post-fMRI scores on the STAI-S in the responder group
(_P_=0.23) or in the non-responder group (_P_=0.09). SUBJECTIVE STATE RATINGS The mean subjective self-rating scores for each emotion-eliciting condition during the medicated and unmedicated
states in the two groups are provided in Table 2. During the medicated and the non-medicated phases, the highest happiness scores were recorded during the presentation of happiness scripts
both by responders and non-responders, whereas the highest irritability scores were recorded during irritability-provoking scripts (all mean scores higher than 3; Table 2). This suggests
that both groups were engaged in responding to the paradigm. Scores for anxiety were low (less than 2) but were systematically higher during the induction of irritability than for the
happiness and neutral conditions (Table 2). There was no significant group effects or _group by treatment_ interaction in the ANOVAs assessing differences in scale scores across the two
emotion-eliciting (irritability or happiness) conditions. This suggests absence of significant differences between responders and non-responders in regard to emotional responses upon
presentation of emotion-provoking personal scripts during the fMRI scanning sessions. FMRI RESULTS There was a significant interaction between clomipramine and group effects in the I−N and
I−H contrasts (Table 3). In the I−N contrast, there was a large cluster of stronger BOLD signal change in responders (compared with non-responders), which encompassed: the posterior portions
of the superior and middle frontal gyri (Brodmann’s area (BA) 8, 9); the pre- and post-central gyri (BA 2–4, 6); and the inferior parietal gyrus (BA 40; Figure 2). Responders also showed
stronger BOLD signal change in the I−H contrast in a cluster involving the medial frontal gyrus (BA 6), the pre- and post-central gyri (BA 2–4) and the supramarginal gyrus (BA 40), as well
as in a cluster encompassing the inferior parietal and angular gyri (BA 37, 39–41), the superior and middle occipital gyri (BA 19) and the middle temporal gyrus (BA 21; Figure 2). There was
no significant interaction effect for the H−N contrast. The spatial maps resulting from the within-group analyses (for the I–N, I–H and H–N contrasts, respectively) are shown in the
Supplementary Material. These maps display brain regions of significantly increased or decreased BOLD signal during the medicated versus unmedicated state separately for the responder group
(Supplementary Table S1) and the non-responder group (Supplementary Table S2). DISCUSSION To our knowledge, this is the first fMRI study evaluating healthy individuals who reported
consistent changes in mood and emotional behavior after chronic use of an antidepressant. When presented with irritability-inducing stimuli while under clomipramine treatment, responders
showed a pattern of brain activity that was significantly different from that of the non-responders under the same conditions. Among the non-responders, task-related BOLD signal changes
during presentation of emotion-provoking autobiographical scripts were lower in the medicated state than after washout, whereas the responders showed higher frontoparietal activity during
the induction of irritability when medicated than after washout. Similarly to these findings fMRI studies using emotion-eliciting stimuli in patients with mood disorders under antidepressant
treatment found clinical response to be associated with increased regional brain activity in prefrontal–parietal and other brain regions.15,17,27,41, 42, 43, 44, 45, 46, 47, 48, 49, 50
Together, this suggests that stronger prefrontal–parietal BOLD signal change during emotion induction is a neural correlate of antidepressant drug effects on mood regulation. However, this
effect may be independent of the therapeutic action of antidepressants, as this and other studies show that such pattern also occurs in healthy individuals.8, 9, 10,12,51,52 The absence of
differences in clomipramine doses and plasma levels, as well as in the suppression of REM sleep (unpublished data on file) between the responder and non-responder groups in this study,
indicates that the brain activity changes in the treatment-responders were not because of pharmacokinetic factors but may result from genetic variations in mood regulation in an
extratherapeutic response to these drugs.53,54 In this vein, it is noteworthy that previous studies of antidepressant response in clinical samples have implicated gene variations unrelated
to vulnerability to mood disorders.55 The differences in brain activity between responders and non-responders were localized to left-lateralized brain clusters primarily involving the
frontal and parietal cortices. This suggests that the neural substrate specifically associated with the response to clomipramine in healthy subjects does not involve the more extensive,
multifocal cortical–subcortical circuitry that is typically engaged during non-personal emotional tasks. However, the small size of our sample may have precluded the detection of less
salient (but still potentially relevant) BOLD signal differences in other brain regions involved in emotional processing. The brain regions in which the BOLD signal differed between
responders and non-responders are considered highly relevant for self-referential emotional tasks.24, 25, 26, 27, 28, 29,56, 57, 58 As these findings are not confounded by therapeutic
effects, they underscore the relevance of frontoparietal regions to emotional processing and to the psychopharmacological effects of clomipramine. The magnitude of the differences in
activity between groups in the frontoparietal region may have been influenced by the choice of a paradigm of autobiographic recall to elicit emotional responses,25,26 and further studies
using other emotion-eliciting paradigms are warranted. Moreover, noteworthy is the finding of brain activity differences between responder and non-responder groups during the emotional
condition of negative valence (irritability), rather than during the happiness condition. Previous fMRI studies of healthy individuals reported higher frontoparietal activity during
recollection of self-relevant negative information than during retrieval of self-relevant positive information.6,8 The valence-specific findings of the present study are consistent with
previous observations that healthy individuals who report beneficial effects from treatment with antidepressants fell less responsive to negative emotional experiences, rather than being
more prone to positive experiences.20 Inspection of the mean subjective self-rating scores showed an overall pattern of higher negative emotion scores (irritability and anxiety) during
presentation of irritability scripts and higher positive emotion scores (happiness ratings) during presentation of happiness scripts. This indicates that subjects responded emotionally to
the paradigm during both conditions. The lack of significant differences between responders and non-responders in emotional response under emotion-provoking personal scripts during fMRI
scanning suggests that the visual scales were not sensitive enough to discriminate, during the fMRI sessions, the daily life emotional changes reported during treatment by the responders to
clomipramine. Likewise, a decoupling between subjective emotional responses and the objective BOLD signal changes elicited during experimental situations has been reported in a number of
previous fMRI studies that used emotion-provoking tasks in healthy individuals.2,4,7,8,11,59,60 The results of the within-group brain activity comparisons in the non-responder group (see
Supplementary Information, Supplementary Table S2), with treatment-related BOLD signal reductions in brain regions considered critical to emotional processing, are similar to those reported
during emotional stimuli in previous fMRI studies of healthy individuals treated with multiple doses of antidepressants.2,7,14,18 As only one-third of healthy subjects typically experience
notable mood and performance changes under treatment with antidepressants,19,61 it is possible that the majority of individuals taking part in the quoted fMRI studies had effects akin to
those of the non-responders in the current experiment. Among the limitations of this study, the small sample, not balanced in gender distribution in the clomipramine responder group, may
have prevented the detection of less salient but significant differences in additional brain regions relevant to emotional processing. Therefore, confirmation of the findings here reported
is warranted. In addition, there was an imbalance between responders and non-responders in terms of the interval between the first and the second fMRI sessions, and in the total duration of
exposure to clomipramine before the second fMRI scanning session (responders received two 4-week courses of clomipramine before their second fMRI scanning session, whereas non-responders
only received a single 4-week course, as they were not included in the second phase of the trial). However, this is unlikely to have confounded the fMRI findings, as all subjects were first
scanned at the end of 4 weeks of treatment with clomipramine, whereas the second scanning session was carried out after 4 weeks of washout in all cases. Finally, possible differences in
nonspecific practice effects from the first to the second fMRI session (medicated versus unmedicated state) were likely minimized by the training session carried out before the first fMRI
session. In conclusion, carefully selected healthy subjects experiencing a consistent positive change in mood and perceived performance after 4 weeks of small, sub-therapeutic doses of
clomipramine treatment, presented a distinct pattern of increased activity in frontoparietal regions during emotion-eliciting stimuli in comparison with non-responders. These findings
underscore the need for a systematic evaluation of subjective mood changes in pharmacological fMRI studies of antidepressants in healthy subjects in order to reduce intersubject variability
in brain activity patterns, and provide further evidence of specific pharmacodynamic basis for the observed differences in mood response in clinical and non-clinical samples. REFERENCES *
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ . Effect of a single dose of citalopram on amygdala response to emotional faces. _Br J Psychiatry_ 2009; 194: 5–40. Article Google
Scholar * Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP . Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. _Int J
Neuropsychopharmacol_ 2009; 12: 1009–1020. Article CAS PubMed PubMed Central Google Scholar * Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U _et al_.
Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. _Neuroimage_ 2010; 49: 1161–1170. Article CAS
PubMed Google Scholar * McCabe C, Mishor Z . Antidepressant medications reduce subcortical—cortical resting-state functional connectivity in healthy volunteers. _Neuroimage_ 2011; 57:
1317–1323. Article CAS PubMed PubMed Central Google Scholar * Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T _et al_. Effects of dopaminergic and serotonergic manipulation
on emotional processing: a pharmacological fMRI study. _Neuroimage_ 2005; 27: 991–1001. Article PubMed Google Scholar * Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R,
Harmer CJ . Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. _Neuroimage_ 2007; 37: 904–911. Article PubMed Google
Scholar * Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ . Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. _Br J Psychiatry_
2007; 190: 531–532. Article PubMed Google Scholar * Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ . The effects of reboxetine on emotional processing in healthy volunteers: an
fMRI study. _Mol Psychiatry_ 2008; 13: 1011–1020. Article CAS PubMed Google Scholar * Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C _et al_. Noradrenergic enhancement
of amygdala responses to fear. _Soc Cogn Affect Neurosci_ 2009; 4: 119–126. Article PubMed PubMed Central Google Scholar * Brühl AB, Jäncke L, Herwig U . Differential modulation of
emotion processing brain regions by noradrenergic and serotonergic antidepressants. _Psychopharmacology (Berl)_ 2011; 216: 389–399. Article Google Scholar * Norbury R, Taylor MJ, Selvaraj
S, Murphy SE, Harmer CJ, Cowen PJ . Short-term antidepressant treatment modulates amygdala response to happy faces. _Psychopharmacology (Berl)_ 2009; 206: 197–204. Article CAS Google
Scholar * Brühl AB, Kaffenberger T, Herwig U . Serotonergic and noradrenergic modulation of emotion processing by single dose antidepressants. _Neuropsychopharmacology_ 2010; 35: 521–533.
Article PubMed Google Scholar * Matthews SC, Simmons AN, Strigo IA, Arce E, Stein MB, Paulus MP . Escitalopram attenuates posterior cingulate activity during self-evaluation in healthy
volunteers. _Psychiatry Res_ 2010; 182: 81–87. Article CAS PubMed PubMed Central Google Scholar * Anderson IM, McKie S, Elliott R, Williams SR, Deakin JF . Assessing human 5-HT function
_in vivo_ with pharmaco-MRI. _Neuropharmacology_ 2008; 55: 1029–1037. Article CAS PubMed Google Scholar * Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ . A meta-analytic study of
changes in brain activation in depression. _Hum Brain Mapp_ 2008; 29: 683–695. Article PubMed PubMed Central Google Scholar * Van Wingen GA, Van Eijndhoven P, Cremers HR, Tendolkar I,
Verkes RJ, Buitelaar JK _et al_. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. _J Psychiatr Res_ 2010; 44: 527–534.
Article PubMed Google Scholar * Davidson RJ, Irwin W, Anderle MJ, Kalin NH . The neural substrates of affective processing in depressed patients treated with venlafaxine. _Am J
Psychiatry_ 2003; 160: 64–75. PubMed Google Scholar * Harmer CJ, Goodwin GM, Cowen PJ . Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant
drug action. _Br J Psychiatry_ 2009; 195: 102–108. Article PubMed Google Scholar * Gentil V, Zilberman ML, Lobo D, Henna E, Moreno RA, Gorenstein C . Clomipramine-induced mood and
perceived performance changes in selected healthy individuals [letter]. _J Clin Psychopharmacol_ 2007; 27: 314–315. Article PubMed Google Scholar * Serretti A, Calati R, Goracci A, Di
Simplicio M, Castrogiovanni P, De Ronchi D . Antidepressants in healthy subjects: what are the psychotropic/psychological effects? _Eur Neuropsychopharmacol_ 2010; 20: 433–453. Article CAS
PubMed Google Scholar * Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S _et al_. Regional metabolic effects of fluoxetine in major depression: serial changes and
relationship to clinical response. _Biol Psychiatry_ 2000; 48: 830–843. Article CAS PubMed Google Scholar * Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ _et al_. Neural
responses to happy facial expressions in major depression following antidepressant treatment. _Am J Psychiatry_ 2007; 164: 599–607. Article PubMed Google Scholar * Drevets WC .
Translating progress in depression research to the clinic: one step at a time on a very long road. _World Psychiatry_ 2010; 9: 162. Article PubMed PubMed Central Google Scholar *
Simplicio D . Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. _Mol Psychiatry_ 2011;
17: 503–510. Article PubMed Google Scholar * Cerqueira CT, Almeida JRC, Gorenstein C, Gentil V, Leite CC, Sato JR _et al_. Engagement of multifocal neural circuits during recall of
autobiographical happy events. _Braz J Med Biol Res_ 2008; 41: 1076–1085. Article CAS PubMed Google Scholar * Cerqueira CT, Almeida JR, Sato JR, Gorenstein C, Gentil V, Leite CC _et al_.
Cognitive control associated with irritability induction: an autobiographical recall fMRI study. _Rev Bras Psiquiatr_ 2010; 32: 109–118. Article PubMed Google Scholar * Price JL, Drevets
WC . Neurocircuitry of mood disorders. _Neuropsychopharmacology_ 2009; 35: 192–216. Article PubMed Central Google Scholar * Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey
ML _et al_. Functional anatomy of autobiographical memory recall deficits in depression. _Psychol Med_ 2011; 29: 1–13. Google Scholar * Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati
P . Medial prefrontal cortex and the self in major depression. _J Affect Disord_ 2012; 136: e1–e11. Article PubMed Google Scholar * Oldfield RC . The assessment and analysis of
handedness: the Edinburgh inventory. _Neuropsychologia_ 1971; 9: 97–113. Article CAS PubMed Google Scholar * First MB, Spitzer RL, Gibbon M, Willians JBW . _Structured Clinical Interview
for DSM-IV Axis I Disorders (SCID version 2.0)_. Biometric research Department, New York State Psychiatric Institute: New York, USA, 1995. Google Scholar * Weissman MM, Wickramaratne P,
Adams P, Wolk S, Verdeli H, Olfson M . Brief screening for family psychiatric history: the family history screen. _Arch Gen Psychiatry_ 2000; 57: 675–682. Article CAS PubMed Google
Scholar * Gentil V, Lotufo-Neto F, Andrade L, Cordás T, Bernik M, Ramos R _et al_. Clomipramine, a betterreferencedrug for panic/agoraphobia. I. Effectiveness comparison with imipramine. _J
Psychopharmacol._ 1993; 7: 316–324. Article CAS PubMed Google Scholar * Gillman PK . Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. _Br J Pharmacol_
2007; 151: 737–748. Article CAS PubMed PubMed Central Google Scholar * Wong DF, Tauscher J, Grunder G . The role of imaging in proof of concept for CNS drug discovery and development.
_Neuropsychopharmacology_ 2009; 34: 187–203. Article CAS PubMed Google Scholar * Campos A, Gonzalez MA, Amor A . The Spanish version of the Vividness of Visual Imagery Questionnaire:
factor structure and internal consistency reliability. _Psychol Rep_ 2002; 90: 503–506. Article PubMed Google Scholar * Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA .
_Manual for the State-Trait Anxiety Inventory_. Consulting Psychologists Press: Palo Alto, CA, 1983. Google Scholar * Bullmore E, Fadili J, Breakspear M, Salvador R, Suckling J, Brammer M .
Wavelets and statistical analysis of functional magnetic resonance images of the human brain. _Stat Methods Med Res_ 2003; 12: 375–399. Article PubMed Google Scholar * Friman O, Borga M,
Lundberg P, Knutsson H . Adaptive analysis of fMRI data. _Neuroimage_ 2003; 19: 837–845. Article PubMed Google Scholar * Bullmore E, Brammer M, Williams SC, Rabe-Hesketh S, Janot N,
David A _et al_. Statistical methods of estimation and inference for functional MR image analysis. _Magn Reson Med_ 1996; 35: 261–277. Article CAS PubMed Google Scholar * Roy M, Harvey
PO, Berlim MT, Mamdani F, Beaulieu MM, Turecki G _et al_. Medial prefrontal cortex activity during memory encoding of pictures and its relation to symptomatic improvement after citalopram
treatment in patients with major depression. _J Psychiatry Neurosci_ 2010; 35: 152–162. PubMed PubMed Central Google Scholar * López-Solà M, Pujol J, Hernández-Ribas R, Harrison BJ,
Contreras-Rodríguez O, Soriano-Mas C _et al_. Effects of duloxetine treatment on brain response to painful stimulation in major depressive disorder. _Neuropsychopharmacology_ 2010; 35:
2305–2317. Article PubMed PubMed Central Google Scholar * Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S _et al_. Changes in regional brain glucose metabolism measured
with positron emission tomography after paroxetine treatment of major depression. _Am J Psychiatry_ 2001; 158: 899–905. Article CAS PubMed Google Scholar * Canli T, Cooney RE, Goldin P,
Shah M, Sivers H, Thomason ME _et al_. Amygdala reactivity to emotional faces predicts improvement in major depression. _Neuroreport_ 2005; 16: 1267–1270. Article PubMed Google Scholar *
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L _et al_. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. _Neuropsychopharmacology_ 2005; 30: 1334–1344.
Article CAS PubMed Google Scholar * Anand A, Li Y, Wang Y, Gardner K, Lowe MJ . Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating
circuit: an FMRI study. _J Neuropsychiatry Clin Neurosci_ 2007; 19: 274–282. Article PubMed PubMed Central Google Scholar * Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT,
Phillips ML . Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. _Am J
Psychiatry_ 2010; 167: 1373–1380. Article PubMed PubMed Central Google Scholar * Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Lehericy S _et al_. Self-referential processing
and the prefrontal cortex over the course of depression: a pilot study. _J Affect Disord_ 2010; 124: 196–201. Article PubMed Google Scholar * Fales CL, Barch DM, Rundle MM, Mintun MA,
Mathews J, Snyder AZ _et al_. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. _J Affect
Disord_ 2009; 112: 206–211. Article CAS PubMed Google Scholar * Hsu DT, Langenecker SA, Kennedy SE, Zubieta JK, Heitzeg MM . fMRI BOLD responses to negative stimuli in the prefrontal
cortex are dependent on levels of recent negative life stress in major depressive disorder. _Psychiatry Res_ 2010; 183: 202–208. Article PubMed PubMed Central Google Scholar * Grefkes C,
Wang LE, Eickhoff SB, Fink GR . Noradrenergic modulation of cortical networks engaged in visuomotor processing. _Cereb Cortex_ 2010; 20: 783–797. Article PubMed Google Scholar * Völlm B,
Richardson P, McKie S, Elliott R, Deakin JF, Anderson IM . Serotonergic modulation of neuronal responses to behavioural inhibition and reinforcing stimuli: an fMRI study in healthy
volunteers. _Eur J Neurosci_ 2006; 23: 552–560. Article PubMed Google Scholar * Fortier E, Noreau A, Lepore F, Boivin M, Perusse D, Rouleau GA _et al_. Early impact of 5-HTTLPR
polymorphism on the neural correlates of sadness. _Neurosci Lett_ 2010; 485: 261–265. Article CAS PubMed Google Scholar * Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz
KE, Kolachana BS _et al_. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. _Nat Neurosci_ 2005; 8: 828–834. Article
CAS PubMed Google Scholar * Savitz JB, Drevets WC . Imaging phenotypes of major depressive disorder: genetic correlates. _Neuroscience_ 2009; 164: 300–330. Article CAS PubMed PubMed
Central Google Scholar * Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC _et al_. Selective alteration of personality and social behavior by serotonergic intervention. _Am J
Psychiatry_ 1998; 155: 373–379. Article CAS PubMed Google Scholar * Lemogne C, Bergouignan L, Boni C, Gorwood P, Pelissolo A, Fossati P . Genetics and personality affect visual
perspective in autobiographical memory. _Conscious Cogn_ 2009; 18: 823–830. Article PubMed Google Scholar * Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B .
Personality change during depression treatment: a placebo-controlled trial. _Arch Gen Psychiatry_ 2009; 66: 1322–1330. Article PubMed PubMed Central Google Scholar * Anderson IM, McKie
S, Elliott R, Williams SR, Deakin JF . Assessing human 5-HT function _in vivo_ with pharmacoMRI. _Neuropharmacology_ 2008; 55: 1029–1037. Article CAS PubMed Google Scholar * de Almeida
JR, Phillips ML, Cerqueira CT, Zilberman M, Lobo D, Henna E _et al_. Neural activity changes to emotional stimuli in healthy individuals under chronic use of clomipramine. _J
Psychopharmacol_ 2009; 24: 1165–1174. Article PubMed PubMed Central Google Scholar * Dumont G, De Visser SJ, Cohen AF, Van Gerven J . Biomarkers for the effects of selective serotonin
reuptake inhibitors (SSRIs) in healthy subjects. _Br J Clin Pharmacol._ 2005; 59: 495–510. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This
study was supported by a grant from the _Fundação de Amparo à Pesquisa do Estado de São Paulo_ (FAPESP, São Paulo Research Foundation; Grant no. 01/00189-9). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department and Institute of Psychiatry, School of Medicine and Hospital das Clínicas, University of São Paulo, São Paulo, Brazil C T Cerqueira, C Gorenstein, V Gentil & G
F Busatto * Department of Cognitive Neuroscience, Federal University of the ABC, Santo André, Brazil J R Sato * Department and Institute of Radiology, School of Medicine, University of São
Paulo, São Paulo, Brazil J R Sato, E Amaro Jr & C C Leite * Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA J R C de Almeida * Laboratory of
Psychopharmacology (LIM 23), School of Medicine, USP, São Paulo, Brazil, C Gorenstein * Department of Pharmacology, Institute of Biomedical Sciences, USP, São Paulo, Brazil C Gorenstein
Authors * C T Cerqueira View author publications You can also search for this author inPubMed Google Scholar * J R Sato View author publications You can also search for this author inPubMed
Google Scholar * J R C de Almeida View author publications You can also search for this author inPubMed Google Scholar * E Amaro Jr View author publications You can also search for this
author inPubMed Google Scholar * C C Leite View author publications You can also search for this author inPubMed Google Scholar * C Gorenstein View author publications You can also search
for this author inPubMed Google Scholar * V Gentil View author publications You can also search for this author inPubMed Google Scholar * G F Busatto View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C T Cerqueira. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest.
ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 117 KB) RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain
permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Cerqueira, C., Sato, J., de Almeida, J. _et al._ Healthy individuals treated with clomipramine: an fMRI study of brain activity during autobiographical recall of
emotions. _Transl Psychiatry_ 4, e405 (2014). https://doi.org/10.1038/tp.2014.47 Download citation * Received: 02 April 2014 * Accepted: 22 April 2014 * Published: 01 July 2014 * Issue Date:
July 2014 * DOI: https://doi.org/10.1038/tp.2014.47 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









:max_bytes(150000):strip_icc():focal(149x0:151x2)/amanda-seyfried-300-5-932e91f351d04f858cc4e76a8562e7a4.jpg)
