- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The mammalian mitochondrial ribosome (mitoribosome) and its associated translational factors have evolved to accommodate greater participation of proteins in mitochondrial
translation. Here we present the 2.68–3.96 Å cryo-EM structures of the human 55S mitoribosome in complex with the human mitochondrial elongation factor G1 (EF-G1mt) in three distinct
conformational states, including an intermediate state and a post-translocational state. These structures reveal the role of several mitochondria-specific (mito-specific) mitoribosomal
proteins (MRPs) and a mito-specific segment of EF-G1mt in mitochondrial tRNA (tRNAmt) translocation. In particular, the mito-specific C-terminal extension in EF-G1mt is directly involved in
translocation of the acceptor arm of the A-site tRNAmt. In addition to the ratchet-like and independent head-swiveling motions exhibited by the small mitoribosomal subunit, we discover
significant conformational changes in MRP mL45 at the nascent polypeptide-exit site within the large mitoribosomal subunit that could be critical for tethering of the elongating mitoribosome
onto the inner-mitochondrial membrane. SIMILAR CONTENT BEING VIEWED BY OTHERS DISTINCT PRE-INITIATION STEPS IN HUMAN MITOCHONDRIAL TRANSLATION Article Open access 10 June 2020 DISTINCT
MECHANISMS OF THE HUMAN MITORIBOSOME RECYCLING AND ANTIBIOTIC RESISTANCE Article Open access 14 June 2021 STRUCTURAL BASIS FOR LATE MATURATION STEPS OF THE HUMAN MITORIBOSOMAL LARGE SUBUNIT
Article Open access 16 June 2021 INTRODUCTION Mitochondria are thought to have originated through an early endosymbiotic event between an α-protobacterium and a primitive eukaryotic host
cell1. However, the structural, functional, and compositional organization of the mitochondrial ribosomes (mitoribosome) is dramatically different from its cytoplasmic and bacterial
counterparts2,3,4,5,6,7. The ribosomal RNA (rRNA) to ribosomal protein ratio in mammalian mitoribosome (~1:2) is reversed as compared to that in the eubacterial ribosomes (~2:1). The first
cryo-EM study of the mammalian mitoribosome identified several unique structural features5 in both its subunits: the large 39S subunit (LSU) and the smaller 28S subunit (SSU). Subsequent
high-resolution structures2,4,8,9 provided molecular description of previously identified features5, such as heavily shielded rRNA cores by mitoribosomal proteins (MRPs), the presence of a
significantly modified entrance of the mRNA channel and nascent polypeptide-exit tunnel (NPET), and a P-site finger. In addition, the high-resolution structures revealed that one of the
mitochondrial tRNAs (tRNAsmt) partially substitutes for the role of bacterial 5S rRNA by becoming a structural component of the mammalian mitoribosomal LSU2,4. Similar to bacterial
translation, the mechanism of the mammalian mitochondrial translation is roughly divided into four stages: initiation, elongation, termination, and ribosome recycling10,11. Each of these
steps are facilitated by translational factors that are homologous to their bacterial counterparts but carry mitochondria-specific (mito-specific) segments10,11. Biochemical12,13,14,15 and
structural8,9,16,17 studies have shown that the mito-specific segments in translational factors play important functions in mitochondrial translation. The distinct structural features in
both the mitoribosome and its binding translational factors therefore suggest unique molecular interactions and mechanism during each mitochondrial translation step. The critical step of
tRNA and mRNA translocation on the ribosome is promoted by elongation factor-G (EF-G) in eubacteria and homologous EF-2 in eukaryotic cytoplasm. In mammalian mitochondria, there are two
isoforms of EF-Gmt: EF-G1mt and EF-G2mt18,19. EF-G1mt catalyzes tRNAmt translocation on the mitoribosome, whereas EF-G2mt is involved exclusively in mitoribosome recycling19. A mutation in
human EF-G1mt leads to fatal hepatoencephalopathy, indicating that this isoform is essential for mitochondrial protein biosynthesis in humans20,21. In addition, defects in mitochondrial
protein synthesis are associated with numerous human diseases that directly involve mutations in MRPs and tRNAsmt22,23,24. The bacterial EF-G is composed of five structural domains, namely G
(or domain I) and domains II – V25,26. The structural and functional aspects of EF-G-catalyzed translocation on bacterial ribosomes have been extensively studied in various functional
states, using both cryo-EM27,28,29,30,31,32,33 and X-ray crystallography34,35,36,37,38. Like other translocases, the mammalian EF-G1mt (molecular weight ~80 kDa) is a single polypeptide that
possesses mito-specific extensions at both its termini with an additional 47 amino acids (aa), including the signal sequences, as compared to its bacterial homologs39. Human EF-G1mt is 751
aa long, where the first 36 residues at the N-terminus constitute the mitochondrial targeting signal39, which is cleaved off in the functional form. The functional human EF-G1mt shows ~45%
sequence identity with its bacterial counterpart, with a major difference being the presence of an 11 aa mito-specific extension at its C terminus. We have determined near-atomic-resolution
cryo-EM structures of the human 55S mitoribosome in complex with the human EF-G1mt to investigate the roles of the mito-specific MRPs and C-terminal extension in EF-G1mt in tRNAmt
translocation in mammalian mitochondria. Our study reveals several distinct features, including mito-specific molecular interactions during EF-G1mt-mediated tRNAmt translocation on the human
mitoribosome. In addition, we identify conformational changes associated with translation elongation at the exit of the NPET within the mitoribosome that could be necessary for facilitating
the release of the nascent polypeptide chain through the NPET and anchoring of the mitoribosome on to the inner mitochondrial membrane. RESULTS AND DISCUSSION OVERALL STRUCTURE OF THE
55S·EF-G1MT COMPLEX To obtain the 55S·EF-G1mt complex a non-hydrolysable analog of GTP, GMPPCP, was used to lock EF-G1mt on the mitoribosomes (see Methods). A cryo-EM structure for the
55S·EF-G1mt·GMPPCP complex with an overall resolution of 2.7 Å (Supplementary Figs. 1 and 2) was obtained (see Methods). 3D classification of all selected 55S mitoribosome images yielded two
major classes that were refined to 2.96 Å and 2.97 Å resolution, respectively, and a minor class that was refined to 3.96 Å resolution (Supplementary Figs. 1 and 2; Supplementary Table 1).
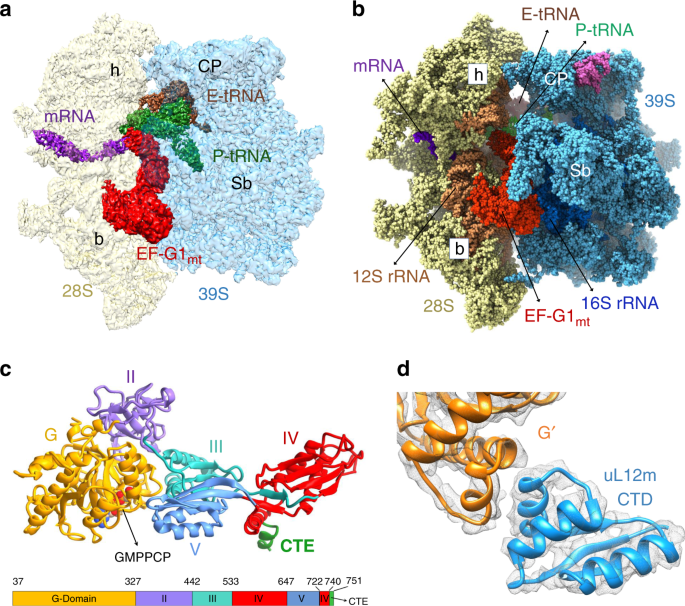
All three 55S mitoribosome maps show well-defined densities for EF-G1mt, but reveal significant differences in the relative orientation of the 28S subunit with respect to the 39S subunit.
The cryo-EM structure with the 28S subunit in its fully rotated state8 is referred to as Class I, the structure with the 28S subunit in its unrotated canonical state2,4 is referred to as
Class III (Fig. 1a, b), and the structure with the 28S subunit an intermediate state between the Class I and Class III conformations is referred to as Class II. Each of these structures
shows variable densities for tRNAsmt in the mitoribosomal peptidyl (P) and exit (E) sites (Fig. 1a, b; and Supplementary Notes). The overall conformation of EF-G1mt on the 55S mitoribosome
in all three maps is analogous to the structure of EF-G in the cytoplasmic ribosomal complexes, where the factor has been trapped either with the help of the antibiotic fusidic acid
(FA)27,31,32,35 or by using a non-hydrolysable GTP analog29,33,34,36,37. In addition to determining the structure of complete EF-G1mt with all of its 715 aa residues that fold into five
globular domains (Fig. 1c), high-resolution features in our cryo-EM maps (Supplementary Fig. 3) allow us to model 75 rRNA residues and 1,082 aa residues that are absent in the currently
available human mitoribosome structure2. Furthermore, we identify species-specific structural differences among mammalian MRPs. The EF-G1mt binding stabilizes the flexible C-terminal domain
(CTD) of the uL10-L12 stalk29 and enables modeling of one copy of the uL12m CTD that interacts with the G′ subdomain of EF-G1mt (Fig. 1d). EF-G1mt induced movement also brings the N-terminal
domain (NTD) of uL11m by 5 Å closer to the uL12m-CTD (Supplementary Fig. 4c), thereby enabling the latter to simultaneously interact with the uL11m and the G′ subdomain to form an arc-like
structure27,29. A direct interaction between uL12m CTD and EF-G1mt suggests uL12m’s role in factor recruitment40,41 during mitochondrial translation. INTERACTIONS OF EF-G1MT WITH THE
GTPASE-ACTIVATING CENTER OF THE MITORIBOSOME Translocation of tRNAs and mRNA is an intrinsic property of the ribosome but binding of EF-G·GTP and subsequent hydrolysis of GTP on EF-G
enhances the rate of translocation by several orders of magnitude42,43. Using our higher resolution map (Complex III), a complete de novo model of the nucleotide-binding pocket and the
interactions of switch I with other domains of EF-G1mt and the adjacent ribosomal components could be constructed. The bound GMPPCP is held in position through a large network of hydrogen
bonds and van der Waals interactions with several highly conserved EF-G1mt residues, notably D56 and K59 from the P-loop, T101 from the switch I region, and H124 from the switch II region
(Fig. 2a). A crucial Mg2+ ion positioned close to γ phosphate of GMPPCP is coordinated by T60 from the P-loop and T101 from the switch I region (Fig. 2b). H124 is known to be essential for
catalyzing the hydrolysis of GTP, as mutation of this crucial residue in bacterial EF-Tu severely inhibits its ribosome-stimulated GTP hydrolysis44,45. In our maps, the catalytic H124 is
oriented towards the γ phosphate of the bound GMPPCP molecule (Fig. 2c), representing an active nucleotide-binding pocket34,36 while its analog is pointed in the opposite direction in a
GDP-state bacterial post-translocation complex (Supplementary Fig. 5)35. The positioning of H124 is stabilized by the universally conserved sarcin-ricin loop (SRL) of the 16S rRNA segment
from the 39S LSU, which plays a central role in activating the translational GTPases34,35,36. Binding of G domain adjacent to the SRL closes the nucleotide-binding pocket of EF-G1mt and the
base A3129 from the SRL is responsible for stabilizing the current activated conformation of H124 through hydrogen-bonding interactions (Fig. 2c, d). Bases A3130 and G3131 from the SRL are
found coordinating a uniquely placed Mg2+ ion which in turn is known to stabilize the P-loop D56 in an active conformation (Fig. 2c, d). As observed in bacteria34,36, the binding of EF-G1mt
to the 55S mitoribosome stabilizes the factor in an active conformation necessary to catalyze the hydrolysis reaction, by ordering the switch I region and positioning of D56 and H124 towards
the active site for GTP hydrolysis. INTERACTIONS OF DOMAIN IV OF EF-G1MT IN THE A SITE OF THE 28S SUBUNIT IN THREE CONFORMATIONAL STATES In all three 55S·EF-G1mt complexes, the EF-G1mt is
held in position by interacting with several components of both the large and small mitoribosomal subunits but domain IV of EF-G1mt and mitoribosomal components interact differently among
these complexes. In the Class I 55S·EF-G1mt complex, the 28S subunit has undergone a ~9.5° counter-clockwise rotation relative to the 39S subunit (Fig. 3a), similar to previously described
ratchet-like inter-subunit reorganization of the bacterial ribosome30,46. In addition to this ratchet-like motion, significant head swiveling32,47 was also observed in the Class I complex,
where the 28S subunit head region has rotated ~3° relative to the body in a roughly orthogonal direction to the ratchet-like motion (Fig. 3a). The Class II complex presents a previously
unknown EF-G-bound conformational intermediate, where the head region of the 28S subunit has swiveled as in the ratcheted Class I complex (Fig. 3b), while the conformation of the 28S body
region is similar to that in the unrotated Class III complex (Fig. 3c). The presence of a Class I complex-like conformational state with ratcheted and head rotated SSU in a previous EF-G1mt
-unbound map2 but the absence of such a conformation in our control maps (Supplementary Fig. 6) suggest that the Class I complex is formed either upon binding of EF-G1mt to a subpopulation
of mitoribosomes that carries only a single tRNA at the E-site2,48 (Fig. 3d), or to a population that carries loosely bound P-site tRNAs that are all translocated to the E-site. Overall, we
find that EF-G1mt binding brings a greater proportion of mitoribosomes into unratcheted state, when compared with the distribution in our control population (Supplementary Fig. 6). The Class
II complex represents the smallest of the three populations and shows a strong density for the E-site tRNA but a somewhat fragmented tRNA density at the P site (Fig. 3e), whereas the Class
III complex shows densities for both P- and E-site tRNAs (Figs. 1a, 3f). Domain IV of the bacterial EF-G is known to play a crucial role in tRNA and mRNA translocation27,49. In all three
Classes, Domain IV of the EF-G1mt is inserted into the 28S subunit decoding center such that it would sterically overlap with the anticodon arm of an A-site tRNA (Fig. 3g–i), as found in
case of analogous bacterial complex27. Minimum mitoribosomal interactions with domain IV occur in Class I complex, an intermediate number of interactions occur in Class II complex, and the
maximum interactions occur in Class III complex (Fig. 3g–i). In the Class III complex, Domain IV makes contacts with multiple 12S rRNA components of the 28S subunit such as helix 24 (h24),
h30, h44, and the anticodon arm of the tRNA bound in the P/P state (Fig. 3i). [We have adopted the bacterial numbering to refer to rRNA helices throughout, as they are identified by a number
prefixed with an ‘h’ for the mitochondrial 12S rRNA in SSU and an ‘H’ for its 16S rRNA in LSU. The rRNA nucleotide numbering are according to Amunts and coworkers2.] In the 28S P-site
region, aa residues S543, G544 and G545 from the loop1 region of domain IV interact with the backbone phosphates of h44 bases C1561 and G1562, while base A1078 from h24 is placed within
hydrogen-bond forming distance from G544 and G545 of domain IV (Fig. 3i). The Class II and Class III maps show density for a P-site tRNA (Fig. 3e, f) bound in the classical P/P state4,5.
However, the P-site density is relatively weak, because it represents an averaged density of the endogenously bound multi-sized tRNAsmt, some of which are known to have much smaller T-loops
compared to their bacterial counterparts50,51. Nevertheless, conserved segments such as anticodon and CCA arm of tRNA could be docked into corresponding densities. Accordingly, nucleotides
33 and 34 from the anticodon of the P-site tRNA are positioned within 3 Å of residues M618 and V619 (Fig. 3i) from the loop3 region of domain IV. In the 28S head region of Class III complex,
the backbone phosphates of the 12S rRNA bases U1209 and U1210 interact with aa residues S576 and N577 from the loop2 region of domain IV (Fig. 3i). The size variability in tRNAsmt also
affect the density corresponding to the anticodon region of the E-site tRNAmt (Supplementary Notes and Supplementary Fig. 7). Simultaneous interactions of domain IV with both the head and
shoulder regions of the 28S subunit and the anticodon region of the tRNA in Complex III would stabilize the tRNA in the P site and prevent the anticodon end of translocated P-site tRNA from
slipping back to the A site, as also suggested by structural studies on bacterial translocation31,32,35,52. The small subunit of a bacterial 70S ribosome is also found in an unrotated
conformation with similar interactions in the crystallographic structure of the 70S·EF-G·GDP·FA post-translocational complex35, suggesting that our Class III 55S·EF-G1mt complex represents
an authentic post-translocation state of the human mitoribosome. The core 12S SSU rRNA regions of the mitochondrial and bacterial ribosomes that are known to interact with A- and P-site
tRNAs in eubacteria are generally conserved5,53, and the relative orientations of bound A- and P-site tRNAs are also similar (Supplementary Fig. 8), despite the presence of a significantly
altered and MRP-enriched environment around the tRNA binding sites in the LSU of the mitoribosome as elaborated under the next heading. The tip of EF-G1mt domain IV in the Class I complex is
positioned ~10 Å away from 12S rRNA helices h24 and h30, closer to the 28S shoulder or the A site than its position in the Class III complex (Fig. 3g, also see Supplementary Fig. 9). Domain
IV does not interact with the 28S head region in Class I complex, and the only 28S subunit element that still interacts with domain IV is the 12S rRNA h44 (Fig. 3g). This is not surprising
since simultaneous interactions of domain IV with both the head and platform components would impede the head rotation, and a combination of subunit ratcheting and head swiveling help
translocate the A- and P-site tRNAs into the P and E sites, respectively31,32,47. Unlike the Class III complex, the Class I map does not have enough density to model a P-site tRNA but
superimposing the Class III P-site tRNA density onto the Class I complex suggests that the interactions of domain IV with anticodon of the P-site tRNA in the P/P state would not be
established in the Class I complex (Fig. 3g). Interestingly, these contacts can be restored if the anticodon end of the P-site tRNA is moved by 6–7 Å towards the A site of the 28S subunit
(Fig. 3g), which indicates that the Class I 55S·EF-G1mt complex represents a key early translocation intermediate where the domain IV has moved only partially into the A site following the
movement of A-site tRNA into an intermediate chimeric ap/P state31, preceding the Class II state (Fig. 3h) that is followed by the Class III state (Fig. 3i). Domain IV of EF-G1mt
synchronizes the ratcheting motion of the ribosome along with the movement of tRNAs, as it appears to closely follow the anticodon arm of the A-site tRNA during its translocation into the P
site31,32,35,52. ROLE OF P-SITE FINGER AND OTHER MRPS THAT DIRECTLY INTERACT WITH TRNASMT AND EF-G1MT One of the major structural differences between the bacterial and mitochondrial
ribosomes is the loss of several rRNA segments in mitochondria that are partially compensated by the acquisition of new MRPs and extensions in homologous MRPs5,54,55,56. This also changes
the compositional landscape of the ribosomal intersubunit space that provides the corridor for the mRNA and tRNA movement on the mammalian mitoribosome during translation elongation. In
mammalian mitochondria, protein bL5 is lost from the P site while bL25 and the A-site finger (23S rRNA helix 38) are lost from the A site2,4,5. The loss of these structural elements that
interact with bound tRNA molecules is compensated by a unique finger-like structural element called the P-site finger (PSF) that interacts with both the A- and P-site bound tRNAs4,5. In the
Class III complex, the PSF is found interacting with both the T-loop and the D-loop of P-site tRNA (Fig. 4a). The role of PSF appears to be to correctly position the A- and P-site tRNAs and
prevent the elbow region of the P-site bound tRNA from reverting back to the A site during and after its translocation from the A to the P site. In comparison to empty 55S mitoribosomes2,4,
the PSF has undergone a significant conformational change and moved closer towards the P-site bound tRNA in all our EF-G1mt-bound complexes (Fig. 4b) as well as in the mammalian
mitochondrial initiation complex9. Tight interactions with the PSF could be one of the reasons for the frequent co-purification of mitoribosomes with a P-site bound tRNAmt5. In our maps, we
found a previously unassigned tubular density in the region between the C-terminus of mito-specific protein mL64 and the PSF8. This extra density is readily attributable to an
α-helix-forming 32 aa residues of the C-terminus of mL64 (Fig. 4c), which extends in the 39S subunit between the P- and E-site tRNAs while interacting with the T-loop regions of both the
tRNAs (Fig. 4a). Along with the mito-specific protein mL48 and the mito-specific segments of MRPs uL11m and uL16m, PSF and the C-terminus of mL64 span all three tRNA binding sites on the 39S
subunit (Fig. 4a), structurally compensating for the absence of some of the bacterial homologs of r-proteins and rRNA components that are known to be involved in tRNA positioning,
stabilization and translocation in the bacterial ribosome. In all three EF-G1mt-bound complexes the uL11m stalk-base region within the mitoribosomal LSU moves by 5 Å towards the domain V of
EF-G1mt (Supplementary Fig. 4a, b), as compared to that in the empty 55S mitoribosomes2,4, in the initiation9 and in mitoribosome recycling complexes8. A similar movement of the uL11m region
was reported for the bacterial 70S·EF-G complexes29. However, in the mammalian mitoribosome the conformational change is associated with a direct contact between the domain IV of EF-G1mt
and the mito-specific segment of uL11m (Fig. 4d). K192 from the mito-specific C-terminus α-helix of uL11m interacts with the E562 from the domain IV of EF-G1mt through a hydrogen-bond
interaction (Fig. 4d). Interestingly, the uniquely placed E562 is absent in EF-G2mt (Supplementary Fig. 10). The presence of E562 and the mito-specific CTE in EF-G1mt and their absence in
EF-G2mt, along with presence of four small insertion segments within corresponding domains II and III of EF-G2mt (Supplementary Fig. 10), appear to be the key differences that confer
specificity to these two factors for their roles in elongation and recycling steps, respectively. ROLE OF THE C-TERMINAL EXTENSION IN EF-G1MT Both the Class I and Class III complexes show an
additional density adjacent to the conserved C-terminal end of the EF-G1mt domain IV that could readily accommodate its mito-specific C-terminal extension (CTE) (Fig. 5a), which is not
resolved in the Class II complex. The lysine-rich CTE folds into an α-helix and extends into the 39S subunit enabling the EF-G1mt to interact with rRNA and tRNAmt segments that would be
inaccessible to the bacterial EF-Gs. The CTE is positioned close to the nucleotides U2606-G2608 segment of the 16S LSU rRNA helix 71 (H71) (Fig. 5a). In its current orientation, the CTE
would overlap with the inner bend of A-site tRNAmt elbow primarily involving tRNAmt’s CCA arm (Fig. 5b), suggesting that the CTE plays a direct role in the movement of the CCA arm of the
A-site bound tRNAmt. The interaction of lysine-rich CTE with H71 would also prevent the reverse translocation of the P-site tRNA to the A site. The fact that EF-G1mt remains active on the
_E. coli_ ribosomes, but _E. coli_ EF-G remains inactive on mitoribosomes57, suggests that the observed interaction of EF-G1mt’s CTE with the mitoribosome and the A-site tRNAmt in our
structure could also be associated with EF-G1mt’s GTPase activity on the ribosome. A significantly altered landscape of the mitoribosomal intersubunit space described in the previous section
and the location of EF-G1mt’s CTE on the mitoribosome suggest that the MRPs and translational factors have coevolved with its unique tRNAs\({}_{{\mathrm{mt}}}\) to structurally and
functionally compensate for the lost bacterial RNA segments. CONFORMATIONAL CHANGES AT THE NASCENT POLYPEPTIDE-EXIT SITE The newly synthesized protein chain exits the ribosome through a
tunnel-like feature in the large subunit5,58,59 known as the nascent polypeptide-exit tunnel (NPET). The NPET originates from the peptidyltransferase center (PTC) and ends on the opposite
side at the solvent interface, which is referred to as the polypeptide-exit site (PES). The structural composition of this tunnel is substantially different between the bacterial and the
mammalian mitochondrial ribosomes2,4,5. Domains I and III of the 23S rRNA that line the bottom portions of NPET in bacteria are greatly reduced in the analogous mitochondrial 16S LSU
rRNA5,53. The loss of these important structural components surrounding the tunnel is compensated through the acquisition of larger bacterial r-protein homologs with extended N and C
termini5,54,56. A mito-specific protein mL45 is also present near the PES2,4. During the initiation phase, the entire NPET is blocked by the insertion of the N-terminus (NT) residues 38–64
of mL45 into the NPET9. The N-terminal region of mL45 also interacts with MRPs uL23m and uL24m near PES. Mutational studies have shown that deletion of the mL45 NT severely inhibits
mitochondrial translation9. Though the blocked NPET might not pose any problem for an initiating mitoribosome, a vacant tunnel would be necessary to accommodate the growing nascent
polypeptide chain during the translation elongation phase. In all our complexes, we found an unassigned density that is connected to the CCA end of the P-site tRNA and reaches close to the
NT of the ribosomal protein mL45 inside the NPET (Fig. 6a). This density that could accommodate up to 5 aa residues can be readily attributed to a nascent peptide chain (NPC). In our
structure, a conserved adenine residue (A2725) from a loop region between the 16S LSU rRNA helices H73 and H74 intercalates between the NT of NPC and aa R40 from the NT of mL45 (Fig. 6a). By
simultaneously interacting with the NPC and NT of mL45, A2725 might play a crucial role in triggering a conformational change in the large mitoribosomal subunit that eventually results in
the retraction of NT of mL45 from the NPET to make room for the growing NPC. Indeed, we observed a significant conformational change involving aa residues R61 to D73 (Fig. 6b). Compared to
their position in the initiation complex9, these residues have moved substantially, ~9 Å away from the tunnel and toward uL24m, which also shifts in conjunction with the mL45 movement. The
EF-G1mt-induced conformational change in the large subunit captured in our structure likely represents a functional state, as mL45 prepares to retrieve its NT from the NPET to allow the
insertion of incoming nascent polypeptide from the PTC side of the NPET. In addition, the residues T101-Y128 located in the core region of mL45 undergo a large conformational change (Fig.
6c) between the initiation9 and our elongation complexes. In the initiation complex, these residues form two separate α-helices with an angle of ~120° between them. Of these, residues
T109–T115 from the N-terminal helix rotate by ~60° to become part of a single long α-helix in the elongation complex, leaving residues S101-R108 in an open conformation. This large
conformational change, involving a secondary structure rearrangement, may be necessary for anchoring of the mammalian mitoribosome to the inner mitochondrial membrane (IMM). mL45 happens to
be the homolog of Mba1, the IMM-associated receptor necessary for the co-translational insertion of nascent polypeptides into the IMM in yeast60. Interestingly, this positively charged
segment (aa residues 101–114) of mL45 has been implicated in mediating the association of 55S mitoribosomes with the negatively charged lipid content of the IMM through charge-based
interactions61, which could be accompanied by the observed conformational change in this study. Furthermore, the C-terminal region of the uL23m that interacts with the mL45 NT has undergone
a large conformational change where its α-helix involving aa residues A123-R137 has moved by ~20 Å toward the mL45 NTE. It appears that this α-helix movement is necessary to clear the path
for the displacement of mL45’s NTE from the exit tunnel (Supplementary Fig. 11a). However, a comparison of the sequences of uL23m in human with other mammals (Supplementary Fig. 11b)
suggests that this major conformational difference could be species-specific. In summary, our study presents the most complete structure for the human 55S mitoribosome, and shows that the
EF-G1mt-bound mitoribosome can adopt at least three different conformations irrespective of the GTP hydrolysis state. The major variation occurs in the relative orientation of its entire 28S
subunit, or only its head domain, suggesting an unusual adaptability of the 28S subunit during translocation (Fig. 3). Direct structural evidence is presented that the mito-specific
components in both the mitoribosome and EF-G1mt are involved in tRNAmt translocation. Our study also shows how mito-specific ribosomal proteins, such as PSF and mL64 in the mitoribosome’s
tRNAmt interaction sites (Fig. 4), and the addition of a mere 11 aa residues in the C-terminus of EF-G1mt (Fig. 5), allow the mitochondrial translation system to adapt to a massive reduction
in mitoribosomal RNA components as compared to their bacterial counterparts. For example, the absence of 23S rRNA helix 38, also known as the A-site finger that dynamically interacts with
both A- and P-site tRNAs during the tRNA translocation in eubacteria, is structurally and functionally compensated by the PSF protein in the mammalian mitoribosome. Similarly, the missing
eubacterial E-site tRNA interacting rRNA components53 are replaced by protein mL64. Finally, the large conformational changes between the initiation and elongation states involving
mito-specific protein mL45 in the NPET’s exit site (Fig. 6), seem to be associated with the mitoribosomal anchoring to the IMM. METHODS ISOLATION OF MITOCHONDRIA FROM HEK CELLS Mitochondria
were isolated from human embryonic kidney cells lacking _N_-acetyl-glucosaminyltransferase I (HEK293S GnTI)8 that were cultured in roller bottles using FreeStyleTM293 media (Gibco, Life
Technologies) supplemented with 5% fetal bovine serum (Gibco, Life Technologies). After centrifugation at 1000 × _g_ for 7 min, the HEK293S GnTI cell-pellet was transferred to a glass
homogenizer and resuspended in buffer containing 50 mM HEPES-KOH pH 7.5, 10 mM KCl, 1.5 mM MgOAc, 70 mM sucrose, 210 mM mannitol, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF. After
homogenization, the supernatant was separated from the cell debris by spinning at 950 × _g_ for 15 min. The supernatant was then spun at 11,000 × _g_ for 15 min, and the resulting pellet
that contains crude mitochondria was resuspended in SEM buffer (250 mM sucrose, 20 mM HEPES-KOH pH 7.5, 1 mM EDTA, and 1 mM EGTA). DNase I (3 units/ml) was added to the crude mitochondria
and incubated at 4 °C for 1 h. A discontinuous gradient was prepared in a Beckman polyallomer tube by layering 2.5 ml of 60%, 4 ml of 32%, 1 ml of 23%, and 1 ml of 15% sucrose solutions in
buffer containing 10 mM HEPES-KOH pH 7.5 and 1 mM EDTA. DNase-treated sample was loaded on the discontinuous gradient and centrifuged for 1 h at 135,000 × _g_ using Ti70 rotor in a Beckman
ultracentrifuge. The brownish-orange layer containing pure mitochondria was carefully separated and re-suspended in SEM buffer. ISOLATION OF MITORIBOSOMES FROM MITOCHONDRIA Mitoribosomes
were isolated8 by adding four volumes of lysis buffer (25 mM HEPES-KOH pH 7.5, 100 mM KCl, 25 mM MgOAc, 1.7% Triton X-100, 2 mM DTT and 1 mM PMSF) to the mitochondrial-pellet and then
incubating for 15 min at 4 °C. The sample was centrifuged at 30,000 × _g_ for 20 min and the supernatant was loaded on top of 1 M sucrose cushion in buffer (20 mM HEPES-KOH pH 7.5, 100 mM
KCl, 20 mM MgOAc, 1% Triton X-100, and 2 mM DTT). After centrifugation for 17 h at 90,000 × _g_ using Ti70 rotor in Beckman ultracentrifuge, a minimal volume of Mitobuffer (20 mM HEPES-KOH
pH 7.5, 100 mM KCl, 20 mM MgOAc, and 2 mM DTT) enough to dissolve the pellet was added. 10–30% continuous sucrose density gradients were prepared in Mitobuffer, using the gradient making
apparatus (C.B.S. Scientific Co.). The resuspended pellet was subjected to 10–30% continuous sucrose density gradient centrifugation at 60,000 × _g_ for 17 h using Sw32 rotor in Beckman
ultracentrifuge. The gradient was fractionated on ISCO gradient analyzer (Teledyne ISCO, Inc), and the fractions corresponding to the mitoribosomes were collected and pooled. Finally, the
pooled mitoribosomes were concentrated by spinning them at 130,000 × _g_ for 6 h using Ti70 rotor, and the pellet was resuspended in Polymix buffer (5 mM HEPES-KOH pH 7.5, 100 mM KCl, 20 mM
MgOAc, 5 mM NH4Cl, 0.5 mM CaCl2, 1 mM DTT, 1 mM spermidine, and 8 mM putrescine)62. CLONING AND EXPRESSION OF HUMAN EF-G1MT An expressed sequence tag coding for human EF-G1mt was obtained
from GeneCopoeia (No. GC-W1058). Using PCR, the sequence corresponding to the mature form of EF-G1mt (amino acids 36–751) was amplified by employing forward
5′-GGAATTCCATATGTCTTCATCAGGGGTGATTCC-3′ and reverse 5′-AACCGCTCGAGTTCTTGGCTTTTCCTTTTTTAAC-3′ primers39. The PCR product was cloned into pET 21c (+) (Novagen) and this vector provides a
sequence encoding six His residues (His-tag) at the C-terminus. The resulting construct was transformed into _E. coli_ ER2267 and subsequently transformed into _E. coli_ BL21(DE3) (RIL) for
over-expression. PURIFICATION OF HUMAN EF-G1MT The cultures were grown to mid-log phase and induced with 50 μM isopropyl-1-thio-d-galactopyranoside (IPTG). After centrifugation at 5,000 rpm
for 15 min at 4 °C, the cells were harvested, shock-frozen, and stored at −80 °C. The frozen cells were disrupted by grinding with double the cell weight of Alumina Type A-5 (Sigma) for a
total of 20 min. The paste was resuspended in Buffer B (50 mM Tris–HCl, pH 7.6, 40 mM KCl, 7 mM MgCl2, 7 mM β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, and 10% glycerol), and
the debris was removed by centrifugation at 10,000 rpm at 4 °C for 10 min. This is followed by DNase I (5 μg/mL) treatment and centrifugation at 15,000 rpm at 4 °C for 20 min. The resulting
supernatant was mixed with 0.6 mL of a 50% slurry of Ni–NTA resin equilibrated in Buffer B and relatively pure EF-G1mt was obtained using affinity chromatography39. In order to achieve
high-level purity, ion exchange chromatography technique was employed where the partially purified EF-G1mt from the Ni–NTA purification was processed on a cation exchange TSKgel SP-5PW
column (TosoHaas, Japan). PREPARATION OF THE HUMAN MITORIBOSOME•EF-G1MT•GMPPCP COMPLEX To obtain the 55S·EF-G1mt complex, a non-hydrolysable analog of GTP, GMPPCP, was used to lock EF-G1mt
on the mitoribosomes. The complex was formed by incubating 150 nM 55S mitoribosomes with 5 μM EF-G1mt and 1 mM GMPPCP (Sigma-Aldrich, USA) at 37 °C for 5 min in the HEPES polymix buffer.
CRYO-ELECTRON MICROSCOPY AND IMAGE PROCESSING In all, 4 μl of the 55S·EF-G1mt•GMPPCP complex was applied to Quantifoil holey copper 1.2/1.3 grids that were pre-coated with a thin layer (~50
Å thick) of home-made continuous carbon film and glow-discharged for 30 s on a plasma sterilizer. After incubating the grids for 15 s at 4 °C and 100% humidity, they were blotted for 4 s and
immediately flash-frozen into the liquid ethane with the help of a Vitrobot (FEI company). Data were collected on a Titan Krios electron microscope (FEI company) equipped with a Gatan K2
summit direct-electron detecting camera at 300 kV. We used a defocus range of −1.0 to −3.0 µm at a calibrated magnification of ×105,000, yielding a pixel size of 1.0961 Å. A dose rate of 7
electrons per pixel per s and an exposure time of 10 s resulted in a total dose of 69.2 e/Å2. All the downstream processing of data was done using CryoSPARC63. Full-frame motion correction
was applied to all 50 movie frames corresponding to each of the 6,671 micrographs. After determining their contrast transfer function (CTF) using CTFFIND464, bad images were deselected. From
the remaining 6649 micrographs, 1,611,0847 particles were picked using the auto-pick function, and after local motion correction, 1,262,274 particles remained. This step was followed by the
reference-free 2D classification and finally 851,131 good particles were retained based on the 2D averages. The initial 3D reconstruction and refinement yielded 2.74 Å resolution 55S map
(Supplementary Fig. 1), with its 39S subunit showing an overall resolution of 2.68 Å (Supplementary Fig. 2), and the local resolution in the core regions of the 39S subunit were found to be
in the 2.5–2.6 Å range (Supplementary Fig. 2). However, the 28S subunit was relatively poorly resolved. Reference-based 3D classification was employed to separate 55S mitoribosomes (289,982
particles) from 39S subunits (408,686 particles) and 28S subunits (152,463 particles). Particles corresponding to the 55S mitoribosomes were further subjected to multiple rounds 3D
classification that yielded three stable classes, Class I (99,804 particles), Class II (25,755 particles), and Class III (150,347 particles), and allowed us to remove a small population of
14,076 bad particles. After 3D refinement Class I, Class II and Class III yielded a final resolution of 2.97 Å, 3.96 Å, and 2.96 Å, respectively, and all of the three classes showed strong
densities that could be readily attributed to a bound EF-G1mt. After masked local refinements, the 28S and 39S subunits from the Class I complex were refined to 3.15 Å and 2.91 Å,
respectively; the 28S and 39S subunits from the Class II complex were refined to 4.82 Å and 3.77 Å, respectively; and the 28S and 39S subunits from the Class III complex were refined to 3.04
Å and 2.87 Å, respectively (Supplementary Fig. 2). The Gold-standard criterion of 0.143 FSC cutoff65 was used to report all resolutions. MODEL BUILDING AND OPTIMIZATION Coordinates
corresponding to the small and large subunits of the published human mitoribosome (PDB ID: 3J9M)2 were used as the initial template. The higher resolution of our maps (Supplementary Fig. 3)
enabled us to build multiple protein and rRNA segments that were not present in the previous human mitoribosome structures2,8,66. Highly resolved secondary structural elements (SSE) and
amino acid side-chain features guided the manual building of the majority of protein models using UCSF Chimera 1.1467 and COOT68. For modeling the relatively low-resolved regions such as the
L7/L12 stalk proteins and the C terminal domain (CTD) of L12 from the large subunit and protein mS39 from the small subunit, homologous structures from the porcine mitoribosome9 were used
as a template. Additional segments (75 rRNA nts and 1082 aa residues) that were absent in the previous human mitoribosome structures were modeled de novo. These new rRNA segments were built
in ModeRNA server69, using corresponding segments wherever available from the porcine mitoribosome9 as a template. For building the P- and E-site tRNAs, the high-resolution structure of
yeast tRNAPhe (PDB ID: 1EhZ)70 was used as template to generate tRNAmtPhe. The tRNAmtPhe was docked manually and rigid body fitted into the corresponding cryo-EM density using Chimera
1.1467. Owing to sub-optimal occupancies and inherent heterogeneity within the endogenously bound tRNAsmt, the resolution corresponding to the tRNAmt densities were relatively low to allow
any sequence-specific modeling of the P- and E-site tRNAsmt. Homology models of EF-G1mt generated in I-TASSER71 were used as the initial template. Regions in the homology model that do not
fully accommodate into the corresponding EF-G1mt density were modeled de novo using Chimera 1.1467 and COOT68. Lower resolution in our cryo-EM maps corresponding to the density of EF-G1mt C
terminal extension (CTE) restricted modeling of this region at the side-chain level but permitted building the carbon backbone guided by recognizable SSEs. For the final optimization of the
models into the cryo-EM densities, we used the “Real-space refinement” function in PHENIX72. The models were validated using Molprobity server73, and the overall statistics of EM
reconstruction and molecular modeling are listed in Supplementary Table 1. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary
linked to this article. DATA AVAILABILITY The data that support this study are available from the corresponding authors upon reasonable request. The cryo-EM maps and atomic coordinates have
been deposited in the Electron Microscopy and PDB Data Bank (wwPDB.org) under accession codes EMD-21233 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-21233] and PDB 6VLZ
[https://doi.org/10.2210/pdb6VLZ/pdb] for the EF-G1mt-bound 55S mitoribosome (Complex I), and EMD-21242 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-21242] and PDB 6VMI
[https://doi.org/10.2210/pdb6VMI/pdb] for the EF-G1mt-bound 55S mitoribosome (Complex III). Cryo-EM maps of the Complex II and bovine 55S mitoribosome have been deposited with accession
codes EMD-22212 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-22212] and EMD-22209 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-22209], respectively. All raw micrographs and particle images used
in 3D reconstructions will be made available through empiar, an electron microscopy public image archive, https://www.ebi.ac.uk/pdbe/emdb/empiar/. REFERENCES * Gray, M. W., Burger, G. &
Lang, B. F. The origin and early evolution of mitochondria. _Genome Biol._ 2, reviews1018.1–reviews1018.5 (2001). Google Scholar * Amunts, A., Brown, A., Toots, J., Scheres, S. H. W. &
Ramakrishnan, V. Ribosome. The structure of the human mitochondrial ribosome. _Science_ 348, 95–98 (2015). ADS CAS PubMed PubMed Central Google Scholar * Desai, N., Brown, A., Amunts,
A. & Ramakrishnan, V. The structure of the yeast mitochondrial ribosome. _Science_ 355, 528–531 (2017). ADS CAS PubMed PubMed Central Google Scholar * Greber, B. J. et al. Ribosome.
The complete structure of the 55S mammalian mitochondrial ribosome. _Science_ 348, 303–308 (2015). ADS CAS PubMed Google Scholar * Sharma, M. R. et al. Structure of the mammalian
mitochondrial ribosome reveals an expanded functional role for its component proteins. _Cell_ 115, 97–108 (2003). CAS PubMed Google Scholar * Florent Waltz, H. S., Bochler, A., Giegé, P.,
Hashem, Y. Cryo-EM structure of the RNA-rich plant mitochondrial ribosome. _bioRxiv_, https://doi.org/10.1101/777342 (2019). * Ramrath, D. J. F. et al. Evolutionary shift toward
protein-based architecture in trypanosomal mitochondrial ribosomes. _Science_ 362, eaau7735 (2018). * Koripella, R. K., Sharma, M. R., Risteff, P., Keshavan, P. & Agrawal, R. K.
Structural insights into unique features of the human mitochondrial ribosome recycling. _Proc. Natl Acad. Sci. USA_ 116, 8283–8288 (2019). CAS PubMed Google Scholar * Kummer, E. et al.
Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. _Nature_ 560, 263–267 (2018). ADS CAS PubMed Google Scholar * Christian, B. E. & Spremulli, L.
L. Mechanism of protein biosynthesis in mammalian mitochondria. _Biochim. Biophys. Acta_. 1819, 1035–1054 (2012). CAS PubMed Google Scholar * Sharma, M. R., Kaushal, P. S., Gupta, M.,
Banavali, N. K. & Agrawal, R. K. In _Translation in_ Mito_chondria and Other Organelles_ (ed. Duchêne A.-M.) Ch. 1, 1–28 (Springer, 2013). * Bhargava, K. & Spremulli, L. L. Role of
the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3. _Nucleic Acids Res._ 33, 7011–7018 (2005). CAS PubMed PubMed Central Google
Scholar * Gaur, R. et al. A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. _Mol. Cell_ 29, 180–190 (2008). CAS PubMed PubMed
Central Google Scholar * Haque, M. E. & Spremulli, L. L. Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis. _J. Mol. Biol._
384, 929–940 (2008). CAS PubMed PubMed Central Google Scholar * Rorbach, J. et al. The human mitochondrial ribosome recycling factor is essential for cell viability. _Nucleic Acids Res._
36, 5787–5799 (2008). CAS PubMed PubMed Central Google Scholar * Koripella, R. K. et al. Structure of human mitochondrial translation initiation factor 3 bound to the small ribosomal
subunit. _iScience_ 12, 76–86 (2019). ADS CAS PubMed PubMed Central Google Scholar * Yassin, A. S. et al. Insertion domain within mammalian mitochondrial translation initiation factor 2
serves the role of eubacterial initiation factor 1. _Proc. Natl Acad. Sci. USA_ 108, 3918–3923 (2011). ADS CAS PubMed Google Scholar * Hammarsund, M. et al. Identification and
characterization of two novel human mitochondrial elongation factor genes, hEFG2 and hEFG1, phylogenetically conserved through evolution. _Hum. Genet._ 109, 542–550 (2001). CAS PubMed
Google Scholar * Tsuboi, M. et al. EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. _Mol. Cell_ 35, 502–510 (2009). CAS PubMed Google Scholar *
Antonicka, H., Sasarman, F., Kennaway, N. G. & Shoubridge, E. A. The molecular basis for tissue specificity of the oxidative phosphorylation deficiencies in patients with mutations in
the mitochondrial translation factor EFG1. _Hum. Mol. Genet._ 15, 1835–1846 (2006). CAS PubMed Google Scholar * Coenen, M. J. et al. Cytochrome c oxidase biogenesis in a patient with a
mutation in COX10 gene. _Ann. Neurol._ 56, 560–564 (2004). CAS PubMed Google Scholar * Abbott, J. A., Francklyn, C. S. & Robey-Bond, S. M. Transfer, RNA. and human disease. _Front.
Genet._ 5, 158 (2014). PubMed PubMed Central Google Scholar * Bugiardini, E. et al. MRPS25 mutations impair mitochondrial translation and cause encephalomyopathy. _Hum. Mol. Genet._ 28,
2711–2719 (2019). CAS PubMed PubMed Central Google Scholar * Pearce, S., Nezich, C. L. & Spinazzola, A. Mitochondrial diseases: translation matters. _Mol. Cell. Neurosci._ 55, 1–12
(2013). CAS PubMed Google Scholar * AEvarsson, A. et al. Three-dimensional structure of the ribosomal translocase: elongation factor G from Thermus thermophilus. _EMBO J._ 13, 3669–3677
(1994). CAS PubMed PubMed Central Google Scholar * Czworkowski, J., Wang, J., Steitz, T. A. & Moore, P. B. The crystal structure of elongation factor G complexed with GDP, at 2.7 A
resolution. _EMBO J._ 13, 3661–3668 (1994). CAS PubMed PubMed Central Google Scholar * Agrawal, R. K., Penczek, P., Grassucci, R. A. & Frank, J. Visualization of elongation factor G
on the Escherichia coli 70S ribosome: the mechanism of translocation. _Proc. Natl Acad. Sci. USA_ 95, 6134–6138 (1998). ADS CAS PubMed Google Scholar * Brilot, A. F., Korostelev, A. A.,
Ermolenko, D. N. & Grigorieff, N. Structure of the ribosome with elongation factor G trapped in the pretranslocation state. _Proc. Natl Acad. Sci. USA_ 110, 20994–20999 (2013). ADS CAS
PubMed Google Scholar * Datta, P. P., Sharma, M. R., Qi, L., Frank, J. & Agrawal, R. K. Interaction of the G’ domain of elongation factor G and the C-terminal domain of ribosomal
protein L7/L12 during translocation as revealed by cryo-EM. _Mol. Cell_ 20, 723–731 (2005). CAS PubMed Google Scholar * Frank, J. & Agrawal, R. K. A ratchet-like inter-subunit
reorganization of the ribosome during translocation. _Nature_ 406, 318–322 (2000). ADS CAS PubMed Google Scholar * Ramrath, D. J. et al. Visualization of two transfer RNAs trapped in
transit during elongation factor G-mediated translocation. _Proc. Natl Acad. Sci. USA_ 110, 20964–20969 (2013). ADS CAS PubMed Google Scholar * Ratje, A. H. et al. Head swivel on the
ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. _Nature_ 468, 713–716 (2010). ADS CAS PubMed PubMed Central Google Scholar * Agrawal, R. K., Heagle, A.
B., Penczek, P., Grassucci, R. A. & Frank, J. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. _Nat. Struct. Biol._ 6,
643–647 (1999). CAS PubMed Google Scholar * Chen, Y., Feng, S., Kumar, V., Ero, R. & Gao, Y. G. Structure of EF-G-ribosome complex in a pretranslocation state. _Nat. Struct. Mol.
Biol._ 20, 1077–1084 (2013). CAS PubMed Google Scholar * Gao, Y. G. et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. _Science_ 326,
694–699 (2009). ADS CAS PubMed PubMed Central Google Scholar * Tourigny, D. S., Fernandez, I. S., Kelley, A. C. & Ramakrishnan, V. Elongation factor G bound to the ribosome in an
intermediate state of translocation. _Science_ 340, 1235490 (2013). PubMed Google Scholar * Zhou, J., Lancaster, L., Donohue, J. P. & Noller, H. F. Crystal structures of EF-G-ribosome
complexes trapped in intermediate states of translocation. _Science_ 340, 1236086 (2013). PubMed PubMed Central Google Scholar * Zhou, J., Lancaster, L., Donohue, J. P. & Noller, H.
F. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. _Science_ 345, 1188–1191 (2014). ADS CAS PubMed PubMed Central Google Scholar * Bhargava,
K., Templeton, P. & Spremulli, L. L. Expression and characterization of isoform 1 of human mitochondrial elongation factor G. _Protein Expr. Purif._ 37, 368–376 (2004). CAS PubMed
Google Scholar * Diaconu, M. et al. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. _Cell_ 121, 991–1004 (2005). CAS PubMed Google
Scholar * Helgstrand, M. et al. The ribosomal stalk binds to translation factors IF2, EF-Tu, EF-G and RF3 via a conserved region of the L12 C-terminal domain. _J. Mol. Biol._ 365, 468–479
(2007). CAS PubMed Google Scholar * Rodnina, M. V., Peske, F., Peng, B. Z., Belardinelli, R. & Wintermeyer, W. Converting GTP hydrolysis into motion: versatile translational
elongation factor G. _Biol. Chem._ 401, 131–142 (2019). PubMed Google Scholar * Rodnina, M. V., Savelsbergh, A., Katunin, V. I. & Wintermeyer, W. Hydrolysis of GTP by elongation factor
G drives tRNA movement on the ribosome. _Nature_ 385, 37–41 (1997). ADS CAS PubMed Google Scholar * Cool, R. H. & Parmeggiani, A. Substitution of histidine-84 and the GTPase
mechanism of elongation factor Tu. _Biochemistry_ 30, 362–366 (1991). CAS PubMed Google Scholar * Daviter, T., Wieden, H. J. & Rodnina, M. V. Essential role of histidine 84 in
elongation factor Tu for the chemical step of GTP hydrolysis on the ribosome. _J. Mol. Biol._ 332, 689–699 (2003). CAS PubMed Google Scholar * Frank, J. & Agrawal, R. K. Ratchet-like
movements between the two ribosomal subunits: their implications in elongation factor recognition and tRNA translocation. _Cold Spring Harb. Symp. Quant. Biol._ 66, 67–75 (2001). CAS PubMed
Google Scholar * Schuwirth, B. S. et al. Structures of the bacterial ribosome at 3.5 A resolution. _Science_ 310, 827–834 (2005). ADS CAS PubMed Google Scholar * Kaushal, P. S.,
Sharma, M. R. & Agrawal, R. K. The 55S mammalian mitochondrial ribosome and its tRNA-exit region. _Biochimie_ 114, 119–126 (2015). CAS PubMed PubMed Central Google Scholar *
Savelsbergh, A., Matassova, N. B., Rodnina, M. V. & Wintermeyer, W. Role of domains 4 and 5 in elongation factor G functions on the ribosome. _J. Mol. Biol._ 300, 951–961 (2000). CAS
PubMed Google Scholar * Hanada, T. et al. Translation ability of mitochondrial tRNAsSer with unusual secondary structures in an in vitro translation system of bovine mitochondria. _Genes
Cells_ 6, 1019–1030 (2001). CAS PubMed Google Scholar * Ohtsuki, T., Kawai, G. & Watanabe, K. The minimal tRNA: unique structure of Ascaris suum mitochondrial tRNA(Ser)(UCU) having a
short T arm and lacking the entire D arm. _FEBS Lett._ 514, 37–43 (2002). CAS PubMed Google Scholar * Pulk, A. & Cate, J. H. Control of ribosomal subunit rotation by elongation factor
G. _Science_ 340, 1235970 (2013). PubMed PubMed Central Google Scholar * Mears, J. A. et al. A structural model for the large subunit of the mammalian mitochondrial ribosome. _J. Mol.
Biol._ 358, 193–212 (2006). CAS PubMed PubMed Central Google Scholar * Koc, E. C. et al. The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of
ribosomal proteins present. _J. Biol. Chem._ 276, 43958–43969 (2001). CAS PubMed Google Scholar * O’Brien, T. W. Evolution of a protein-rich mitochondrial ribosome: implications for human
genetic disease. _Gene_ 286, 73–79 (2002). PubMed Google Scholar * Suzuki, T. et al. Structural compensation for the deficit of rRNA with proteins in the mammalian mitochondrial ribosome.
Systematic analysis of protein components of the large ribosomal subunit from mammalian mitochondria. _J. Biol. Chem._ 276, 21724–21736 (2001). CAS PubMed Google Scholar * Eberly, S. L.,
Locklear, V. & Spremulli, L. L. Bovine mitochondrial ribosomes. Elongation factor specificity. _J. Biol. Chem._ 260, 8721–8725 (1985). CAS PubMed Google Scholar * Ban, N., Nissen,
P., Hansen, J., Moore, P. B. & Steitz, T. A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. _Science_ 289, 905–920 (2000). ADS CAS PubMed Google
Scholar * Frank, J. et al. A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome. _Nature_ 376, 441–444 (1995). ADS CAS PubMed Google Scholar * Lorenzi,
I. et al. Ribosome-associated Mba1 escorts Cox2 from insertion machinery to maturing assembly intermediates. _Mol. Cell Biol._ 36, 2782–2793 (2016). CAS PubMed PubMed Central Google
Scholar * Englmeier, R., Pfeffer, S. & Forster, F. Structure of the human mitochondrial ribosome studied in situ by cryoelectron tomography. _Structure_ 25, 1574–1581 (2017). CAS
PubMed Google Scholar * Koripella, R. K. et al. A conserved histidine in switch-II of EF-G moderates release of inorganic phosphate. _Sci. Rep._ 5, 12970 (2015). ADS PubMed Google
Scholar * Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. _Nat. Methods_ 14, 290–296 (2017).
CAS PubMed Google Scholar * Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. _J. Struct. Biol._ 192, 216–221 (2015). PubMed
PubMed Central Google Scholar * Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron
cryomicroscopy. _J. Mol. Biol._ 333, 721–745 (2003). CAS PubMed Google Scholar * Brown, A. et al. Structure of the large ribosomal subunit from human mitochondria. _Science_ 346, 718–722
(2014). ADS CAS PubMed PubMed Central Google Scholar * Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. _J. Comput. Chem._ 25,
1605–1612 (2004). CAS PubMed Google Scholar * Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. _Acta Crystallogr. D Biol. Crystallogr._ 66, 486–501
(2010). CAS PubMed PubMed Central Google Scholar * Rother, M. et al. ModeRNA server: an online tool for modeling RNA 3D structures. _Bioinformatics_ 27, 2441–2442 (2011). CAS PubMed
Google Scholar * Shi, H. & Moore, P. B. The crystal structure of yeast phenylalanine tRNA at 1.93 A resolution: a classic structure revisited. _RNA_ 6, 1091–1105 (2000). CAS PubMed
PubMed Central Google Scholar * Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. _Nat. Protoc._ 5, 725–738
(2010). CAS PubMed PubMed Central Google Scholar * Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol.
Crystallogr._ 66, 213–221 (2010). CAS PubMed PubMed Central Google Scholar * Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. _Acta
Crystallogr. D Biol. Crystallogr._ 66, 12–21 (2010). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank ArDean Leith for help with computation. We acknowledge the use
of the Wadsworth Center’s Media and Culture core facility, for help in producing large volumes of HEK293S GnTI cells, and the Wadsworth Center’s and New York Structural Biology Center’s
(NYSBC’s) EM facilities. NYSBC EM facilities are supported by grants from the Simons Foundation (349247), NYSTAR, the NIH (GM103310), and the Agouron Institute (F00316). This work was
supported by the NIH grant R01 GM61576 (to R.K.A.). AUTHOR INFORMATION Author notes * Kalpana Bhargava Present address: High Energy Material Research Lab, Defense Research and Development
Organization, Sutarwadi, Pashan, Pune, Maharashtra, 411021, India * Partha P. Datta Present address: Department of Biological Sciences, Indian Institute of Science Education and Research
Kolkata, Mohanpur, West Bengal, 741246, India * Prem S. Kaushal Present address: Regional Centre for Biotechnology, 3rd Milestone, Faridabad-Gurgaon Expressway, PO Box # 3, Faridabad,
Haryana, 121001, India * These authors contributed equally: Ravi Kiran Koripella and Manjuli R. Sharma. AUTHORS AND AFFILIATIONS * Division of Translational Medicine, Wadsworth Center, New
York State Department of Health, Empire State Plaza, Albany, NY, 12201, USA Ravi Kiran Koripella, Manjuli R. Sharma, Partha P. Datta, Prem S. Kaushal, Pooja Keshavan, Nilesh K. Banavali
& Rajendra K. Agrawal * Department of Chemistry, Campus Box 3290, University of North Carolina, Chapel Hill, NC, USA Kalpana Bhargava & Linda L. Spremulli * Department of Biomedical
Sciences, University at Albany, SUNY, Albany, NY, 12201-0509, USA Nilesh K. Banavali & Rajendra K. Agrawal Authors * Ravi Kiran Koripella View author publications You can also search for
this author inPubMed Google Scholar * Manjuli R. Sharma View author publications You can also search for this author inPubMed Google Scholar * Kalpana Bhargava View author publications You
can also search for this author inPubMed Google Scholar * Partha P. Datta View author publications You can also search for this author inPubMed Google Scholar * Prem S. Kaushal View author
publications You can also search for this author inPubMed Google Scholar * Pooja Keshavan View author publications You can also search for this author inPubMed Google Scholar * Linda L.
Spremulli View author publications You can also search for this author inPubMed Google Scholar * Nilesh K. Banavali View author publications You can also search for this author inPubMed
Google Scholar * Rajendra K. Agrawal View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.K.A. conceived this study. K.B. and L.L.S.
contributed to reagents, including purified EF-G1mt and clones for EF-G1mt; P.K. purified human mitoribosomes and human EF-G1mt; R.K.K. prepared the human 55S-EF-G1mt complex. R.K.K.,
M.R.S., and N.K.B. collected cryo-EM data and performed image processing. M.R.S., P.P.D., and P.S.K. performed purification and reconstructions of bovine mitoribosomes, R.K.K., M.R.S., and
N.K.B. performed molecular modeling, R.K.K., M.R.S., and R.K.A. analyzed the data and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Rajendra K. Agrawal. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution
to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Koripella, R.K., Sharma, M.R., Bhargava, K. _et al._ Structures of the human
mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation. _Nat Commun_ 11, 3830 (2020). https://doi.org/10.1038/s41467-020-17715-2 Download
citation * Received: 13 April 2020 * Accepted: 15 July 2020 * Published: 31 July 2020 * DOI: https://doi.org/10.1038/s41467-020-17715-2 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative