- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
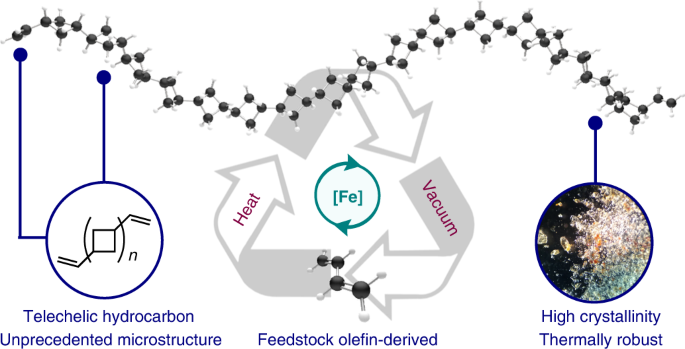
ABSTRACT Closed-loop recycling offers the opportunity to mitigate plastic waste through reversible polymer construction and deconstruction. Although examples of chemical recycling of
polymers are known, few have been applied to materials derived from abundant commodity olefinic monomers, which are the building blocks of ubiquitous plastic resins. Here we describe a [2+2]
cycloaddition/oligomerization of 1,3-butadiene to yield a previously unrealized telechelic microstructure of (1,_N_′-DIVINYL)OLIGOCYCLOBUTANE. This material is thermally stable, has
stereoregular segments arising from chain-end control, and exhibits high crystallinity even at low molecular weight. Exposure of the oligocyclobutane to vacuum in the presence of the
pyridine(diimine) iron precatalyst used to synthesize it resulted in deoligomerization to generate pristine butadiene, demonstrating a rare example of closed-loop chemical recycling of an
oligomeric material derived from a commodity hydrocarbon feedstock. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time
Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS UPCYCLING POLYETHYLENE INTO CLOSED-LOOP RECYCLABLE POLYMERS THROUGH TITANOSILICATE CATALYZED C-H OXIDATION AND
IN-CHAIN HETEROATOM INSERTION Article Open access 24 October 2024 TAILORED SYNTHESIS OF CIRCULAR POLYOLEFINS Article 11 March 2025 BIFUNCTIONAL AND RECYCLABLE POLYESTERS BY CHEMOSELECTIVE
RING-OPENING POLYMERIZATION OF A Δ-LACTONE DERIVED FROM CO2 AND BUTADIENE Article Open access 08 October 2024 DATA AVAILABILITY All data necessary to support the conclusions of this paper
are provided in the Supplementary Information, including optimized DFT coordinates and energetics, calculated free energies and MD equilibrated coordinates. CHANGE HISTORY * _ 22 FEBRUARY
2021 In the version of this Article originally published, the Supplementary Information PDF was missing. This has now been corrected. _ REFERENCES * Geyer, R., Jambeck, J. R. & Law, K.
L. Production, use and fate of all plastics ever made. _Sci. Adv._ 3, e1700782 (2017). Article PubMed PubMed Central Google Scholar * Rahimi, A. & García, J. M. Chemical recycling of
waste plastics for new materials production. _Nat. Rev. Chem._ 1, 0046 (2017). Article Google Scholar * Garcia, J. M. & Robertson, M. L. The future of plastics recycling. _Science_
358, 870–872 (2017). Article CAS PubMed Google Scholar * Seeley, M. E., Song, B., Passie, R. & Hale, R. C. Microplastics affect sedimentary microbial communities and nitrogen
cycling. _Nat. Commun._ 11, 2372 (2020). Article CAS PubMed PubMed Central Google Scholar * Sardon, H. & Dove, A. P. Plastics recycling with a difference. _Science_ 360, 380–381
(2018). Article CAS PubMed Google Scholar * Hopewell, J., Dvorak, R. & Kosior, E. Plastics recycling: challenges and opportunities. _Philos. Trans. R. Soc. Lond. B Biol. Sci._ 364,
2115–2126 (2009). Article CAS PubMed PubMed Central Google Scholar * Zhang, X., Fevre, M., Jones, G. O. & Waymouth, R. M. Catalysis as an enabling science for sustainable polymers.
_Chem. Rev._ 118, 839–885 (2018). Article CAS PubMed Google Scholar * Helms, B. A. & Russell, T. P. Reaction: polymer chemistries enabling cradle-to-cradle life cycles for plastics.
_Chem_ 1, 816–818 (2016). Article CAS Google Scholar * Hong, M. & Chen, E. Y. X. Chemically recyclable polymers: a circular economy approach to sustainability. _Green Chem._ 19,
3692–3706 (2017). Article CAS Google Scholar * Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. _Angew. Chem. Int.
Ed._ 41, 898–952 (2002). Article Google Scholar * Liu, T. et al. Eugenol-derived biobased epoxy: shape memory, repairing and recyclability. _Macromolecules_ 50, 8588–8597 (2017). Article
CAS Google Scholar * Ogden, W. A. & Guan, Z. Recyclable, strong and highly malleable thermosets based on boroxine networks. _J. Am. Chem. Soc._ 140, 6217–6220 (2018). Article CAS
PubMed Google Scholar * Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. _Science_ 360, 398–403 (2018). Article
CAS PubMed Google Scholar * Christensen, P. R., Scheuermann, A. M., Loeffler, K. E. & Helms, B. A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds.
_Nat. Chem._ 11, 442–448 (2019). Article CAS PubMed Google Scholar * Lian, Z., Bhawal, B. N., Yu, P. & Morandi, B. Palladium-catalyzed carbon–sulfur or carbon–phosphorus bond
metathesis by reversible arylation. _Science_ 356, 1059–1063 (2017). Article CAS PubMed Google Scholar * García, J. M. Catalyst design challenges for the future of plastics recycling.
_Chem_ 1, 813–815 (2016). Article Google Scholar * Feldman, D. Polymer history. _Des. Monomers Polym._ 11, 1–15 (2008). Article CAS Google Scholar * Amghizar, I., Vandewalle, L. A., Van
Geem, K. M. & Marin, G. B. New trends in olefin production. _Eng. J._ 3, 171–178 (2017). CAS Google Scholar * Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer
for an ideal, circular polymer economy. _Nat. Rev. Mater._ 5, 501–516 (2020). Article CAS Google Scholar * Pastine, S. J. Reaction: design with the end in mind. _Chem_ 1, 818–819 (2016).
Article CAS Google Scholar * Long, T. E. Reaction: benign by design demands innovation. _Chem_ 2, 7–8 (2017). Article CAS Google Scholar * Russell, S. K., Lobkovsky, E. & Chirik,
P. J. Iron-catalyzed intermolecular [2_π_+2_π_] cycloaddition. _J. Am. Chem. Soc._ 133, 8858–8861 (2011). Article CAS PubMed Google Scholar * Hoyt, J. M., Schmidt, V. A., Tondreau, A. M.
& Chirik, P. J. Iron-catalyzed intermolecular [2+2] cycloadditions of unactivated alkenes. _Science_ 349, 960–963 (2015). Article CAS PubMed Google Scholar * Hoyt, J. M., Sylvester,
K. T., Semproni, S. P. & Chirik, P. J. Synthesis and electronic structure of bis(imino)pyridine iron metallacyclic intermediates in iron-catalyzed cyclization reactions. _J. Am. Chem.
Soc._ 135, 4862–4877 (2013). Article CAS PubMed Google Scholar * Schmidt, V. A., Hoyt, J. M., Margulieux, G. W. & Chirik, P. J. Cobalt-catalyzed [2_π_+2_π_] cycloadditions of
alkenes: scope, mechanism and elucidation of electronic structure of catalytic intermediates. _J. Am. Chem. Soc._ 137, 7903–7914 (2015). Article CAS PubMed PubMed Central Google Scholar
* Hall, H. K. Jr Synthesis and polymerisation of polycyclic compounds with strained C–C single bonds. _Br. Polym. J._ 4, 371–389 (1972). Article CAS Google Scholar * Hall, H. K. Jr
& Ykman, P. Addition polymerization of cyclobutene and bicyclobutane monomers. _J. Polym. Sci. Macromol. Rev._ 11, 1–45 (1976). Article CAS Google Scholar * Wu, C. C. & Lenz, R.
W. Thermal and autoxidation reactions of poly-3-methylenecyclobutene and poly-1-methyl-3-methylenecyclobutene. _J. Polym. Sci. A Polym. Chem._ 10, 3555–3567 (1972). Article CAS Google
Scholar * Danopoulos, A. A., Wright, J. A. & Motherwell, W. B. Molecular N2 complexes of iron stabilised by N-heterocyclic ‘pincer’ dicarbene ligands. _Chem. Commun_. 784–786 (2005);
https://doi.org/10.1039/b415562a * Darmon, J. M. et al. Electronic structure determination of pyridine N-heterocyclic carbene iron dinitrogen complexes and neutral ligand derivatives.
_Organometallics_ 33, 5423–5433 (2014). Article CAS PubMed PubMed Central Google Scholar * Pagar, V. V. & RajanBabu, T. V. Tandem catalysis for asymmetric coupling of ethylene and
enynes to functionalized cyclobutanes. _Science_ 361, 68–72 (2018). Article CAS PubMed PubMed Central Google Scholar * Parsutkar, M. M., Pagar, V. V. & RajanBabu, T. V. Catalytic
enantioselective synthesis of cyclobutenes from alkynes and alkenyl derivatives. _J. Am. Chem. Soc._ 141, 15367–15377 (2019). Article CAS PubMed PubMed Central Google Scholar * Bandrup,
J., Immergut, E. & Grulke, E. _Polymer Handbook_ 4th edn (Wiley-Blackwell, 2003). * Kennedy, C. R., Zhong, H., Joannou, M. V. & Chirik, P. J. Pyridine(diimine) iron diene complexes
relevant to catalytic [2+2]-cycloaddition reactions. _Adv. Synth. Catal._ 362, 404–416 (2020). Article CAS PubMed Google Scholar * Kozuch, S. & Shaik, S. How to conceptualize
catalytic cycles? The energetic span model. _Acc. Chem. Res._ 44, 101–110 (2011). Article CAS PubMed Google Scholar * Pangborn, A. B., Giardello, M. A., Grubbs, R. H., Rosen, R. K. &
Timmers, F. J. Safe and convenient procedure for solvent purification. _Organometallics_ 15, 1518–1520 (1996). Article CAS Google Scholar * Wreford, S. S. & Whitney, J. F. Magnesium
butadiene as a reagent for the preparation of transition-metal butadiene complexes: molecular structure of bis(η-butadiene)[1,2-bis(dimethylphosphino)ethane]hafnium. _Inorg. Chem._ 20,
3918–3924 (1981). Article CAS Google Scholar * Britovsek, G. J. P. et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(imino)pyridyl ligands: synthesis, structures
and polymerization studies. _J. Am. Chem. Soc._ 121, 8728–8740 (1999). Article CAS Google Scholar * Russell, S. K., Darmon, J. M., Lobkovsky, E. & Chirik, P. J. Synthesis of
aryl-substituted bis(imino)pyridine iron dinitrogen complexes. _Inorg. Chem._ 49, 2782–2792 (2010). Article CAS PubMed Google Scholar * Bart, S. C., Lobkovsky, E. & Chirik, P. J.
Preparation and molecular and electronic structures of iron(0) dinitrogen and silane complexes and their application to catalytic hydrogenation and hydrosilation. _J. Am. Chem. Soc._ 126,
13794–13807 (2004). Article CAS PubMed Google Scholar * Russell, S. K., Milsmann, C., Lobkovsky, E., Weyhermüller, T. & Chirik, P. J. Synthesis, electronic structure and catalytic
activity of reduced bis(aldimino)pyridine iron compounds: experimental evidence for ligand participation. _Inorg. Chem._ 50, 3159–3169 (2011). Article CAS PubMed Google Scholar *
Bouwkamp, M. W., Bowman, A. C., Lobkovsky, E. & Chirik, P. J. Iron-catalyzed [2_π_+2_π_] cycloaddition of α,ω-dienes: the importance of redox-active supporting ligands. _J. Am. Chem.
Soc._ 128, 13340–13341 (2006). Article CAS PubMed Google Scholar * Rummelt, S. M. et al. Synthesis, structure and hydrogenolysis of pyridine dicarbene iron dialkyl complexes.
_Organometallics_ 38, 3159–3168 (2019). Article CAS PubMed PubMed Central Google Scholar * Cirera, J., Via-Nadal, M. & Ruiz, E. Benchmarking density functional methods for
calculation of state energies of first row spin-crossover molecules. _Inorg. Chem._ 57, 14097–14105 (2018). Article CAS PubMed Google Scholar * Jensen, K. P. Bioinorganic chemistry
modeled with the TPSSh density functional. _Inorg. Chem._ 47, 10357–10365 (2008). Article CAS PubMed Google Scholar * Srinivasan, S., Rappe, A. M. & Soroush, M. in _Computational
Quantum Chemistry_: _Insights into Polymerization_ _Reactions_ Ch. 4 (Elsevier, 2019). * Pápai, M. & Vanko, G. On predicting Mössbauer parameters of iron-containing molecules with
density-functional theory. _J. Chem. Theory Comput._ 9, 5004–5020 (2013). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to S. Klemenz
and the Schoop laboratory for initial assistance with powder diffraction, as well as D. Gregory for assistance with TGA/GCMS and DSC. M.M.B. thanks K. Conover for assistance with
high-temperature NMR experiments. M.M.B., C.R.K. and P.J.C. thank Firmenich for initial support of this work. M.M.B. and C.R.K. thank the NIH for Ruth L. Kirschstein National Research
Service Awards (F32 GM134610 and GM126640). All authors thank ExxonMobil for support of this research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, Princeton
University, Princeton, NJ, USA Megan Mohadjer Beromi, C. Rose Kennedy & Paul J. Chirik * ExxonMobil Chemical Company, Baytown, TX, USA Jarod M. Younker, Alex E. Carpenter, Sarah J.
Mattler & Joseph A. Throckmorton Authors * Megan Mohadjer Beromi View author publications You can also search for this author inPubMed Google Scholar * C. Rose Kennedy View author
publications You can also search for this author inPubMed Google Scholar * Jarod M. Younker View author publications You can also search for this author inPubMed Google Scholar * Alex E.
Carpenter View author publications You can also search for this author inPubMed Google Scholar * Sarah J. Mattler View author publications You can also search for this author inPubMed Google
Scholar * Joseph A. Throckmorton View author publications You can also search for this author inPubMed Google Scholar * Paul J. Chirik View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS C.R.K. and P.J.C. conceived the project. C.R.K. and M.M.B. performed experiments regarding the synthesis and partial characterization of
oligomers. A.E.C. and S.J.M. conducted full NMR characterization and peak assignments. M.M.B., A.E.C. and J.A.T. performed experiments on the thermal stability and crystallinity of the
oligomer. J.M.Y. conducted TST/DFT and molecular mechanics calculations. M.M.B. and A.E.C. performed the chemical recycling experiments. CORRESPONDING AUTHOR Correspondence to Paul J.
Chirik. ETHICS DECLARATIONS COMPETING INTERESTS C.R.K. and P.J.C. are inventors on patent application US 2019/0211142 A1 titled ‘Oligomeric and polymeric species comprising cyclobutane
units’. J.M.Y., A.E.C., S.J.M. and J.A.T. are employees of ExxonMobil Chemical Company. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks the anonymous reviewers for
their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–22, Tables 1–9 and references 1–34. Spectroscopic data for the oligocyclobutanes, chemical/stereochemical NMR shift
assignments, DFT NMR simulations, WAXS data and MD simulations, TGA and DSC data, spectroscopic data for chemical recycling, gelation tests and DFT simulations of the reaction profile and
associated discussion. SUPPLEMENTARY DATA 1 Optimized coordinates for the divinyloligocyclobutane dimer, trimer and butadiene using the B3LYP-D3 functional. SUPPLEMENTARY DATA 2 Optimized
coordinates for the iron catalyst stationary points on the singlet energy surface using the B3LYP-D3 functional. SUPPLEMENTARY DATA 3 Optimized coordinates for the divinyloligocyclobutane
dimer, trimer and butadiene using the M06L functional. SUPPLEMENTARY DATA 4 Optimized coordinates for the iron catalyst stationary points on the singlet energy surface using the M06L
functional. SUPPLEMENTARY DATA 5 Optimized coordinates for the divinyloligocyclobutane dimer, trimer, and butadiene using the TPSSh functional. SUPPLEMENTARY DATA 6 Optimized coordinates for
the iron catalyst stationary points using the broken symmetry (1,1) restrictions at the TPSSh level of theory. SUPPLEMENTARY DATA 7 Optimized coordinates for the iron catalyst stationary
points using the broken symmetry (3,1) restrictions at the TPSSh level of theory. SUPPLEMENTARY DATA 8 Optimized coordinates for the iron catalyst stationary points on the singlet energy
surface using the TPSSh functional. SUPPLEMENTARY DATA 9 Optimized coordinates for the iron catalyst stationary points on the triplet energy surface using the TPSSh functional. SUPPLEMENTARY
DATA 10 Optimized coordinates for the iron catalyst stationary points on the quintet energy surface using the TPSSh functional. SUPPLEMENTARY DATA 11 Associated mae files for the calculated
NMR shifts. SUPPLEMENTARY DATA 12 The resultant coordinates of the optimized geometries of the single strand oligomer. SUPPLEMENTARY DATA 13 The resultant coordinates of the optimized
geometries of the supercell of the oligomer strands. SUPPLEMENTARY DATA 14 The raw data for the resultant coordinates of the optimized geometries of the single strand oligomer and supercell
of the strands. SUPPLEMENTARY DATA 15 Combined spreadsheet comparing all functionals for all points in reaction profile. SUPPLEMENTARY DATA 16 Combined spreadsheet comparing all spin
manifolds for all points in reaction profile. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mohadjer Beromi, M., Kennedy, C.R., Younker, J.M. _et al._
Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. _Nat. Chem._ 13, 156–162 (2021). https://doi.org/10.1038/s41557-020-00614-w Download citation *
Received: 25 February 2020 * Accepted: 28 October 2020 * Published: 25 January 2021 * Issue Date: February 2021 * DOI: https://doi.org/10.1038/s41557-020-00614-w SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative







