- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT For patients with metastatic prostate cancer, the 5-year survival rate of 31% points to a need for novel therapies and improvement of existing modalities. We propose that p53 gene
therapy and chemotherapy, when combined, will provide superior tumor cell killing for the treatment of prostate carcinoma. To this end, we have developed the AdRGD-PGp53 vector which offers
autoregulated expression of p53, resulting in enhanced tumor cell killing in vitro and in vivo. Here, we combined AdRGD-PGp53 along with the chemotherapy drugs used in the clinical treatment
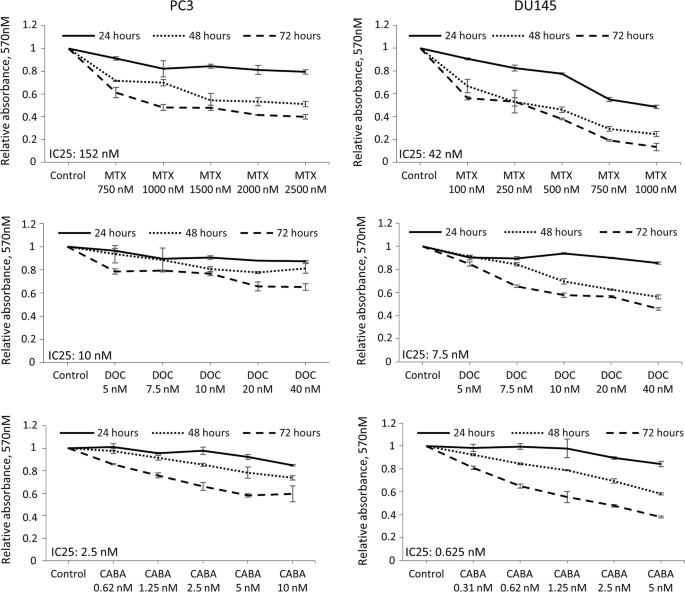
of prostate carcinoma, mitoxantrone, docetaxel, or cabazitaxel. Our results indicate that all drugs increase phosphorylation of p53, leading to improved induction of p53 targets. In vitro
experiments reveal that AdRGD-PGp53 sensitizes prostate cancer cells to each of the drugs tested, conferring increased levels of cell death. In a xenograft mouse model of in situ gene
therapy, AdRGD-PGp53 treatment, when combined with cabazitaxel, drastically reduced tumor progression and increased survival rates to 100%. Strikingly, we used a sub-therapeutic dose of
cabazitaxel thus avoiding leukopenia, yet still showed potent anti-tumor effects when combined with AdRGD-PGp53 in this mouse model. The AdRGD-PGp53 approach warrants further development for
its application in gene therapy of prostate carcinoma. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 6 print issues and online access $259.00 per year only $43.17 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SYNTHETIC LETHAL COMBINATION OF CHK1 AND WEE1 INHIBITION FOR TREATMENT OF
CASTRATION-RESISTANT PROSTATE CANCER Article 25 January 2024 TARGETING ADAR1 WITH A SMALL MOLECULE FOR THE TREATMENT OF PROSTATE CANCER Article 10 February 2025 TOPOISOMERASE II ALPHA
INHIBITION CAN OVERCOME TAXANE-RESISTANT PROSTATE CANCER THROUGH DNA REPAIR PATHWAYS Article Open access 15 November 2021 REFERENCES * Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017.
CA Cancer J Clin. 2017;67:7–30. PubMed Google Scholar * Debruyne F. Hormonal therapy of prostate cancer. Semin Urol Oncol. 2002;20(3Suppl 1):4–9. PubMed Google Scholar * McCullough AR.
Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(Suppl 2):S3–S10. PubMed PubMed Central Google Scholar * Maia MC, Hansen AR. A comprehensive review of immunotherapies in
prostate cancer. Crit Rev Oncol Hematol. 2017;113:292–303. PubMed Google Scholar * Liu LF. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–75. CAS PubMed
Google Scholar * Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and
prednisone. J Clin Oncol. 1999;17:1654–63. CAS PubMed Google Scholar * Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus
prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. CAS PubMed Google
Scholar * Berry W, Dakhil S, Modiano M, Gregurich M, Asmar L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone
refractory prostate cancer. J Urol. 2002;168:2439–43. CAS PubMed Google Scholar * Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind,
controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol.
2003;21:3335–42. CAS PubMed Google Scholar * Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–91. CAS PubMed
Google Scholar * Schimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chemother
Pharmacol. 1999;43:165–72. CAS PubMed Google Scholar * Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–33. CAS PubMed Google Scholar
* Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
CAS PubMed Google Scholar * Cisternino S, Bourasset F, Archimbaud Y, Semiond D, Sanderink G, Scherrmann JM. Nonlinear accumulation in the brain of the new taxoid TXD258 following
saturation of P-glycoprotein at the blood-brain barrier in mice and rats. Br J Pharmacol. 2003;138:1367–75. CAS PubMed PubMed Central Google Scholar * Attard G, Greystoke A, Kaye S, De
Bono J. Update on tubulin-binding agents. Pathol Biol (Paris). 2006;54:72–84. CAS Google Scholar * de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus
cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. PubMed
Google Scholar * Strauss BE, Costanzi-Strauss E. pCLPG: a p53-driven retroviral system. Virology. 2004;321:165–72. CAS PubMed Google Scholar * Bajgelman MC, Strauss BE. Development of an
adenoviral vector with robust expression driven by p53. Virology. 2008;371:8–13. CAS PubMed Google Scholar * Bajgelman MC, Medrano RF, Carvalho AC, Strauss BE. AAVPG: a vigilant vector
where transgene expression is induced by p53. Virology. 2013;447:166–71. CAS PubMed Google Scholar * Tamura RE, da Silva Soares RB, Costanzi-Strauss E, Strauss BE. Autoregulated
expression of p53 from an adenoviral vector confers superior tumor inhibition in a model of prostate carcinoma gene therapy. Cancer Biol Ther. 2016;17:1221–30. CAS PubMed PubMed Central
Google Scholar * Tamura RE, Hunger A, Fernandes DC, Laurindo FR, Costanzi-Strauss E, Strauss BE. Induction of oxidants distinguishes susceptibility of prostate carcinoma cell lines to p53
gene transfer mediated by an improved adenoviral vector. Hum Gene Ther. 2017;28:639–53. CAS PubMed Google Scholar * Merkel CA, Medrano RF, Barauna VG, Strauss BE. Combined p19Arf and
interferon-beta gene transfer enhances cell death of B16 melanoma in vitro and in vivo. Cancer Gene Ther. 2013;20:317–25. CAS PubMed Google Scholar * Peng HH, Wu S, Davis JJ, Wang L, Roth
JA, Marini FC 3rd, et al. A rapid and efficient method for purification of recombinant adenovirus with arginine-glycine-aspartic acid-modified fibers. Anal Biochem. 2006;354:140–7. CAS
PubMed PubMed Central Google Scholar * Merkel CA, da Silva Soares RB, de Carvalho AC, Zanatta DB, Bajgelman MC, Fratini P, et al. Activation of endogenous p53 by combined p19Arf gene
transfer and nutlin-3 drug treatment modalities in the murine cell lines B16 and C6. BMC Cancer. 2010;10:316. PubMed PubMed Central Google Scholar * Tomayko MM, Reynolds CP. Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–54. CAS PubMed Google Scholar * Radhakrishnan S, Miranda E, Ekblad M, Holford A, Pizarro MT,
Lemoine NR, et al. Efficacy of oncolytic mutants targeting pRb and p53 pathways is synergistically enhanced when combined with cytotoxic drugs in prostate cancer cells and tumor xenografts.
Hum Gene Ther. 2010;21:1311–25. CAS PubMed Google Scholar * Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell.
1997;91:325–34. CAS PubMed Google Scholar * Loughery J, Cox M, Smith LM, Meek DW. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive
promoters. Nucleic Acids Res. 2014;42:7666–80. CAS PubMed PubMed Central Google Scholar * Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of
cancers. Hum Gene Ther. 2005;16:1016–27. CAS PubMed Google Scholar * Guo W, Song H. Development of gene therapeutics for head and neck cancer in china: from bench to bedside. Hum Gene
Ther. 2018;29:180–7. CAS PubMed Google Scholar * Ko SC, Gotoh A, Thalmann GN, Zhau HE, Johnston DA, Zhang WW, et al. Molecular therapy with recombinant p53 adenovirus in an
androgen-independent, metastatic human prostate cancer model. Hum Gene Ther. 1996;7:1683–91. CAS PubMed Google Scholar * Asgari K, Sesterhenn IA, McLeod DG, Cowan K, Moul JW, Seth P, et
al. Inhibition of the growth of pre-established subcutaneous tumor nodules of human prostate cancer cells by single injection of the recombinant adenovirus p53 expression vector. Int J
Cancer J Int du Cancer. 1997;71:377–82. CAS Google Scholar * Eastham JA, Hall SJ, Sehgal I, Wang J, Timme TL, Yang G, et al. In vivo gene therapy with p53 or p21 adenovirus for prostate
cancer. Cancer Res. 1995;55:5151–5. CAS PubMed Google Scholar * Tamura RE, Hunger A, Fernandes D, Laurindo F, Costanzi-Strauss E, Strauss BE Induction of oxidants distinguishes
susceptibility of prostate carcinoma cell lines to p53 gene transfer mediated by an improved adenoviral vector. Hum Gene Ther 2017;28:639-53. CAS PubMed Google Scholar * Sasaki R,
Shirakawa T, Zhang ZJ, Tamekane A, Matsumoto A, Sugimura K, et al. Additional gene therapy with Ad5CMV-p53 enhanced the efficacy of radiotherapy in human prostate cancer cells. Int J Radiat
Oncol Biol Phys. 2001;51:1336–45. CAS PubMed Google Scholar * Cowen D, Salem N, Ashoori F, Meyn R, Meistrich ML, Roth JA, et al. Prostate cancer radiosensitization in vivo with
adenovirus-mediated p53 gene therapy. Clin Cancer Res. 2000;6:4402–8. CAS PubMed Google Scholar * Gjerset R, Haghighi A, Lebedeva S, Mercola D. Gene therapy approaches to sensitization of
human prostate carcinoma to cisplatin by adenoviral expression of p53 and by antisense jun kinase oligonucleotide methods. Methods Mol Biol. 2001;175:495–520. CAS PubMed Google Scholar *
Chuang JC, Sheu GT, Wang PC, Liao FT, Liu WS, Huang CF, et al. Docetaxel and 5-fluorouracil induce human p53 tumor suppressor gene transcription via a short sequence at core promoter
element. Toxicol Vitr. 2012;26:678–85. CAS Google Scholar * Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, et al. Functional p53 determines docetaxel sensitivity in prostate cancer
cells. Prostate. 2013;73:418–27. CAS PubMed Google Scholar * Gan L, Wang J, Xu H, Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling.
Prostate. 2011;71:1158–66. CAS PubMed Google Scholar * Nozawa M, Mukai H, Takahashi S, Uemura H, Kosaka T, Onozawa Y, et al. Japanese phase I study of cabazitaxel in metastatic
castration-resistant prostate cancer. Int J Clin Oncol. 2015;20:1026–34. CAS PubMed Google Scholar * Heidenreich A, Bracarda S, Mason M, Ozen H, Sengelov L, Van Oort I, et al. Safety of
cabazitaxel in senior adults with metastatic castration-resistant prostate cancer: results of the European compassionate-use programme. Eur J Cancer. 2014;50:1090–9. CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS We thank Roger Chammas and his staff for ongoing support and critical discussions. We thank Otto Luiz Dutra Cerqueira for assistance during the
preparation of this manuscript. This work was a collaborative effort with Sanofi-Aventis which facilitated the purchase of the cabazitaxel used here. FUNDING Financial support was received
from the São Paulo Research Foundation, FAPESP (RET, 2011/21256–8; BES, 2013/25167–5 and 2015/26580–9) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (RET,
442738/2014–5; 302888/2017–9, BES). Financial support was provided by Sanofi-Aventis (ISS PRECLL 06945) in order to obtain the cabazitaxel used in this study. AUTHOR INFORMATION Author notes
* Rodrigo Esaki Tamura Present address: Department of Biological Sciences, Federal University of São Paulo, Diadema, SP, Brazil AUTHORS AND AFFILIATIONS * Viral Vector Laboratory, Center
for Translational Investigation in Oncology/LIM24, Cancer Institute of São Paulo, School of Medicine, University of São Paulo, SP, Brazil Rodrigo Esaki Tamura, Marlous G. Lana & Bryan E.
Strauss * Gene Therapy Laboratory, Department of Cell and Developmental Biology, Biomedical Sciences Institute, University of São Paulo, SP, Brazil Eugenia Costanzi-Strauss Authors *
Rodrigo Esaki Tamura View author publications You can also search for this author inPubMed Google Scholar * Marlous G. Lana View author publications You can also search for this author
inPubMed Google Scholar * Eugenia Costanzi-Strauss View author publications You can also search for this author inPubMed Google Scholar * Bryan E. Strauss View author publications You can
also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Bryan E. Strauss. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no
conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tamura, R.E., Lana, M.G., Costanzi-Strauss, E. _et al._
Combination of cabazitaxel and p53 gene therapy abolishes prostate carcinoma tumor growth. _Gene Ther_ 27, 15–26 (2020). https://doi.org/10.1038/s41434-019-0071-x Download citation *
Received: 05 December 2018 * Revised: 22 February 2019 * Accepted: 08 March 2019 * Published: 29 March 2019 * Issue Date: February 2020 * DOI: https://doi.org/10.1038/s41434-019-0071-x SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative