- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND/OBJECTIVES The growing interest of medical community about sarcopenia resulted in the production of several clinical practice guidelines (CPGs), with an unavoidable
variability in terms of the overall quality of those publications. Our aim is to evaluate the quality of CPGs on sarcopenia using the AGREE II instrument. SUBJECTS/METHODS We performed an
online literature search for sarcopenia CPGs using different databases. Four independent reviewers evaluated the quality of CPGs using the AGREE II instrument. To classify the quality of
each guideline, we defined specific thresholds of final score: high-quality if five or more domains scored >60%; average-quality if three or four domains scored >60%; low-quality if ≤2
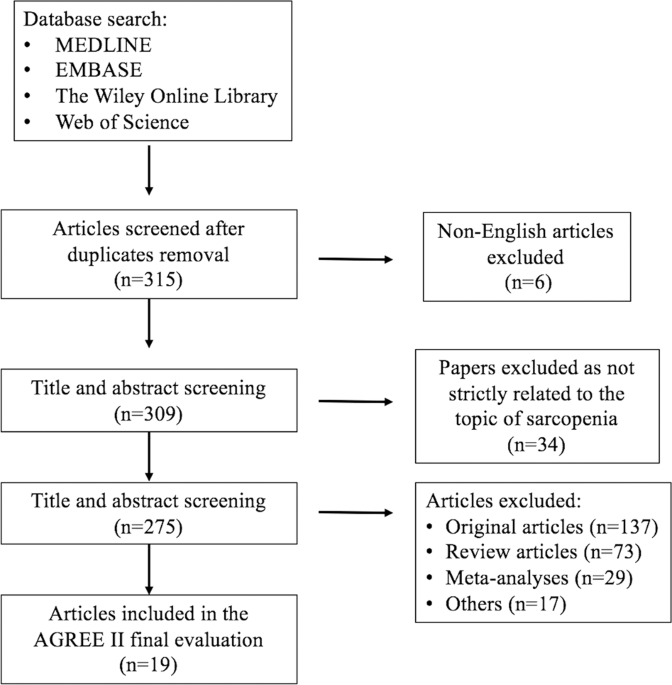
domains scored >60%. RESULTS Our literature search yielded 315 articles, and after applying exclusion criteria our final analysis included 19 CPGs. The overall quality of CPGs was
remarkable, as 13/19 (68.4%) were considered of “high-quality” CPGs, with more than four domains reached a score higher than 60%. “Scope and Purpose” and “Clarity of Presentations” had the
best domain results (78.4% and 73.8%, respectively), while the two domains with the lowest scores were “Rigor of Development” and “Applicability” (61.5% and 58.7%, respectively).
Interobserver variability ranged between moderate (0.624) and fair (0.275). CONCLUSIONS Our study showed that the overall quality of CPGs about sarcopenia was noteworthy, as more than
two-third of paper obtained a “high-quality” score. The domain “applicability” had the lowest score, suggesting that emphasis should be put on possible strategies for helping other doctors
to implement guideline recommendations in clinical practice. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TOWARD THE RECOGNITION AND MANAGEMENT OF SARCOPENIA IN ROUTINE CLINICAL CARE
Article 11 November 2021 THE EFFECTIVENESS OF OPTIMAL EXERCISE-BASED STRATEGY FOR PATIENTS WITH HIP FRACTURE: A SYSTEMATIC REVIEW AND BAYESIAN NETWORK META-ANALYSIS Article Open access 29
June 2023 SARCOPENIA IN HOSPITALIZED GERIATRIC PATIENTS: INSIGHTS INTO PREVALENCE AND ASSOCIATED PARAMETERS USING NEW EWGSOP2 GUIDELINES Article Open access 15 October 2020 REFERENCES *
Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50(Nov 1):1231–3. Article Google Scholar * Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in
daily practice: assessment and management. BMC Geriatr. 2016;16(Dec 5):170. Article Google Scholar * Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia:
revised European consensus on definition and diagnosis. Age Ageing. 2019;48(Jan 1):16–31. Article Google Scholar * Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NEP, et al.
Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(Jul):391–6. Article Google Scholar * Iolascon G, Di Pietro G, Gimigliano F, Mauro GL, Moretti A,
Giamattei MT, et al. Physical exercise and sarcopenia in older people: position paper of the Italian Society of Orthopaedics and Medicine (OrtoMed). Clin Cases Min Bone Metab.
2014;11(Sep):215–21. Google Scholar * De Spiegeleer A, Beckwée D, Bautmans I, Petrovic M, Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics
(BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs
Aging. 2018;35(Aug 25):719–34. Article Google Scholar * Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Van Kan GA, et al. Biomarkers of sarcopenia in clinical
trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3(Sep):181–90. Article Google Scholar * Petak S, Barbu CG, Yu EW, Fielding R,
Mulligan K, Sabowitz B, et al. The Official Positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom. 2013;16(Oct):508–19.
Article Google Scholar * Kung J, Miller RR, Mackowiak PA. Failure of clinical practice guidelines to meet institute of medicine standards: two more decades of little, if any, progress.
Arch Intern Med. 2012;172(Nov 26):1628–33. Article Google Scholar * Grilli R, Magrini N, Penna A, Mura G, Liberati A. Practice guidelines developed by specialty societies: the need for a
critical appraisal. Lancet. 2000;355(Jan 8):103–6. Article CAS Google Scholar * Kredo T, Bernhardsson S, Machingaidze S, Young T, Louw Q, Ochodo E, et al. Guide to clinical practice
guidelines: the current state of play. Int J Qual Heal Care. 2016;28(Feb):122–8. Article Google Scholar * Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, Lampert U, Eikermann M.
Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. Zhang H-L, editor. PLoS One. 2017;12(Mar 30):e0174831. *
Sardanelli F, Bashir H, Berzaczy D, Cannella G, Espeland A, Flor N, et al. The role of imaging specialists as authors of systematic reviews on diagnostic and interventional imaging and its
impact on scientific quality: report from the EuroAIM Evidence-based Radiology Working Group. Radiology 2014;272(Aug):533–40. Article Google Scholar * EIBIR. European network for the
assessment of imaging in medicine [Internet]. [cited 27 Dec 2016]. http://www.eibir.org/scientific-activities/joint-initiatives/euroaim/ * Sconfienza LM. Sarcopenia: ultrasound today,
smartphones tomorrow? Eur Radiol. 2019;29(Jan 12):1–2. Article Google Scholar * Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in
systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(Dec 6):245. Article Google Scholar * AGREE Enterprise website [Internet]. [cited 5 Jun 2019].
https://www.agreetrust.org/ * Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ
2010;182(Dec 14):E839–42. Article Google Scholar * Ou Y, Goldberg I, Migdal C, Lee PP. A critical appraisal and comparison of the quality and recommendations of glaucoma clinical practice
guidelines. Ophthalmology 2011;118(Jun):1017–23. Article Google Scholar * Armstrong JJ, Rodrigues IB, Wasiuta T, MacDermid JC. Quality assessment of osteoporosis clinical practice
guidelines for physical activity and safe movement: an AGREE II appraisal. Arch Osteoporos. 2016;11(Dec 13):6. Article Google Scholar * Messina C, Bignotti B, Tagliafico A, Orlandi D,
Corazza A, Sardanelli F, et al. A critical appraisal of the quality of adult musculoskeletal ultrasound guidelines using the AGREE II tool: an EuroAIM initiative. Insights Imaging 2017;8(Oct
28):491–7. Article Google Scholar * Messina C, Bignotti B, Bazzocchi A, Phan CM, Tagliafico A, Guglielmi G, et al. A critical appraisal of the quality of adult dual-energy X-ray
absorptiometry guidelines in osteoporosis using the AGREE II tool: an EuroAIM initiative. Insights Imaging 2017;8(Jun 21):311–7. Article Google Scholar * Sekercioglu N, Al-Khalifah R,
Ewusie JE, Elias RM, Thabane L, Busse JW, et al. A critical appraisal of chronic kidney disease mineral and bone disorders clinical practice guidelines using the AGREE II instrument. Int
Urol Nephrol. 2017;49(Feb 1):273–84. Article CAS Google Scholar * Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on
definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39(Jul 1):412–23. Article Google Scholar * Nishikawa H, Shiraki M, Hiramatsu
A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 2016;46(Sep):951–63. Article Google Scholar * Reginster J-Y, Cooper C, Rizzoli R, Kanis JA, Appelboom G, Bautmans I, et al. Recommendations for the
conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res. 2016;28(Feb):47–58. Article Google Scholar * Kendler DL, Borges JLC, Fielding RA, Itabashi A,
Krueger D, Mulligan K, et al. The Official Positions of the International Society for clinical densitometry: indications of use and reporting of DXA for body composition. J Clin Densitom.
Jan;16:496–507. * Vellas B, Pahor M, Manini T, Rooks D, Guralnik JM, Morley J, et al. Designing pharmaceutical trials for sarcopenia in frail older adults: EU/US task force recommendations.
J Nutr Health Aging. 2013;17(Aug 6):612–8. Article CAS Google Scholar * Suominen MH, Jyvakorpi SK, Pitkala KH, Finne-Soveri H, Hakala P, Mannisto S, et al. Nutritional guidelines for
older people in Finland. J Nutr Health Aging. 2014;18(Dec 23):861–7. Article CAS Google Scholar * Abellan van Kan G, André E, Bischoff Ferrari HA, Boirie Y, Onder G, Pahor M, et al. Carla
Task Force on Sarcopenia: propositions for clinical trials. J Nutr Health Aging. 2009;13(Oct):700–7. Article CAS Google Scholar * Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean
RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(May):547–58. Article
Google Scholar * Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir
Assoc. 2014;15(Feb):95–101. Article Google Scholar * Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr.
2017;36(Feb):11–48. Article Google Scholar * Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia
(ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–61. Article CAS Google Scholar * Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et
al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(Feb):49–64. Article CAS Google Scholar * Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC,
Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(Oct):1187–96. Article CAS Google Scholar * van den Berg T,
Engelhardt EG, Haanstra TM, Langius JAE, van Tulder MW. Methodology of clinical nutrition guidelines for adult cancer patients. J Parenter Enter Nutr. 2012;36(May 13):316–22. Article Google
Scholar * Zeng L, Zhang L, Hu Z, Ehle EA, Chen Y, Liu L, et al. Systematic review of evidence-based guidelines on medication therapy for upper respiratory tract infection in children with
AGREE instrument. Schildgen O, editor. PLoS One. 2014;9 (Feb 20):e87711. * Gitto S, Bisdas S, Emili I, Nicosia L, Pescatori LC, Bhatia K. Clinical practice guidelines on ultrasound-guided
fine needle aspiration biopsy of thyroid nodules: a critical appraisal using AGREE II. Endocrine. 2019;65(2):371–78. Article CAS Google Scholar * Doniselli FM, Zanardo M, Manfrè L, Papini
GDE, Rovira A, Sardanelli F, et al. A critical appraisal of the quality of low back pain practice guidelines using the AGREE II tool and comparison with previous evaluations: a EuroAIM
initiative. Eur Spine J. 2018;27(Nov 15):2781–90. Article Google Scholar * Lawrenson JG, Evans JR, Downie LE. A critical appraisal of national and international clinical practice
guidelines reporting nutritional recommendations for age-related macular degeneration: are recommendations evidence-based? Nutrients 2019;11(Apr 11):823. Article CAS Google Scholar *
Sabharwal S, Patel NK, Gauher S, Holloway I, Athanasiou T, Athansiou T. High methodologic quality but poor applicability: assessment of the AAOS guidelines using the AGREE II instrument.
Clin Orthop Relat Res. 2014;472(Jun 25):1982–8. Article Google Scholar * Sabharwal S, Patel V, Nijjer SS, Kirresh A, Darzi A, Chambers JC, et al. Guidelines in cardiac clinical practice:
evaluation of their methodological quality using the AGREE II instrument. J R Soc Med. 2013;106(Aug 28):315–22. Article Google Scholar * Anker SD, Morley JE, von Haehling S. Welcome to the
ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(Dec):512–4. Article Google Scholar * Norris SL, Holmer HK, Ogden LA, Burda BU. Conflict of interest in clinical practice
guideline development: a systematic review. Mintzes B, editor. PLoS One. 2011;6 (Oct 19):e25153. * Norris SL, Holmer HK, Ogden LA, Selph SS, Fu R. Conflict of interest disclosures for
clinical practice guidelines in the national guideline clearinghouse. Ross JS, editor. PLoS One. 2012;7 (Nov 7):e47343. * Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial
conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ 2011;343(Oct 11):d5621–d5621. Article Google Scholar
Download references ACKNOWLEDGEMENTS This work has been conducted within the framework of the EuroAIM, research platform of the European Institute for Biomedical Research
(http://www.eibir.org/scientific-activities/joint-initiatives/euroaim/). This study was supported by the Italian Ministry of Health (Ricerca Corrente). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * IRCCS Istituto Ortopedico Galeazzi, Via Riccardo Galeazzi 4, 20161, Milan, Italy Carmelo Messina, Jacopo Antonino Vitale, Luigi Pedone, Vito Chianca, Domenico Albano &
Luca Maria Sconfienza * Dipartimento di Scienze Biomediche per la Salute, Università degli Studi di Milano, Via Carlo Pascal, 36, 20133, Milan, Italy Carmelo Messina, Salvatore Gitto &
Luca Maria Sconfienza * Scuola di Specializzazione in Radiodiagnostica, Università degli Studi di Milano, Via Festa del Perdono 7, 20122, Milan, Italy Ilaria Vicentin * Sezione di Scienze
Radiologiche, Dipartimento di Biomedicina, Neuroscienze e Diagnostica Avanzata, Università degli Studi di Palermo, Via del Vespro 127, 90127, Palermo, Italy Domenico Albano Authors * Carmelo
Messina View author publications You can also search for this author inPubMed Google Scholar * Jacopo Antonino Vitale View author publications You can also search for this author inPubMed
Google Scholar * Luigi Pedone View author publications You can also search for this author inPubMed Google Scholar * Vito Chianca View author publications You can also search for this author
inPubMed Google Scholar * Ilaria Vicentin View author publications You can also search for this author inPubMed Google Scholar * Domenico Albano View author publications You can also search
for this author inPubMed Google Scholar * Salvatore Gitto View author publications You can also search for this author inPubMed Google Scholar * Luca Maria Sconfienza View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed to meet the ICMJE Recommendations for authorship, having provided: 1.
Substantial contributions to the conception and design (CM, DA), acquisition (IV), or analysis and interpretation of data (LP, SG, JV, VC). 2. Drafting the paper (CM, DA) or revising it
critically for important intellectual content (CM, LMS). 3. Final approval of the version to be published (all authors). 4. All authors agreed to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CORRESPONDING AUTHOR Correspondence to Carmelo Messina.
ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Messina, C., Vitale, J.A., Pedone, L. _et al._ Critical appraisal of papers reporting recommendation on sarcopenia using the AGREE II tool: a EuroAIM initiative. _Eur J
Clin Nutr_ 74, 1164–1172 (2020). https://doi.org/10.1038/s41430-020-0638-z Download citation * Received: 11 November 2019 * Revised: 27 March 2020 * Accepted: 07 April 2020 * Published: 27
April 2020 * Issue Date: August 2020 * DOI: https://doi.org/10.1038/s41430-020-0638-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(738x236:740x238)/david-beckham-fallen-tree-010423-1-7c60134c3e984fd1a19f9c4f29e00721.jpg)