- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Immune evasion is one of the critical hallmarks of malignant tumors, especially non-small cell lung cancer (NSCLC). Emerging findings have illustrated the roles of
N6-methyladenosine (m6A) on NSCLC immune evasion. Here, this study investigated the function and underlying mechanism of m6A reader YTH domain family protein 3 (YTHDF3) on NSCLC immune
evasion. YTHDF3 was found to be highly expressed in NSCLC tissue and act as an independent prognostic factor for overall survival. Functionally, up-regulation of YTHDF3 impaired the CD8+ T
antitumor activity to deteriorate NSCLC immune evasion, while YTHDF3 silencing recovered the CD8+ T antitumor activity to inhibit immune evasion. Besides, YTHDF3 up-regulation reduced the
apoptosis of NSCLC cells. Mechanistically, PD-L1 acted as the downstream target for YTHDF3, and YTHDF3 could upregulate the transcription stability of PD-L1 mRNA. Overall, YTHDF3 targeted
PD-L1 to promote NSCLC immune evasion partially through escaping effector cell cytotoxicity CD8+ T mediated killing and antitumor immunity. In summary, this study provides an essential
insight for m6A modification on CD8+ T cell-mediated antitumor immunity in NSCLC, which might inspire an innovation for lung cancer tumor immunotherapy. SIMILAR CONTENT BEING VIEWED BY
OTHERS RNA METHYLATION OF CD47 MEDIATES TUMOR IMMUNOSUPPRESSION IN EGFR-TKI RESISTANT NSCLC Article 03 February 2025 KEAP1 PROMOTES ANTI-TUMOR IMMUNITY BY INHIBITING PD-L1 EXPRESSION IN
NSCLC Article Open access 27 February 2024 WNK3 INHIBITION ELICITS ANTITUMOR IMMUNITY BY SUPPRESSING PD-L1 EXPRESSION ON TUMOR CELLS AND ACTIVATING T-CELL FUNCTION Article Open access 10
November 2022 INTRODUCTION Non-small-cell lung cancer (NSCLC) accounts for a large proportion (80–85%) of the whole lung cancer, which is the most commonly diagnosed malignancy and remains
the leading cause of tumor-death worldwide [1, 2]. The 5-year survival rate of NSCLC approximately maintain at only 20% [3, 4]. Clinically, cancer immunotherapy could restore or enhance the
effector function of CD8+ T cells or other immune cells. Therefore, a better understanding for NSCLC immunotherapy is of significant importance. Immune evasion acts as an important hallmark
of malignant tumors, which refers to the phenomenon that cancer cells evasion the recognition and killing of the immune cells [5]. Immune evasion processes continue to evolve in NSCLC
invasive stage and are associated with inter/intra-tumor heterogeneity, which contributes to immunotherapy of checkpoint blockade (ICB) [6, 7]. Tumor immune microenvironment (TME) is deeply
correlated to the tumor immunotherapy efficacy, whose characteristics could significantly affect the tumor progression and metastasis [8]. TME is crucial for the development of NSCLC, in
which NSCLC cells interacted with immune cells (T cells, B cells) to facilitate immune evasion. For example, in NSCLC, ILT4 overexpression suppresses the tumor immunity by impairing T cell
response and recruiting M2-like TAMs, preventing immunosuppression and enhancing the efficacy of PD-L1 inhibitor in EGFR wild-type NSCLC [9]. Programmed cell death protein ligands-1 (PD-L1)
is one of the PD-1 ligands, which has been shown to be a valuable biomarker for the tumor prognosis [10]. PD-L1 expression is mainly expressed in cancer cells, antigen-presenting cells
(APCs) or tumor-infiltrating cells in many cancers [11]. In NSCLC, the functions of PD-L1 have been diffusely reported. For example, PD-L1 expression is regulated by oncogenic drivers, e.g.
epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) in NSCLC [12]. Besides, PD-L1 expression is correlated to shorter survival in advanced/metastatic NSCLC [13].
Thus, PD-L1 plays an essential role in tumor immune evasion. N6-methyladenosine (m6A) has been reported to be abnormally expressed in numerous cancers, including NSCLC. In this study, this
research investigated the role of YTHDF3 on NSCLC immune evasion via m6A modification-dependent manner. YTHDF3 could accelerate the immune evasion partially through escaping effector cell
cytotoxicity CD8+ T mediated killing and antitumor immunity. Mechanistically, YTHDF3 could upregulate the transcription stability of PD-L1 mRNA. Overall, the study provided an essential
insight for epigenetics m6A modification and CD8+ T cell-mediated antitumor immunity in NSCLC. RESULTS YTHDF3 HIGHLY EXPRESSED IN NSCLC TISSUE AND ACTED AS AN INDEPENDENT PROGNOSTIC FACTOR
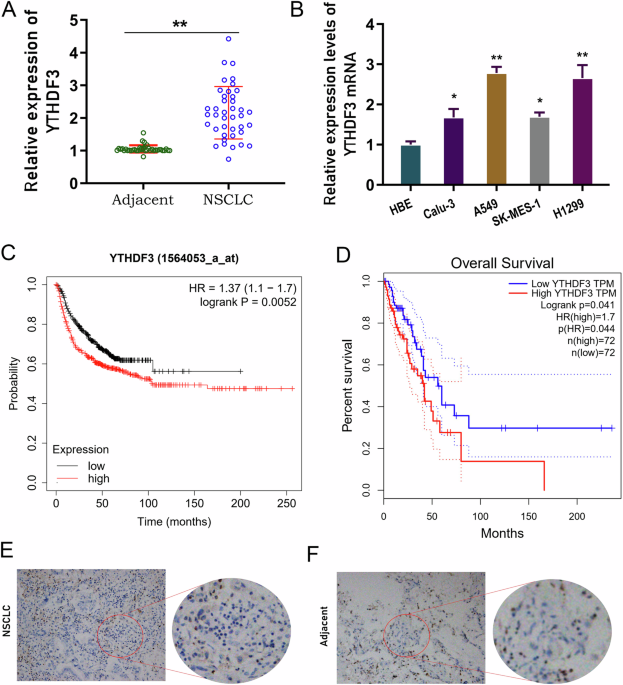
FOR OVERALL SURVIVAL Firstly, the clinical function of YTHDF3 in NSCLC was investigated. In the enrolled cancer samples, YTHDF3 highly expressed in NSCLC tissue as comparing with normal
tissue (Fig. 1A). In the NSCLC cells (Calu-3, A549, SK-MES-1, H1299), YTHDF3 was also highly expressed as comparing with normal cells (HBE) (Fig. 1B). Overall survival analysis revealed that
high-expression YTHDF3 indicated the poor prognosis for NSCLC patients, acting as an independent prognostic factor for NSCLC overall survival (Fig. 1C, D). In the NSCLC tissue,
immunohistochemical analysis revealed that YTHDF3 was highly expressed in the tumor tissue as comparing with adjacent normal tissue (Fig. 1E, F). Overall, these findings revealed that YTHDF3
highly expressed in NSCLC tissue and acted as an independent prognostic factor for overall survival. YTHDF3 SILENCING RECOVERED CD8+ T ANTITUMOR ACTIVITY TO INHIBIT NSCLC IMMUNE EVASION To
test the function of YTHDF3 on NSCLC immune evasion, series of experiments were performed as following. Firstly, the YTHDF3 silencing was conducted in NSCLC cells (A549 cells) (Fig. 2A). In
NSCLC cells, apoptosis analysis revealed that YTHDF3 silencing increased the apoptotic rate in A549 cells with shRNA transfection (Fig. 2B). To test the CD8+ T antitumor activity to NSCLC
cells, co-culture system was constructed (Fig. 2C). NSCLC cells were incubated with activated CD8+ T cells for 48 h, and then the cytokines secreted by CD8+ T cells were detected, including
IFN-γ, TNF-α, Granzyme-B and perforin. Results indicated that CD8+ T cells secreted significantly higher amounts of IFN-γ (Fig. 2D), TNF-α (Fig. 2E), Granzyme-B (Fig. 2F) and perforin (Fig.
2G) upon co-cultured with YTHDF3 silencing transfected A549 cells. After incubation with activated CD8+ T cells, the cytotoxicity was measured by LDH release assays, and the results
indicated that CD8+ T cells exerted higher cytotoxicity activity upon co-cultured with YTHDF3 silencing transfected A549 cells (Fig. 2H). YTHDF3 silencing caused a significant lower
expression of cell-surface PD-L1 expression in NSCLC cells (Fig. 2I, J). Overall, these findings revealed that YTHDF3 silencing recovered CD8+ T antitumor activity to inhibit NSCLC immune
evasion. YTHDF3 UPREGULATION IMPAIRED THE CD8+ T ANTITUMOR ACTIVITY TO DETERIORATE IMMUNE EVASION Given YTHDF3 silencing recovered CD8+ T antitumor activity, the subsequent assays were
performed to test the roles of YTHDF3 overexpression on NSCLC cells. The overexpression transfection of YTHDF3 was constructed in H1299 cells (Fig. 3A). Apoptosis analysis revealed that
YTHDF3 overexpression reduced the apoptotic rate in H1299 cells with YTHDF3 overexpression transfection (Fig. 3B). In the co-culture system of CD8+ T and NSCLC cells, the cytokines secreted
by CD8+ T cells were detected, including IFN-γ, TNF-α, Granzyme-B and perforin. Results indicated that CD8+ T cells secreted significantly lower amounts of IFN-γ (Fig. 3C), TNF-α (Fig. 3D),
Granzyme-B (Fig. 3E) and perforin (Fig. 3F) upon co-cultured with YTHDF3 overexpression transfected H1299 cells. After incubation with activated CD8+ T cells, the cytotoxicity was measured
by LDH release assays, and the results indicated that CD8+ T cells exerted lower cytotoxicity activity upon co-cultured with YTHDF3 overexpression transfected H1299 cells (Fig. 3G).
Moreover, YTHDF3 overexpression caused a significant higher expression of cell-surface PD-L1 expression in NSCLC cells (Fig. 3H, I). Overall, these findings revealed that YTHDF3
up-regulation impaired the CD8+ T antitumor activity to deteriorate immune evasion. PD-L1 WAS THE TARGET OF YTHDF3 VIA M6A-MODIFIED MANNER To test the depth mechanism by which YTHDF3
regulated NSCLC immune evasion, the potential downstream target of YTHDF3 was investigated. Firstly, in clinical samples, the correlation within YTHDF3 and PD-L1 exerted positive correlation
(Fig. 4A). The possible m6A motif towards YTHDF3 was identified as GGACU (Fig. 4B). In silico predictive tool indicated that there were several m6A modified sites on PD-L1gene (Fig. 4C). In
NSCLC cells, the m6A modified level was detected and results showed that m6A modification was higher in tumor cells (Fig. 4D). The sublocation of YTHDF3 and PD-L1 was detected using the
FISH assay, and results indicated that YTHDF3 and PD-L1 were co-located in the A549 cells’ cytoplasm (Fig. 4E). Overall, these findings revealed that PD-L1 was the target of YTHDF3 via
m6A-modified manner. YTHDF3 ENHANCED THE PD-L1 MRNA STABILITY To determine the function of YTHDF3 on PD-L1 mRNA’s fate, the depth mechanism assays were performed in this part. RIP assay
using anti-m6A antibody revealed that YTHDF3 silencing reduced the molecular interaction within YTHDF3 and PD-L1 (Fig. 5A), and YTHDF3 overexpression enhanced the molecular interaction
within YTHDF3 and PD-L1 (Fig. 5B). Then, the RNA stability analysis using RNA decay assay showed that YTHDF3 silencing reduced the half-life time for PD-L1 mRNA (Fig. 5C), and YTHDF3
overexpression enhanced the half-life time for PD-L1 mRNA (Fig. 5D). Then, luciferase reporters with PD-L1 3′UTR wild-type (WT) sequences and corresponding mutant (Mut) with putative mutated
m6A sites were constructed (Fig. 5E). Luciferase activity assay indicated that YTHDF3 silencing reduced the luciferase activity with co-transfected with PD-L1-WT plasmid (Fig. 5F), and
YTHDF3 overexpression enhanced it (Fig. 5G). Overall, these findings revealed that YTHDF3 enhanced the PD-L1 mRNA stability. YTHDF3 TARGETED PD-L1 TO REPRESS CD8+ T ANTITUMOR ACTIVITY TO
INDUCE NSCLC IMMUNE EVASION To further investigate the function of YTHDF3 on PD-L1-dependent CD8+ T antitumor activity, rescue assays were performed. Firstly, the YTHDF3 overexpression
co-transfection could upregulate the PD-L1 protein level (Fig. 6A). In the co-culture system of CD8+ T and NSCLC cells, the cytokines secreted by CD8+ T cells were detected, including IFN-γ,
TNF-α, Granzyme-B and perforin. Results indicated that CD8+ T cells secreted significantly lower amounts of IFN-γ (Fig. 6B), TNF-α (Fig. 6C), Granzyme-B (Fig. 6D) and perforin (Fig. 6E)
upon co-cultured with PD-L1 siRNA (si-PD-L1) transfected H1299 cells. Besides, the YTHDF3 overexpression co-transfection could recover them. After incubation with activated CD8+ T cells, the
cytotoxicity was measured by LDH release assays, and the results indicated that CD8+ T cells exerted lower cytotoxicity activity upon co-cultured with PD-L1 silencing (si-PD-L1) transfected
NSCLC cells (H1299 cells) (Fig. 6F). Meanwhile, YTHDF3 overexpression co-transfection could recover it. Moreover, PD-L1 silencing (si-PD-L1) caused a significant lower expression of
cell-surface PD-L1 expression in NSCLC cells (Fig. 6G, H), while YTHDF3 overexpression co-transfection could recover it. Overall, these findings revealed that YTHDF3 targeted PD-L1 to
repress CD8+ T antitumor activity to induce NSCLC immune evasion. YTHDF3 SILENCING REPRESSED THE TUMOR GROWTH AND PROMOTED THE CD8+ T CELLS INFILTRATION To investigate the role of YTHDF3 on
NSCLC immune evasion, the in vivo assay was performed using the LLC cells in C57BL/6 mice (Fig. 7A). The results demonstrated that YTHDF3 silencing repressed the tumor volume (Fig. 7B) and
weight (Fig. 7C). Then, in the tumor tissue, the CD8+ T lymphocytes were marked by CD8 positive staining, and results indicated that YTHDF3 silencing group had higher CD8+ T cells
infiltration (Fig. 7D, E). Therefore, the in vivo data indicated that YTHDF3 silencing repressed the tumor growth and promoted the CD8+ T cells infiltration. DISCUSSION Tumor immunotherapy
is to mobilize the patient’s own immune system to kill tumor cells, so as to achieve the role of cancer treatment [14]. From the perspective of immunity response, cancer immunotherapy could
restore or enhance the effector function of CD8+ T cells in the tumor microenvironment [15]. Thus, addressing the potential regulatory mechanism underlying NSCLC progression to develop new
therapeutic strategies for NSCLC is of vital importance. Given the critical function of m6A modification on tumor immune evasion [16, 17], this study performed series of assays to test the
function of m6A reader YTHDF3 on NSCLC immune evasion. The result indicates that YTHDF3 was found to be highly expressed in NSCLC tissue and acted as an independent prognostic factor for
overall survival. More and more studies have reported the correlation between m6A and tumor prognosis. For instance, m6A reader YTHDF2 expression is negatively correlated to HCC patients’
survival in both data from this study clinical data and Cancer Genome Atlas (TCGA) database [18]. Besides, m6A methyltransferase METTL3 acts as a marker for poor small cell lung cancer
prognosis, and it is highly expressed in chemoresistant small cell lung cancer cells [19]. Thus, the key enzymes are closely correlated to lung cancer prognosis. More literatures have
indicated the critical function of CD8+ T cells on tumor immune response, including NSCLC [20, 21]. For instance, CD8+ T cells from NSCLC patients expressed an analogous gene expression
program, which is distinct from conventional T cell exhaustion. CD8+ T cell differentiation limits the response of CD8+ T cells to immune checkpoint blockade, thereby contributing to immune
checkpoint blockade failure in a subset T cell-infiltrated lung cancer [22]. In this research, our findings indicated that YTHDF3 up-regulation impaired the CD8+ T antitumor activity to
deteriorate NSCLC immune evasion, while YTHDF3 silencing recovered the CD8+ T antitumor activity to inhibit immune evasion. Therefore, our findings suggested the function of CD8+ T cell on
lung cancer. Programmed death-ligand 1 (PD-L1) is a critical element for tumor immune escape microenvironment, which mediates the escape of tumor cells from CD8+ T cells’ killing. Low PD-L1
expression is more likely to experience better treatment benefit from anti-PD-1/PD-L1 agents (pembrolizumab, nivolumab, durvalumab, avelumab, atezolizumab) in advanced NSCLC. In NSCLC, the
roles of PD-L1 have been widely reported through multitudinous mechanism [23]. PD-L1 could play a critical role as predictive biomarker for anti-PD-1/PD-L1 monotherapy with lower tumor PD-L1
expression to alternative treatments, such as combination immunotherapies. Higher PD-L1 expression in NSCLC is correlated to shorter survival in advanced/metastatic NSCLC, acting as a
prognostic factor [24]. Here, in present research, results indicated that PD-L1 acted as the downstream target for YTHDF3, and YTHDF3 could upregulate the transcription stability of PD-L1
mRNA. With the assistance of YTHDF3, its downstream target’s fate could be modified via m6A-dependent manner. For example, YTHDF3 positively regulates NSCLC cells’ migration, invasion, and
EMT in triple-negative breast cancer cells through enhancing ZEB1 mRNA stability in an m6A-dependent manner [25]. Overall, YTHDF3 targeted PD-L1 to promote NSCLC immune evasion partially
through escaping effector cell cytotoxicity CD8+ T mediated killing and antitumor immunity. In summary, the study suggests that YTHDF3 could accelerate the NSCLC immune evasion partially
through repressing cytotoxicity CD8+ T mediated killing and antitumor immunity. The findings might provide an essential insight for epigenetics m6A modification and CD8+ T cell-mediated
antitumor immunity in NSCLC (Fig. 8), which might provide a point for NSCLC immunotherapy. MATERIALS AND METHODS CLINICAL SPECIMEN COLLECTION The clinical tissues, as well as their paired
adjacent normal tissues, were collected from lung cancer patients who underwent surgical treatment in First Affiliated Hospital of Shenzhen University from 2020 to 2022. The
clinicopathological characteristic of the cancer patients were listed in Table 1. All these clinical experiments in the present study had been approved by the Ethics Committee of First
Affiliated Hospital of Shenzhen University. Besides, the written informed consents had been obtained from all the participants. The study was performed according to the ethical standards of
the Declaration of Helsinki. CELL LINES, CULTURE AND VECTORS TRANSFECTION This study utilized human NSCLC cell lines (Calu-3, A549, SK-MES-1, H1299) and control cells (human normal bronchial
epithelial cell, HBE) were provided from American Type Culture Collection (ATCC). Under standard condition (5% CO2, 37 °C), all the NSCLC cells and control cells were cultured in Roswell
Park Memorial Institute 1640 (RPMI-1640) culture medium. To silence or enhance the indicated target, the vectors for YTHDF3 overexpression (YTHDF3-oe) and downregulation (YTHDF3-sh) were
designed and manufactured by GenePharma (Shanghai, China). In addition, the PD-L1 inhibitor (si-PD-L1) and controls (si-NC) were designed and obtained from Ribobio (Guangzhou, China). The
detailed transfection information was performed according to the above manufacture’s protocols. RNA ISOLATION, QRT‑PCR, AND WESTERN BLOT Total RNA was isolated from NSCLC issue samples and
cultured cell lines using TRIzol Reagent it (Invitrogen) according to the manufacturer’s protocols. RNA samples were reversely transcribed into cDNA by PrimeScript RTTM Reagent Kit (TaKaRa)
based on instruction. The qPCR was performed by SYBR Premix Taq (Applied Biosystems, US). The quantification of levels of mRNAs was performed by normalization to that of internal reference
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The RNA relative expression levels (fold change) were analyzed by utilizing 2−ΔΔCt methods. Primers sequences and oligonucleotides
sequences were included in Additional File Supplementary Table S1. For western blot analysis, NSCLC cells were lysed utilizing radioimmunoprecipitation assay (RIPA) buffer (Yeasen, Shanghai,
China). Cell lysate was centrifuged (15 min, 12,000 × _g_, 4 °C) and then the supernatants were collected. The collected protein was added to 10% SDS-PAGE gel and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) by semidry blotters. Being blocked by 5% nonfat milk (1 h, room temperature), the membranes were incubated primary
antibodies (anti-YTHDF3, 1:1000, Abcam, cat no. ab220161; anti-PD-L1, 1:1000, Abcam, cat no. ab213524) overnight at 4 °C. An GAPDH antibody (1:1000) served as the loading control. The
protein amount was visualized by densitometry using enhanced chemiluminescence reagent. IMMUNOHISTOCHEMISTRY STAINING AND FLOW CYTOMETRY APOPTOSIS ANALYSIS For the tissue
immunohistochemistry staining, cancer tissue paraffin sections were fixed by formalin and following deparaffinization and rehydration. For the incubation of primary antibody, anti-YTHDF3
(anti-YTHDF3, 1:1000 dilution, Abcam, cat no. ab220161) was incubated. The slide without primary antibody incubation acted as negative control. For the flow cytometry apoptosis analysis, the
fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) was utilized for double staining with FITC Annexin V Apoptosis Detection Kit (BD Biosciences) according to the
manufacturer’s recommendation. CD8+ T CELL ISOLATION AND CO-CULTURE SYSTEM CD8+ T cells were obtained from healthy volunteer donors’ peripheral blood mononuclear cells (PBMCs). The PBMCs
were isolated and purified using Easy-Sep™ Direct Human CD8+ T Cell Isolation Kit (STEMCELL Technologies, Vancouver, Canada, US, Cat no. #15063). For the activation, human CD8+ T cells were
seeded into 24-well plates and induced with anti-CD3/anti-CD28 antibodies (2 µl/well) and IL-2 (20 ng/mL) (BD Biosciences, Franklin Lakes, NJ, USA) and incubated for 48 h. For the in vitro
co-culture system, the activated CD8+ T cells were co-cultured with adhered cancer cells at 5:1 ratio (effector to target) for 48 h. ENZYME‑LINKED IMMUNOSORBENT ASSAY (ELISA) The ELISA assay
was performed to detect the concentrations CD8+ T cells secreted cytokines, including IFN-γ, TNF-α, Granzyme-B and Perforin with commercial kit in accordance with the manufacturer’s
guideline. The kits were following: IFN-γ, BD Pharmingen, cat no. 550583; TNF-α, eBioscience, cat no. BMS223HS; Granzyme-B, eBioscience, cat no. BMS2027; Perforin, eBioscience, cat no.
BMS2306. CYTOTOXICITY ASSAY The CD8+ T cell-mediated cytotoxicity on NSCLC cells was determined by lactate dehydrogenase (LDH)-based cytotoxicity assay. After co-culture of NSCLC cells and
CD8+ T cells, the culture supernatants were collected for LDH release using Cytotoxicity Detection Kit PLUS (Sigma-Aldrich, catalog no. 04744926001) according to the manufacturer’s
recommendation. SURFACE PD-L1 EXPRESSION ANALYSIS To measure cellular surface PD-L1 expression, NSCLC cells were resuspended in PBS buffer, and incubated with primary anti-PD-L1 antibodies
(BioLegend) according to standard protocols for flow cytometry. Consequently, flow cytometric data were analyzed using the FlowJo software program. M6A MODIFIED LEVEL The total RNA was
extracted from NSCLC cells using TRIzol reagent kit (Invitrogen). RNA sample (200 ng) were added into 96-well plates and each well was added with diluted capture antibody and diluted
enhancer solution (100 mL). The termination solution was supplemented to each well on the microplate reader at 450 nm. The m6A level in total RNA was detected using an m6A RNA methylation
detection kit (cat no. ab185912, Abcam). FLUORESCENCE IN SITU HYBRIDIZATION (FISH) FISH was performed using an mRNA in situ hybridization kit (Shanghai GenePharma Co., Ltd, China). NSCLC
cells samples were incubated with probe buffer overnight at 37 °C in a dark moist chamber. After being washed twice in 50% formamide for 5 min, the slices were incubated with the regents and
sealed with DAPI parafilm. Labeled cells were imaged by immunofluorescence under a confocal fluorescence microscopy (OLYMPUS FV1000 confocal microscopy, Japan). RNA IMMUNOPRECIPITATION RNA
immunoprecipitation (RIP) experiments were performed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) based on the manufacturer’s recommendation.
Briefly, 5 × 106 cells were collected and resuspended in RIPA lysis buffer (300 μL) with RNase inhibitor and protease inhibitor. The cell lysates (200 μL) were incubated with control IgG
antibody or anti-YTHDF3 (1:1000, Abcam, cat no. ab220161) or with A/G magnetic beads overnight at 4 °C. The immunoprecipitated RNA was purified and evaluated by qPCR. LUCIFERASE REPORTER
ASSAY For firefly luciferase construction, cDNAs containing PD-L1 genome 3′-UTR sequence were cloned into pGL3-control vectors (Promega). Besides, cytosine (C) was replaced to marked
adenosine (A) in m6A motif for mutant. NSCLC cells were co-transfected with PD-L1 wild-type (0.5 μg) or mutated reporter plasmids (25 ng, renilla luciferase reporter vector). After 24 h,
cells were harvested and the luciferase activity was detected using Dual-Glo Luciferase system (Promega). The luciferase activity was normalized to Renilla activity. RNA STABILITY ASSAY
NSCLC cells were seeded in the 6-well plates and incubated overnight. At indicated time, NSCLC cells were treated with actinomycin D (5 μg/mL, MedChemExpress, Cat no. HY-17559) for 0, 3, 6,
9 h. Total RNA was isolated from NSCLC cells using TRIzol kit and relative level was quantified by qRT-PCR, which was calculated and normalized by GAPDH. IN VIVO ANIMAL ASSAY C57BL/6 mice
were provided from SLAC (Shanghai, China) and then housed in pathogen-free condition. Mice were inoculated with total of 106 LLC cells that transfected sh-NC or sh-YTHDF3. The volume and
weight were calculated as protocols. This assay was approved by Ethics Committee of First Affiliated Hospital of Shenzhen University. STATISTICAL ANALYSIS All experiments were performed via
three independent assays, which was shown as mean ± standard deviation (SD). Statistic was assessed using Student’s _t_-test or one-way analysis of variance (ANOVA) following Tukey’s
post-hoc tests. Data analysis was performed using GraphPad Prism software version 9.0. and SPSS software version 22.0. _p_ < 0.05 was supposed to statistically significant. DATA
AVAILABILITY The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Alexander M, Kim SY, Cheng
H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198:897–907. Article PubMed PubMed Central Google Scholar * Duma N, Santana-Davila R, Molina JR. Non-small cell lung
cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40. Article CAS PubMed Google Scholar * Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR,
Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20:497–530. Article Google Scholar * Herbst RS,
Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54. Article CAS PubMed Google Scholar * Liu Z, Wang T, She Y, Wu K, Gu S, Li L,
et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer.
Mol Cancer. 2021;20:105. Article CAS PubMed PubMed Central Google Scholar * Mithoowani H, Febbraro M. Non-small-cell lung cancer in 2022: a review for general practitioners in
oncology. Curr Oncol. 2022;29:1828–39. Article PubMed PubMed Central Google Scholar * Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol.
2022;40:586–97. Article CAS PubMed Google Scholar * Hong W, Xue M, Jiang J, Zhang Y, Gao X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug
resistance and immune evasion in non-small cell lung cancer (NSCLC). J Exp Clin Cancer Res. 2020;39:149. Article CAS PubMed PubMed Central Google Scholar * Chen X, Gao A, Zhang F, Yang
Z, Wang S, Fang Y, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.
Theranostics. 2021;11:3392–416. Article CAS PubMed PubMed Central Google Scholar * Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical
impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. Article CAS PubMed Google Scholar * Li W, Wu F, Zhao S, Shi P, Wang S, Cui D. Correlation between
PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev 2022;67:49–57. Article CAS PubMed Google Scholar *
Bassanelli M, Sioletic S, Martini M, Giacinti S, Viterbo A, Staddon A, et al. Heterogeneity of PD-L1 expression and relationship with biology of NSCLC. Anticancer Res. 2018;38:3789–96.
Article CAS PubMed Google Scholar * Brody R, Zhang Y, Ballas M, Siddiqui MK, Gupta P, Barker C, et al. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer. 2017;112:200–15. Article PubMed Google Scholar * Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m(6)A regulator-mediated
methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19:53. Article CAS PubMed PubMed Central Google Scholar *
Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P, et al. NK cells and ILCs in tumor immunotherapy. Mol Asp Med. 2021;80:100870. Article CAS Google Scholar * Zhao W, Liu J,
Wu J, Ma X, Wang X, Zhang L, et al. High-throughput microarray reveals the epitranscriptome-wide landscape of m(6)A-modified circRNA in oral squamous cell carcinoma. BMC Genomics.
2022;23:611. Article CAS PubMed PubMed Central Google Scholar * Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes
immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82:1660–77.e10. Article CAS PubMed Google Scholar * Zhang C, Huang S, Zhuang H, Ruan S, Zhou Z, Huang K, et al.
YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene. 2020;39:4507–18. Article CAS PubMed Google
Scholar * Sun Y, Shen W, Hu S, Lyu Q, Wang Q, Wei T, et al. METTL3 promotes chemoresistance in small cell lung cancer by inducing mitophagy. J Exp Clin Cancer Res. 2023;42:65. Article
PubMed PubMed Central Google Scholar * Papa-Gobbi R, Muglia CI, Rocca A, Curciarello R, Sambuelli AM, Yantorno M, et al. Spatiotemporal regulation of galectin-1-induced T-cell death in
lamina propria from Crohn’s disease and ulcerative colitis patients. Apoptosis 2021;26:323–37. Article CAS PubMed Google Scholar * Wang Y, Jin P, Wang X. N(6)-methyladenosine regulator
YTHDF1 represses the CD8+ T cell-mediated antitumor immunity and ferroptosis in prostate cancer via m(6)A/PD-L1 manner. Apoptosis. 2024;29:142–153. * Horton BL, Morgan DM, Momin N, Zagorulya
M, Torres-Mejia E, Bhandarkar V, et al. Lack of CD8(+) T cell effector differentiation during priming mediates checkpoint blockade resistance in non-small cell lung cancer. Sci Immunol.
2021;6:eabi8800. Article CAS PubMed PubMed Central Google Scholar * Eguren-Santamaria I, Sanmamed MF, Goldberg SB, Kluger HM, Idoate MA, Lu BY, et al. PD-1/PD-L1 blockers in NSCLC brain
metastases: challenging paradigms and clinical practice. Clin Cancer Res. 2020;26:4186–97. Article CAS PubMed Google Scholar * Wang J, Xu Y, Rao X, Zhang R, Tang J, Zhang D, et al.
BRD4-IRF1 axis regulates chemoradiotherapy-induced PD-L1 expression and immune evasion in non-small cell lung cancer. Clin Transl Med. 2022;12:e718. Article CAS PubMed PubMed Central
Google Scholar * Lin Y, Jin X, Nie Q, Chen M, Guo W, Chen L, et al. YTHDF3 facilitates triple-negative breast cancer progression and metastasis by stabilizing ZEB1 mRNA in an
m(6)A-dependent manner. Ann Transl Med. 2022;10:83. Article CAS PubMed PubMed Central Google Scholar Download references FUNDING This work was supported by the Science and Technology
Development Fund Project of Shenzhen (Grant No. JCYJ20220530150813029) and the Team-based Medical Science Research Program (Grant No.2024YZZ12). AUTHOR INFORMATION Author notes * These
authors contributed equally: Yisheng Luo, Chao Zeng, Zezhong Ouyang. AUTHORS AND AFFILIATIONS * Department of Thoracic Surgery, The First Affiliated Hospital of Shenzhen University, Shenzhen
Second People’s Hospital, Shenzhen, 518000, Guangdong Province, China Yisheng Luo, Zezhong Ouyang, Wenbin Zhu, Jiazhi Wang, Zhiyin Chen, Chunyang Xiao, Guodong Wu, Liang Li, Youhui Qian
& Hao Wu * Department of Respiratory and Critical Care Medicine, Peking University Shenzhen Hospital, Shenzhen, 518000, Guangdong Province, China Chao Zeng * National Regional Key
Technology Engineering Laboratory for Medical Ultrasound, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, School of Biomedical Engineering, Shenzhen University
Medical School, Shenzhen University, Shenzhen, 518000, Guangdong Province, China Xin Chen * Guangdong Provincial Key Laboratory of Systems Biology and Synthetic Biology for Urogenital
Tumors, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital (Shenzhen Institute of Translational Medicine), Shenzhen, 518000, Guangdong Province, China
Yuchen Liu Authors * Yisheng Luo View author publications You can also search for this author inPubMed Google Scholar * Chao Zeng View author publications You can also search for this author
inPubMed Google Scholar * Zezhong Ouyang View author publications You can also search for this author inPubMed Google Scholar * Wenbin Zhu View author publications You can also search for
this author inPubMed Google Scholar * Jiazhi Wang View author publications You can also search for this author inPubMed Google Scholar * Zhiyin Chen View author publications You can also
search for this author inPubMed Google Scholar * Chunyang Xiao View author publications You can also search for this author inPubMed Google Scholar * Guodong Wu View author publications You
can also search for this author inPubMed Google Scholar * Liang Li View author publications You can also search for this author inPubMed Google Scholar * Youhui Qian View author publications
You can also search for this author inPubMed Google Scholar * Xin Chen View author publications You can also search for this author inPubMed Google Scholar * Yuchen Liu View author
publications You can also search for this author inPubMed Google Scholar * Hao Wu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Yisheng
Luo, Chao Zeng, Zezhong Ouyang performed the assays. Wenbin Zhu, Jiazhi Wang, Zhiyin Chen, Chunyang Xiao, Guodong Wu, Liang Li, Youhui Qian, Xin Chen wrote the main manuscript and prepared
figures. Yuchen Liu, Hao Wu was responsible for the funding. All authors reviewed the manuscript. CORRESPONDING AUTHORS Correspondence to Yuchen Liu or Hao Wu. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests ETHICS APPROVAL AND CONSENT TO PARTICIPATE Written informed consent was obtained from each patient, and the study had been approved by
the Ethical Committee of First Affiliated Hospital of Shenzhen University. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Luo, Y., Zeng, C., Ouyang, Z. _et al._ YTH domain family protein 3 accelerates
non-small cell lung cancer immune evasion through targeting CD8+ T lymphocytes. _Cell Death Discov._ 10, 320 (2024). https://doi.org/10.1038/s41420-024-02084-2 Download citation * Received:
15 May 2024 * Revised: 13 June 2024 * Accepted: 21 June 2024 * Published: 11 July 2024 * DOI: https://doi.org/10.1038/s41420-024-02084-2 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative