- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The PIWI-interacting RNA (piRNA) pathway protects genome integrity in part through establishing repressive heterochromatin at transposon loci. Silencing requires piRNA-guided
targeting of nuclear PIWI proteins to nascent transposon transcripts, yet the subsequent molecular events are not understood. Here, we identify SFiNX (silencing factor interacting nuclear
export variant), an interdependent protein complex required for Piwi-mediated cotranscriptional silencing in _Drosophila_. SFiNX consists of Nxf2–Nxt1, a gonad-specific variant of the
heterodimeric messenger RNA export receptor Nxf1–Nxt1 and the Piwi-associated protein Panoramix. SFiNX mutant flies are sterile and exhibit transposon derepression because piRNA-loaded Piwi
is unable to establish heterochromatin. Within SFiNX, Panoramix recruits heterochromatin effectors, while the RNA binding protein Nxf2 licenses cotranscriptional silencing. Our data reveal
how Nxf2 might have evolved from an RNA transport receptor into a cotranscriptional silencing factor. Thus, NXF variants, which are abundant in metazoans, can have diverse molecular
functions and might have been coopted for host genome defense more broadly. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PANORAMIX SUMOYLATION ON CHROMATIN CONNECTS THE PIRNA PATHWAY TO THE CELLULAR HETEROCHROMATIN
MACHINERY Article 16 February 2022 TASOR IS A PSEUDO-PARP THAT DIRECTS HUSH COMPLEX ASSEMBLY AND EPIGENETIC TRANSPOSON CONTROL Article Open access 02 October 2020 TEX15 IS AN ESSENTIAL
EXECUTOR OF MIWI2-DIRECTED TRANSPOSON DNA METHYLATION AND SILENCING Article Open access 27 July 2020 DATA AVAILABILITY All sequencing data used for this study (Supplementary Table 12) have
been deposited at NCBI GEO (GSE120617). The mass spectrometry data have been deposited to the ProteomeXchange Consortium via PRIDE (PXD011201)84. Coordinate and structure factors of the
UBA-linker-helix and the dmNxf2–Nxt1 complex are available from the Protein Data Bank (PDB 6OPF and 6MRK). Source data for Fig. 1a are available in Supplementary Table 1, Fig. 1f,g and
Supplementary Fig. 1d,e in Supplementary Table 2, Fig. 2c and Supplementary Fig. 2d in Supplementary Table 3, Supplementary Fig. 2i,k in Supplementary Table 4 and Fig. 7d in Supplementary
Table 6. Source data for Figs. 3d,e, 5h and 7f are available online. CODE AVAILABILITY All custom code is based on the publicly available code used in ref. 64 with modifications indicated in
the Methods section. REFERENCES * Fedoroff, N. V. Presidential address. Transposable elements, epigenetics, and genome evolution. _Science_ 338, 758–767 (2012). Article CAS Google Scholar
* Slotkin, R. K. & Martienssen, R. Transposable elements and the epigenetic regulation of the genome. _Nat. Rev. Genet._ 8, 272–285 (2007). Article CAS Google Scholar * Yang, P.,
Wang, Y. & Macfarlan, T. S. The role of KRAB-ZFPs in transposable element repression and mammalian evolution. _Trends Genet._ 33, 871–881 (2017). Article CAS Google Scholar * Grewal,
S. I. RNAi-dependent formation of heterochromatin and its diverse functions. _Curr. Opin. Genet. Dev._ 20, 134–141 (2010). Article CAS Google Scholar * Castel, S. E. & Martienssen, R.
A. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. _Nat. Rev. Genet._ 14, 100–112 (2013). Article CAS Google Scholar * Holoch, D. &
Moazed, D. RNA-mediated epigenetic regulation of gene expression. _Nat. Rev. Genet._ 16, 71–84 (2015). Article CAS Google Scholar * Shimada, Y., Mohn, F. & Buhler, M. The RNA-induced
transcriptional silencing complex targets chromatin exclusively via interacting with nascent transcripts. _Genes Dev._ 30, 2571–2580 (2016). Article CAS Google Scholar * Dumesic, P. A. et
al. Stalled spliceosomes are a signal for RNAi-mediated genome defense. _Cell_ 152, 957–968 (2013). Article CAS Google Scholar * Reyes-Turcu, F. E., Zhang, K., Zofall, M., Chen, E. &
Grewal, S. I. Defects in RNA quality control factors reveal RNAi-independent nucleation of heterochromatin. _Nat. Struct. Mol. Biol._ 18, 1132–1138 (2011). Article CAS Google Scholar *
Teixeira, F. K. et al. piRNA-mediated regulation of transposon alternative splicing in the soma and germ line. _Nature_ 552, 268–272 (2017). Article CAS Google Scholar * Czech, B. et al.
piRNA-guided genome defense: from biogenesis to silencing. _Annu Rev. Genet_. 52, 131–157 (2018). Article CAS Google Scholar * Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. &
Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. _Nat. Rev. Genet_. 20, 89–108 (2018). Article Google Scholar * Wang, S. H. & Elgin, S. C. Drosophila Piwi functions
downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line. _Proc. Natl Acad. Sci. USA_ 108, 21164–21169 (2011). Article CAS Google
Scholar * Sienski, G., Donertas, D. & Brennecke, J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. _Cell_ 151,
964–980 (2012). Article CAS Google Scholar * Rozhkov, N. V., Hammell, M. & Hannon, G. J. Multiple roles for Piwi in silencing _Drosophila_ transposons. _Genes Dev._ 27, 400–412
(2013). Article CAS Google Scholar * Le Thomas, A. et al. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. _Genes Dev._ 27, 390–399
(2013). Article Google Scholar * Donertas, D., Sienski, G. & Brennecke, J. Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. _Genes
Dev._ 27, 1693–1705 (2013). Article Google Scholar * Ohtani, H. et al. DmGTSF1 is necessary for Piwi-piRISC-mediated transcriptional transposon silencing in the _Drosophila_ ovary. _Genes
Dev._ 27, 1656–1661 (2013). Article CAS Google Scholar * Muerdter, F. et al. A genome-wide RNAi screen draws a genetic framework for transposon control and primary piRNA biogenesis in
_Drosophila_. _Mol. Cell_ 50, 736–748 (2013). Article CAS Google Scholar * Sienski, G. et al. Silencio/CG9754 connects the Piwi-piRNA complex to the cellular heterochromatin machinery.
_Genes Dev._ 29, 2258–2271 (2015). Article CAS Google Scholar * Yu, Y. et al. Panoramix enforces piRNA-dependent cotranscriptional silencing. _Science_ 350, 339–342 (2015). Article CAS
Google Scholar * Rodriguez-Navarro, S. & Hurt, E. Linking gene regulation to mRNA production and export. _Curr. Opin. Cell Biol._ 23, 302–309 (2011). Article CAS Google Scholar *
Stewart, M. Polyadenylation and nuclear export of mRNAs. _J. Biol. Chem._ 294, 2977–2987 (2019). Article CAS Google Scholar * Herold, A. et al. TAP (NXF1) belongs to a multigene family of
putative RNA export factors with a conserved modular architecture. _Mol. Cell Biol._ 20, 8996–9008 (2000). Article CAS Google Scholar * Herold, A., Klymenko, T. & Izaurralde, E.
NXF1/p15 heterodimers are essential for mRNA nuclear export in _Drosophila_. _RNA_ 7, 1768–1780 (2001). CAS PubMed PubMed Central Google Scholar * Herold, A., Teixeira, L. &
Izaurralde, E. Genome-wide analysis of nuclear mRNA export pathways in _Drosophila_. _EMBO J._ 22, 2472–2483 (2003). Article CAS Google Scholar * Niki, Y., Yamaguchi, T. & Mahowald,
A. P. Establishment of stable cell lines of _Drosophila_ germ-line stem cells. _Proc. Natl Acad. Sci. USA_ 103, 16325–16330 (2006). Article CAS Google Scholar * Saito, K. et al. A
regulatory circuit for piwi by the large Maf gene traffic jam in _Drosophila_. _Nature_ 461, 1296–1299 (2009). Article CAS Google Scholar * Handler, D. et al. The genetic makeup of the
drosophila piRNA pathway. _Mol. Cell_ 50, 762–777 (2013). Article CAS Google Scholar * Czech, B., Preall, J. B., McGinn, J. & Hannon, G. J. A transcriptome-wide RNAi screen in the
_Drosophila_ ovary reveals factors of the germline piRNA pathway. _Mol. Cell_ 50, 749–761 (2013). Article CAS Google Scholar * Brown, J. B. et al. Diversity and dynamics of the
_Drosophila_ transcriptome. _Nature_ 512, 393–399 (2014). Article CAS Google Scholar * Wilkie, G. S. et al. Small bristles, the _Drosophila_ ortholog of NXF-1, is essential for mRNA
export throughout development. _RNA_ 7, 1781–1792 (2001). CAS PubMed PubMed Central Google Scholar * Caporilli, S., Yu, Y., Jiang, J. & White-Cooper, H. The RNA export factor, Nxt1,
is required for tissue specific transcriptional regulation. _PLoS Genet._ 9, e1003526 (2013). Article CAS Google Scholar * Valkov, E., Dean, J. C., Jani, D., Kuhlmann, S. I. &
Stewart, M. Structural basis for the assembly and disassembly of mRNA nuclear export complexes. _Biochim Biophys. Acta_ 1819, 578–592 (2012). Article CAS Google Scholar * Grant, R. P.,
Neuhaus, D. & Stewart, M. Structural basis for the interaction between the Tap/NXF1 UBA domain and FG nucleoporins at 1A resolution. _J. Mol. Biol._ 326, 849–858 (2003). Article CAS
Google Scholar * Kohler, A. & Hurt, E. Exporting RNA from the nucleus to the cytoplasm. _Nat. Rev. Mol. Cell Biol._ 8, 761–773 (2007). Article Google Scholar * Braun, I. C., Herold,
A., Rode, M. & Izaurralde, E. Nuclear export of mRNA by TAP/NXF1 requires two nucleoporin-binding sites but not p15. _Mol. Cell Biol._ 22, 5405–5418 (2002). Article CAS Google Scholar
* Grant, R. P., Hurt, E., Neuhaus, D. & Stewart, M. Structure of the C-terminal FG-nucleoporin binding domain of Tap/NXF1. _Nat. Struct. Biol._ 9, 247–251 (2002). Article CAS Google
Scholar * Fribourg, S., Braun, I. C., Izaurralde, E. & Conti, E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear
export factor. _Mol. Cell_ 8, 645–656 (2001). Article CAS Google Scholar * Liker, E., Fernandez, E., Izaurralde, E. & Conti, E. The structure of the mRNA export factor TAP reveals a
cis arrangement of a non-canonical RNP domain and an LRR domain. _EMBO J._ 19, 5587–5598 (2000). Article CAS Google Scholar * Aibara, S. et al. Structural characterization of the
principal mRNA-export factor Mex67-Mtr2 from _Chaetomium thermophilum_. _Acta Crystallogr. F._ 71, 876–888 (2015). Article CAS Google Scholar * Tutucci, E. & Stutz, F. Keeping mRNPs
in check during assembly and nuclear export. _Nat. Rev. Mol. Cell Biol._ 12, 377–384 (2011). Article CAS Google Scholar * Teplova, M., Wohlbold, L., Khin, N. W., Izaurralde, E. &
Patel, D. J. Structure-function studies of nucleocytoplasmic transport of retroviral genomic RNA by mRNA export factor TAP. _Nat. Struct. Mol. Biol._ 18, 990–998 (2011). Article CAS Google
Scholar * Aibara, S., Katahira, J., Valkov, E. & Stewart, M. The principal mRNA nuclear export factor NXF1:NXT1 forms a symmetric binding platform that facilitates export of retroviral
CTE-RNA. _Nucleic Acids Res._ 43, 1883–1893 (2015). Article CAS Google Scholar * Viphakone, N. et al. TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export. _Nat. Commun._ 3,
1006 (2012). Article Google Scholar * Ninova, M. et al. The SUMO ligase Su(var)2-10 links piRNA-guided target recognition to chromatin silencing. Preprint at _b_ _ioRxiv_
https://www.biorxiv.org/content/10.1101/533091v2 (2019). * Heath, C. G., Viphakone, N. & Wilson, S. A. The role of TREX in gene expression and disease. _Biochem. J._ 473, 2911–2935
(2016). Article CAS Google Scholar * Muller-McNicoll, M. & Neugebauer, K. M. How cells get the message: dynamic assembly and function of mRNA-protein complexes. _Nat. Rev. Genet_ 14,
275–287 (2013). Article Google Scholar * Post, C., Clark, J. P., Sytnikova, Y. A., Chirn, G. W. & Lau, N. C. The capacity of target silencing by _Drosophila_ PIWI and piRNAs. _RNA_ 20,
1977–1986 (2014). Article CAS Google Scholar * Fabry, M. H. et al. piRNA-guided co-transcriptional silencing coopts nuclear export factors. _Elife_ 8, e47999 (2019). Article Google
Scholar * Murano, K. et al. Nuclear RNA export factor variant initiates piRNA-guided co-transcriptional silencing. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/605725v1
(2019). * Zhao, K. et al. A Pandas complex adapted for piRNA-guided transposon silencing. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/608273v3 (2019). * Stutz, F. &
Izaurralde, E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. _Trends Cell Biol._ 13, 319–327 (2003). Article CAS Google Scholar * Gramates, L. S. et al. FlyBase at
25: looking to the future. _Nucleic Acids Res._ 45, D663–D671 (2017). Article CAS Google Scholar * ElMaghraby, M. F. et al. A heterochromatin-specific RNA export pathway facilitates
piRNA production. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/596171v1 (2019). * Pan, J. et al. Inactivation of Nxf2 causes defects in male meiosis and age-dependent
depletion of spermatogonia. _Dev. Biol._ 330, 167–174 (2009). Article CAS Google Scholar * Gokcezade, J., Sienski, G. & Duchek, P. Efficient CRISPR/Cas9 plasmids for rapid and
versatile genome editing in _Drosophila_. _G3_ 4, 2279–2282 (2014). Article Google Scholar * Pfeiffer, B. D. et al. Tools for neuroanatomy and neurogenetics in _Drosophila_. _Proc. Natl
Acad. Sci. USA_ 105, 9715–9720 (2008). Article CAS Google Scholar * Mohn, F., Sienski, G., Handler, D. & Brennecke, J. The rhino-deadlock-cutoff complex licenses noncanonical
transcription of dual-strand piRNA clusters in _Drosophila_. _Cell_ 157, 1364–1379 (2014). Article CAS Google Scholar * Hayashi, R. et al. Genetic and mechanistic diversity of piRNA
3’-end formation. _Nature_ 539, 588–592 (2016). Article CAS Google Scholar * Jayaprakash, A. D., Jabado, O., Brown, B. D. & Sachidanandam, R. Identification and remediation of biases
in the activity of RNA ligases in small-RNA deep sequencing. _Nucleic Acids Res_. 39, e141 (2011). Article CAS Google Scholar * Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L.
Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. _Genome Biol._ 10, R25 (2009). Article Google Scholar * Quinlan, A. R. & Hall, I. M. BEDTools: a
flexible suite of utilities for comparing genomic features. _Bioinformatics_ 26, 841–842 (2010). Article CAS Google Scholar * Andersen, P. R., Tirian, L., Vunjak, M. & Brennecke, J. A
heterochromatin-dependent transcription machinery drives piRNA expression. _Nature_ 549, 54–59 (2017). Article CAS Google Scholar * Morlan, J. D., Qu, K. & Sinicropi, D. V. Selective
depletion of rRNA enables whole transcriptome profiling of archival fixed tissue. _PLoS ONE_ 7, e42882 (2012). Article CAS Google Scholar * Dobin, A. et al. STAR: ultrafast universal
RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS Google Scholar * Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and
bias-aware quantification of transcript expression. _Nat. Methods_ 14, 417–419 (2017). Article CAS Google Scholar * Pimentel, H., Bray, N. L., Puente, S., Melsted, P. & Pachter, L.
Differential analysis of RNA-seq incorporating quantification uncertainty. _Nat. Methods_ 14, 687–690 (2017). Article CAS Google Scholar * Lee, T. I., Johnstone, S. E. & Young, R. A.
Chromatin immunoprecipitation and microarray-based analysis of protein location. _Nat. Protoc._ 1, 729–748 (2006). Article CAS Google Scholar * Heinz, S. et al. Simple combinations of
lineage-determining transcription factors prime _cis_-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010). Article CAS Google Scholar * Kent,
W. J., Zweig, A. S., Barber, G., Hinrichs, A. S. & Karolchik, D. BigWig and BigBed: enabling browsing of large distributed datasets. _Bioinformatics_ 26, 2204–2207 (2010). Article CAS
Google Scholar * Dorfer, V. et al. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. _J. Proteome Res._ 13, 3679–3684 (2014). Article CAS
Google Scholar * Doblmann, J. et al. apQuant: accurate label-free quantification by quality filtering. _J. Proteome Res._ 4, 535–541 (2018). Google Scholar * Battye, T. G., Kontogiannis,
L., Johnson, O., Powell, H. R. & Leslie, A. G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. _Acta Crystallogr. D._ 67, 271–281 (2011). Article CAS
Google Scholar * Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. _Acta Crystallogr. D._ 58, (1948–1954 (2002). Google Scholar *
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. _Acta Crystallogr. D._ 53, 240–255 (1997). Article CAS Google
Scholar * Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. _Acta Crystallogr. D._ 66, 486–501 (2010). Article CAS Google Scholar * Chen, V. B. et
al. MolProbity: all-atom structure validation for macromolecular crystallography. _Acta Crystallogr. D._ 66, 12–21 (2010). Article CAS Google Scholar * Engler, C., Kandzia, R. &
Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. _PLoS ONE_ 3, e3647 (2008). Article Google Scholar * Trowitzsch, S., Bieniossek, C., Nie, Y.,
Garzoni, F. & Berger, I. New baculovirus expression tools for recombinant protein complex production. _J. Struct. Biol._ 172, 45–54 (2010). Article CAS Google Scholar * Fan, S. B. et
al. Using pLink to analyze cross-linked peptides. _Curr. Protoc. Bioinforma._ 49, 1–19 (2015). Google Scholar * Combe, C. W., Fischer, L. & Rappsilber, J. xiNET: cross-link network
maps with residue resolution. _Mol. Cell Proteom._ 14, 1137–1147 (2015). Article CAS Google Scholar * Gautier, R., Douguet, D., Antonny, B. & Drin, G. HELIQUEST: a web server to
screen sequences with specific alpha-helical properties. _Bioinformatics_ 24, 2101–2102 (2008). Article CAS Google Scholar * Vizcaino, J. A. et al. 2016 update of the PRIDE database and
its related tools. _Nucleic Acids Res._ 44, D447–D456 (2016). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank K. Meixner for experimental support, P. Duchek and
J. Gokcezade for generating CRISPR edited and transgenic flies, the VBCF NGS unit for deep sequencing, VBCF Protein Technologies Facility for protein expression, VBCF VDRC unit for fly
stocks, the MFPL monoclonal facility for antibodies and the TRiP and Bloomington stock centers for flies. We thank A. Koehler and G. Riddihough (both at www.lifescienceeditors.com) for
comments on the manuscript. We thank the Brennecke laboratory, particularly P. Andersen, for support and feedback. The Brennecke laboratory is supported by the Austrian Academy of Sciences,
the European Community (grant no. ERC-2015-CoG—682181) and the Austrian Science Fund (grant nos. F 4303 and W1207). J. Batki was supported by the Boehringer Ingelheim Fonds. X-ray
diffraction studies were conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines, which are supported by NIGMS grant no. P30 GM124165 and US
Department of Energy grant no. DE-AC02-06CH11357. The Pilatus 6 M detector on 24-ID-C beam line is funded by a NIH-ORIP HEI grant (no. S10 RR029205). MSKCC core facilities are supported by
grant no. P30 CA008748. This work was supported by funds from NIH U19-CA179564 and the Maloris Foundation (to D.J.P.) and MSKCC core grant P30 CA008748. AUTHOR INFORMATION Author notes *
These authors contributed equally: Julia Batki, Jakob Schnabl, Juncheng Wang. AUTHORS AND AFFILIATIONS * Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA),
Vienna BioCenter, Vienna, Austria Julia Batki, Jakob Schnabl, Dominik Handler, Veselin I. Andreev, Christian E. Stieger, Maria Novatchkova, Lisa Lampersberger, Kotryna Kauneckaite, Karl
Mechtler & Julius Brennecke * Structural Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA Juncheng Wang, Wei Xie & Dinshaw J. Patel * Institute of Molecular
Pathology (IMP), Vienna BioCenter, Vienna, Austria Christian E. Stieger, Maria Novatchkova & Karl Mechtler Authors * Julia Batki View author publications You can also search for this
author inPubMed Google Scholar * Jakob Schnabl View author publications You can also search for this author inPubMed Google Scholar * Juncheng Wang View author publications You can also
search for this author inPubMed Google Scholar * Dominik Handler View author publications You can also search for this author inPubMed Google Scholar * Veselin I. Andreev View author
publications You can also search for this author inPubMed Google Scholar * Christian E. Stieger View author publications You can also search for this author inPubMed Google Scholar * Maria
Novatchkova View author publications You can also search for this author inPubMed Google Scholar * Lisa Lampersberger View author publications You can also search for this author inPubMed
Google Scholar * Kotryna Kauneckaite View author publications You can also search for this author inPubMed Google Scholar * Wei Xie View author publications You can also search for this
author inPubMed Google Scholar * Karl Mechtler View author publications You can also search for this author inPubMed Google Scholar * Dinshaw J. Patel View author publications You can also
search for this author inPubMed Google Scholar * Julius Brennecke View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J. Batki, J.S., V.I.A.,
L.L. and K.K. performed all molecular biology and fly experiments. D.H., J.S. and J. Batki performed the computational analyses, C.E.S. and K.M. performed the X-link mass spectrometry
analysis. M.N. generated the phylogenetic comparisons of NXF proteins. J.W. generated, purified and grew crystals of the UBA-linker-helix and the Nxf2 NTF2l–Nxt1 complex, performed the X-ray
crystallographic analyses and performed the SEC–MALS assay with W.X., under the supervision of D.J.P. The paper was written by J. Batki, J.S. and J. Brennecke with input from J.W. and
D.J.P. CORRESPONDING AUTHOR Correspondence to Julius Brennecke. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW
INFORMATION: Anke Sparmann was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. PUBLISHER’S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 RELATED TO
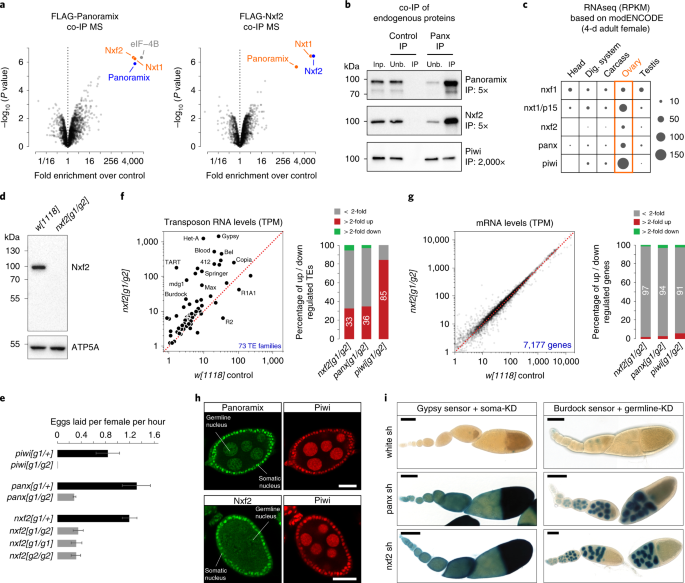
FIG. 1. A, Average peptide peak intensities for indicated proteins in Panoramix or Nxf2 immunoprecipitates (data from experiment shown in Fig. 1a; respective peptide intensities measured in
control samples were subtracted). B, Cartoon depicting frameshift positions caused by the guide RNA-induced insertions/deletions in the Nxf2 protein. C, Morphology of representative ovaries
from flies of indicated genotypes (scale bar: 1mm). D, E, Scatter plots showing steady state ovarian RNA levels (transcripts per million) of genes (D) and transposons (E) in _panx or piwi_
mutants compared to control. F, Confocal images of egg chambers (scale bar: 20 μm) from _panoramix_ (left panel) or _nxf2_ (right panel) mutant flies co-stained for Panoramix or Nxf2,
respectively, with Piwi. The remaining staining in the _panoramix_ mutant egg chambers corresponds to a background staining in the surrounding muscle sheet. SUPPLEMENTARY FIGURE 2 RELATED TO
FIG. 2. A, Confocal images of ovarioles (scale bar: 20μm) from _w[1118]_ control and _nxf2_ mutant flies stained for Piwi (inverted grayscale). B, Confocal images showing egg chambers
(scale bar: 20μm) from flies expressing GFP-tagged Nxf2 in addition to indicated germline-specific gene knockdowns (inverted grayscale). C, Box plot showing fold change (compared to control
knockdown) of piRNA levels in ovaries depleted of indicated genes specifically in the germline (box plot definition as in Fig. 2b). D, Volcano plot showing changes (as effect size) in steady
state transposon levels from RNA-seq experiments (n=3 biological replicates). The plot compares _piwi_ knockdown versus control knockdown. E-G, Density profiles showing normalized reads
from H3K9me3 ChIP-seq (top), or RNA Pol II ChIP-seq (bottom) experiments mapping to the _mdg1_ (E), _burdock_ (F) or _F-element_ (G) transposon consensus sequences (knockdowns indicated). H,
Box plots showing fold changes (compared to control knockdown) of H3K9me3 ChIP-seq signal (top) or input DNA (bottom) in 1kb tiles in OSCs depleted of indicated genes. Piwi dependent and
independent regions are shown separately (box plot definition as in Fig. 2b). I, Volcano plots showing differential gene expression analysis from OSC RNA-seq experiments (gene knockdowns
indicated; n=3 biological replicates). J, Western blots showing knockdown efficiencies of two different _nxf2_ siRNAs in OSCs. ATP5A served as loading control. K, Volcano plots showing
differential expression analysis of genes (left) and transposons (right) from OSC RNA-seq experiments (gene knockdowns indicated; n=3 biological replicates). The plots compare the two tested
independent siRNAs targeting _nxf2_. Source data: panel D, I, K: Supplementary Table 3, 4; uncropped blot images: Supplementary Data Set 1. SUPPLEMENTARY FIGURE 3 RELATED TO FIG. 3. A, Bar
graph showing genomic copy number of indicated genes based on digital droplet PCR. Two clonal reporter OSC lines were analyzed and clone 1, which harbors a single insertion, was used
throughout the study. B, Box plots showing the λN-control normalized GFP intensity in OSCs at indicated days after transfection of λN-tagged Panoramix protein; n=2500 cells; box plot
definition as in Fig. 3b). C-F, Western blots showing levels of indicated FLAG-tagged fusion proteins (λN or Gal4) in OSC lysates (related to Fig. 3c, f, g, h; Actin served as loading
control). Source data: uncropped blot images: Supplementary Data Set 1. SUPPLEMENTARY FIGURE 4 RELATED TO FIG. 4. A, Bar graph showing fold change of RNA-seq levels of indicated genes in
mutant ovaries compared to control ovaries (n=1). B, Confocal images showing OSCs (scale bar: 10μm) with indicated, transiently transfected FLAG-tagged Panoramix constructs. C, Left: Shown
is the entire size exclusion chromatogram (Fig. 4f) of the affinity-purified Strep-Panoramix eluate (mAU = milli-absorbance unit). To the right, an SDS-PAGE of the 7 peak fractions from the
second detected peak is shown (Coomassie blue staining), indicating that this peak does not contain protein. D, Calibration curve of the HiLoad 16/60 Superdex 200 size exclusion
chromatography column. Grey dots indicate protein standards based on which the calibration curve was calculated (black dashed line). The recombinant SFiNX complex is indicated based on the
elution volume in Fig. 4f. (V0: void volume of the column; Ve: measured elution volume; Mw: molecular weight.) Source data: uncropped gel images: Supplementary Data Set 1. SUPPLEMENTARY
FIGURE 5 RELATED TO FIG. 5. A-C, Western blot analysis of GFP or GFP-Nxf2 immunoprecipitation experiments using lysate from S2 cells transiently co-transfected with indicated FLAG-Panoramix
expressing plasmids (relative amount loaded in immunoprecipitation lanes: 3x). D, Protein sequence alignment of Panoramix (308-446) where the experimentally tested residues are marked in
blue. Shown below are predicted secondary structural elements and the conservation score for each position. E, Helical wheel representation of the predicted amphipathic α-helix (322-339)
within Panoramix. The point mutations introduced to abolish the Nxf2 interaction are indicated in purple color. F, Western blot analysis of lysate from S2 cells transiently transfected with
indicated FLAG-Panoramix expressing plasmids. Actin served as loading control. G, Box plots showing GFP intensity in S2 cells 2 days after transfection with plasmids expressing indicated
fusion proteins or empty control (numbers indicate fold-change in median GFP intensity normalized to GFP-only control; box plot definition as in Fig. 3b). H, Western blot analysis of
immunoprecipitation experiments (bait: stabilized degron mutant GFP-Panoramix) using lysate from S2 cells transiently co-transfected with indicated FLAG-Nxf2 expressing plasmids (relative
amount loaded in immunoprecipitation lanes: 3x). I, Co-purification of His-SUMO-Panoramix helix with untagged Nxf2 UBA domain without linker by two rounds of Ni-NTA affinity purification.
Source data: uncropped blot and gel images: Supplementary Data Set 1. SUPPLEMENTARY FIGURE 6 RELATED TO FIG. 5. A, Cartoon view of four UBA-linker-helix monomers in the crystal asymmetric
unit. The linkers between the UBA domain and the Panoramix helix are largely invisible and are shown as dashed lines. B, Molecular weight of UBA-linker-Panoramix helix measured by SEC-MALS
assay. The red line represents the SEC-MALS calculated molecular weight, which is 12.01±0.41 kDa. The theoretical molecular weight is 11.06 kDa, indicating that UBA-linker-Panoramix helix is
a monomer in solution. C, Protein sequence alignment of the Nxf2 and Nxf1 UBA domains from indicated insect species. The relevant mutated residues are marked in red. Shown below are
predicted secondary structural elements and the conservation score for each position. D, Western blot analysis of GFP-Nxf2 or GFP-Nxf1 immunoprecipitation experiments using lysate from S2
cells transiently co-transfected with indicated FLAG-Panoramix expressing plasmids (relative amount loaded in immunoprecipitation lanes: 3x). E, Left: Confocal images showing OSCs (scale
bar: 10 μm) with indicated, transiently transfected FLAG-tagged, siRNA-resistant Panoramix constructs. Right: Western blots showing levels of indicated proteins in OSC lysates with indicated
knockdowns and transiently transfected FLAG-tagged, siRNA-resistant Panoramix constructs (ATP5A served as loading control). F, Left: Confocal images showing OSCs (scale bar: 10 μm) with
indicated, transiently transfected FLAG-tagged, siRNA-resistant Nxf2 constructs. Right: Western blots showing levels of indicated proteins in OSC lysates with indicated knockdowns and
transiently transfected FLAG-tagged, siRNA-resistant Nxf2 constructs (ATP5A served as loading control). G, H, Western blots showing levels of indicated fusion proteins (left: λN, right:
Gal4) in lysates of transiently transfected OSCs (related to Fig. 5i, j; Actin served as loading control). Source data: uncropped blot images: Supplementary Data Set 1. SUPPLEMENTARY FIGURE
7 RELATED TO FIG. 6. Front and back views of the crystal structure of dmNxf2’s NTF2-like domain (purple and green) in complex with dmNxt1 (yellow and orange). Two NTF2-like domain–Nxt1
heterodimers were observed in the crystal asymmetric unit due to crystal packing. Invisible loops in the structure are shown as dashed curves. SUPPLEMENTARY FIGURE 8 RELATED TO FIG. 7. A,
Binding curve for the EMSA calculated from the phosphorimage shown in Fig. 7b; n=3; error bars: s.d.). B, Protein sequence alignment of the RRM/LRR domain of hsNXF1 and the first RRM/LRR
domain of dmNxf2 (1st unit). Identical residues are highlighted by red squares. The key hsNXF1 residues involved in CTE RNA binding are indicated by blue triangles; red triangles mark
residues which were mutated in dmNxf2 for the EMSA assay in Supplementary Fig. 8d. C, Coomassie stained SDS-PAGE showing the recombinant 1st unit of Nxf2, its point mutant variant, and the
GB1 control peptide. D, Phosphorimage showing an Electrophoretic Mobility Shift Assay (EMSA) with labeled single stranded RNA (ssRNA) and increasing amount of the purified Nxf2 protein, its
point mutant variant, and the GB1 control peptide. E, Confocal images depicting somatic cells of egg chambers (scale bar: 20 μm) from flies with indicated genotypes. Expression of the rescue
transgenes was tested by GFP fluorescence, transposon derepression was assessed by _mdg1_ FISH. F, Western blots showing levels of indicated proteins in OSC lysates with indicated
knockdowns and transiently transfected, siRNA-resistant rescue constructs (relates to experiment shown in Fig. 7e; ATP5A served as loading control). G, Western blots showing levels of
indicated λN-fusion proteins in OSC lysates (related to Fig. 7g; Actin served as loading control). H, Maximum likelihood phylogenetic tree of NXF sequences from indicated species inferred
with iqtree. Numbers represent ultrafast bootstrap branch support values. Scale bar indicates expected number of substitutions per codon site. Source data: uncropped blot and gel images:
Supplementary Data Set 1. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–8, Supplementary Tables 6–13 REPORTING SUMMARY SUPPLEMENTARY TABLE 1 co-IP-MS-volcano plot
SUPPLEMENTARY TABLE 2 RNA-seq-CRISPR_mutants SUPPLEMENTARY TABLE 3 DGE-OSC-TE-table SUPPLEMENTARY TABLE 4 DGE-OSC-gene-table SUPPLEMENTARY TABLE 5 XL–MS SUPPLEMENTARY DATASET 1 Uncropped
blot and gel images SUPPLEMENTARY DATASET 2 Flow cytometry gating strategy SOURCE DATA SOURCE DATA FIG. 3 SOURCE DATA FIG. 5 SOURCE DATA FIG. 7 RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Batki, J., Schnabl, J., Wang, J. _et al._ The nascent RNA binding complex SFiNX licenses piRNA-guided heterochromatin formation. _Nat Struct
Mol Biol_ 26, 720–731 (2019). https://doi.org/10.1038/s41594-019-0270-6 Download citation * Received: 26 April 2019 * Accepted: 17 June 2019 * Published: 05 August 2019 * Issue Date: August
2019 * DOI: https://doi.org/10.1038/s41594-019-0270-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(738x236:740x238)/david-beckham-fallen-tree-010423-1-7c60134c3e984fd1a19f9c4f29e00721.jpg)



:max_bytes(150000):strip_icc():focal(149x0:151x2)/miley-cyrus-1-300-6-c634ce84395f4c5692773fc26dea5609.jpg)