- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Expectations have a powerful influence on how we experience the world. Neurobiological and computational models of learning suggest that dopamine is crucial for shaping expectations
of reward and that expectations alone may influence dopamine levels. However, because expectations and reinforcers are typically manipulated together, the role of expectations _per se_ has
remained unclear. We separated these two factors using a placebo dopaminergic manipulation in individuals with Parkinson's disease. We combined a reward learning task with functional
magnetic resonance imaging to test how expectations of dopamine release modulate learning-related activity in the brain. We found that the mere expectation of dopamine release enhanced
reward learning and modulated learning-related signals in the striatum and the ventromedial prefrontal cortex. These effects were selective to learning from reward: neither medication nor
placebo had an effect on learning to avoid monetary loss. These findings suggest a neurobiological mechanism by which expectations shape learning and affect. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS LITHIUM MODULATES STRIATAL REWARD ANTICIPATION AND PREDICTION ERROR CODING IN HEALTHY VOLUNTEERS Article 30 October 2020 DOPAMINE RELEASE IN HUMAN ASSOCIATIVE STRIATUM DURING REVERSAL
LEARNING Article Open access 02 January 2024 THE PREDICTION-ERROR HYPOTHESIS OF SCHIZOPHRENIA: NEW DATA POINT TO CIRCUIT-SPECIFIC CHANGES IN DOPAMINE ACTIVITY Article Open access 29
September 2021 REFERENCES * Goetz, C.G. et al. Placebo influences on dyskinesia in Parkinson's disease. _Mov. Disord._ 23, 700–707 (2008). Article PubMed PubMed Central Google
Scholar * Goetz, C.G. et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. _Mov. Disord._ 23, 690–699 (2008).
Article PubMed Google Scholar * McRae, C. et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. _Arch. Gen. Psychiatry_
61, 412–420 (2004). Article PubMed Google Scholar * Benedetti, F. et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. _Nat.
Neurosci._ 7, 587–588 (2004). Article CAS PubMed Google Scholar * de la Fuente-Fernández, R. et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's
disease. _Science_ 293, 1164–1166 (2001). Article PubMed Google Scholar * Lidstone, S.C. et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. _Arch.
Gen. Psychiatry_ 67, 857–865 (2010). Article CAS PubMed Google Scholar * Voon, V. et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. _Neuron_ 65, 135–142
(2010). Article CAS PubMed PubMed Central Google Scholar * de la Fuente-Fernández, R. et al. Dopamine release in human ventral striatum and expectation of reward. _Behav. Brain Res._
136, 359–363 (2002). Article PubMed Google Scholar * Schweinhardt, P., Seminowicz, D.A., Jaeger, E., Duncan, G.H. & Bushnell, M.C. The anatomy of the mesolimbic reward system: a link
between personality and the placebo analgesic response. _J. Neurosci._ 29, 4882–4887 (2009). Article CAS PubMed PubMed Central Google Scholar * Zubieta, J.K. & Stohler, C.S.
Neurobiological mechanisms of placebo responses. _Ann. NY Acad. Sci._ 1156, 198–210 (2009). Article CAS PubMed Google Scholar * Pessiglione, M., Seymour, B., Flandin, G., Dolan, R.J.
& Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behavior in humans. _Nature_ 442, 1042–1045 (2006). Article CAS PubMed PubMed Central Google Scholar *
Sutton, R.S. & Barto, A.G. _Reinforcement Learning: an Introduction_ (Bradford Book, 1998). * Barto, A.G. Reinforcement learning and dynamic programming. in _Analysis, Design and
Evaluation of Man-Machine Systems, Vol. 1,2_ (ed. Johannsen, G.) 407–412 (1995). * Daw, N.D. in Trial-by-trial data analysis using computational models. _Decision Making, Affect, Learning:
Attention and Performance XXIII, Vol. 1_ (eds. Delgado, M.R., Phelps, E.A. & Robbins, T.W.) 3–38 (Oxford University Press, 2011). * Daw, N.D., O'Doherty, J.P., Dayan, P., Seymour,
B. & Dolan, R.J. Cortical substrates for exploratory decisions in humans. _Nature_ 441, 876–879 (2006). Article CAS PubMed PubMed Central Google Scholar * Hare, T.A.,
O'Doherty, J., Camerer, C.F., Schultz, W. & Rangel, A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors.
_J. Neurosci._ 28, 5623–5630 (2008). Article CAS PubMed PubMed Central Google Scholar * Levy, D.J. & Glimcher, P.W. The root of all value: a neural common currency for choice.
_Curr. Opin. Neurobiol._ 22, 1027–1038 (2012). Article CAS PubMed PubMed Central Google Scholar * Plassmann, H., O'Doherty, J.P. & Rangel, A. Appetitive and aversive goal
values are encoded in the medial orbitofrontal cortex at the time of decision making. _J. Neurosci._ 30, 10799–10808 (2010). Article CAS PubMed PubMed Central Google Scholar * McClure,
S.M., Berns, G.S. & Montague, P.R. Temporal prediction errors in a passive learning task activate human striatum. _Neuron_ 38, 339–346 (2003). Article CAS PubMed Google Scholar *
O'Doherty, J.P., Dayan, P., Friston, K., Critchley, H. & Dolan, R.J. Temporal difference models and reward-related learning in the human brain. _Neuron_ 38, 329–337 (2003). Article
CAS PubMed Google Scholar * Daw, N.D. & Doya, K. The computational neurobiology of learning and reward. _Curr. Opin. Neurobiol._ 16, 199–204 (2006). Article CAS PubMed Google
Scholar * Rushworth, M.F., Mars, R.B. & Summerfield, C. General mechanisms for decision making. _Curr. Opin. Neurobiol._ 19, 75–83 (2009). Article CAS PubMed Google Scholar *
Chowdhury, R. et al. Dopamine restores reward prediction errors in old age. _Nat. Neurosci._ 16, 648–653 (2013). Article CAS PubMed PubMed Central Google Scholar * Schönberg, T., Daw,
N.D., Joel, D. & O'Doherty, J.P. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. _J. Neurosci._ 27,
12860–12867 (2007). Article CAS PubMed PubMed Central Google Scholar * Schönberg, T. et al. Selective impairment of prediction error signaling in human dorsolateral but not ventral
striatum in Parkinson's disease patients: evidence from a model-based fMRI study. _Neuroimage_ 49, 772–781 (2010). Article PubMed Google Scholar * Behrens, T.E., Hunt, L.T.,
Woolrich, M.W. & Rushworth, M.F. Associative learning of social value. _Nature_ 456, 245–249 (2008). Article CAS PubMed PubMed Central Google Scholar * Li, J., Delgado, M.R. &
Phelps, E.A. How instructed knowledge modulates the neural systems of reward learning. _Proc. Natl. Acad. Sci. USA_ 108, 55–60 (2011). Article PubMed Google Scholar * Niv, Y., Edlund,
J.A., Dayan, P. & O'Doherty, J.P. Neural prediction errors reveal a risk-sensitive reinforcement-learning process in the human brain. _J. Neurosci._ 32, 551–562 (2012). Article CAS
PubMed PubMed Central Google Scholar * Kirsch, I. Response expectancy as a determinant of experience and behavior. _Am. Psychol._ 40, 1189–1202 (1985). Article Google Scholar * Wager,
T.D. et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. _Science_ 303, 1162–1167 (2004). Article CAS PubMed Google Scholar * Pessiglione, M. et al.
Subliminal instrumental conditioning demonstrated in the human brain. _Neuron_ 59, 561–567 (2008). Article CAS PubMed PubMed Central Google Scholar * Schmidt, L., Palminteri, S.,
Lafargue, G. & Pessiglione, M. Splitting motivation: unilateral effects of subliminal incentives. _Psychol. Sci._ 21, 977–983 (2010). Article PubMed Google Scholar * Benedetti, F. How
the doctor's words affect the patient's brain. _Eval. Health Prof._ 25, 369–386 (2002). Article PubMed Google Scholar * Fournier, J.C. et al. Antidepressant drug effects and
depression severity: a patient-level meta-analysis. _J. Am. Med. Assoc._ 303, 47–53 (2010). Article CAS Google Scholar * Kirsch, I. & Sapirstein, G. Listening to Prozac but hearing
placebo: a meta-analysis of antidepressant medication. _Prev. Treat._ 1, 2a (1998). Google Scholar * Bódi, N. et al. Reward-learning and the novelty-seeking personality: a between- and
within-subjects study of the effects of dopamine agonists on young Parkinson's patients. _Brain_ 132, 2385–2395 (2009). Article PubMed PubMed Central Google Scholar * Frank, M.J.,
Seeberger, L.C. & O'Reilly, R.C. By carrot or by stick: cognitive reinforcement learning in parkinsonism. _Science_ 306, 1940–1943 (2004). Article CAS PubMed Google Scholar *
Palminteri, S. et al. Pharmacological modulation of subliminal learning in Parkinson's and Tourette's syndromes. _Proc. Natl. Acad. Sci. USA_ 106, 19179–19184 (2009). Article
PubMed PubMed Central Google Scholar * Maia, T.V. Reinforcement learning, conditioning, and the brain: successes and challenges. _Cogn. Affect. Behav. Neurosci._ 9, 343–364 (2009).
Article PubMed Google Scholar * Gold, J.M. et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence.
_Arch. Gen. Psychiatry_ 69, 129–138 (2012). Article PubMed PubMed Central Google Scholar * Bamford, N.S. et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal
terminals. _Neuron_ 42, 653–663 (2004). Article CAS PubMed Google Scholar * Wang, W. et al. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens
core. _J. Physiol. (Lond.)_ 590, 3743–3769 (2012). Article CAS Google Scholar * Bamford, N.S. et al. Dopamine modulates release from corticostriatal terminals. _J. Neurosci._ 24,
9541–9552 (2004). Article CAS PubMed PubMed Central Google Scholar * Colloca, L. et al. Learning potentiates neurophysiological and behavioral placebo analgesic responses. _Pain_ 139,
306–314 (2008). Article PubMed Google Scholar * Voudouris, N.J., Peck, C.L. & Coleman, G. The role of conditioning and verbal expectancy in the placebo response. _Pain_ 43, 121–128
(1990). Article CAS PubMed Google Scholar * Kordower, J.H. et al. Disease duration and the integrity of the nigrostriatal system in disease. _Brain_ 136, 2419–2431 (2013). Article
PubMed PubMed Central Google Scholar * Cools, R., Barker, R.A., Sahakian, B.J. & Robbins, T.W. Enhanced or impaired cognitive function in Parkinson's disease as a function of
dopaminergic medication and task demands. _Cereb. Cortex_ 11, 1136–1143 (2001). Article CAS PubMed Google Scholar * Frank, M.J., Seeberger, L.C. & O'Reilly, R.C. By carrot or by
stick: cognitive reinforcement learning in parkinsonism. _Science_ 306, 1940–1943 (2004). Article CAS PubMed Google Scholar * Shohamy, D., Myers, C.E., Geghman, K.D., Sage, J. &
Gluck, M.A. L-dopa impairs learning, but spares generalization, in Parkinson's disease. _Neuropsychologia_ 44, 774–784 (2006). Article PubMed Google Scholar * Shohamy, D., Myers,
C.E., Grossman, S., Sage, J. & Gluck, M.A. The role of dopamine in cognitive sequence learning: evidence from Parkinson's disease. _Behav. Brain Res._ 156, 191–199 (2005). Article
CAS PubMed Google Scholar * Benedetti, F. et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. _Nat. Neurosci._ 7, 587–588
(2004). Article CAS PubMed Google Scholar * Benedetti, F. et al. Electrophysiological properties of thalamic, subthalamic and nigral neurons during the anti-parkinsonian placebo
response. _J. Physiol. (Lond.)_ 587, 3869–3883 (2009). Article CAS Google Scholar * Starkstein, S.E. et al. Reliability, validity, and clinical correlates of apathy in Parkinson's
disease. _J. Neuropsychiatry Clin. Neurosci._ 4, 134–139 (1992). Article CAS PubMed Google Scholar * Spielberger, C.D. _State-Trait Anxiety Inventory: Bibliography, 2nd edn._ (Consulting
Psychologists Press, 1989). * Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and
recommendations. _Mov. Disord._ 18, 738–750 (2003). * Sutton, R.S. & Barto, A.G. _Reinforcement Learning: an Introduction_ (Bradford Book, 1998). * Lindquist, M.A., Spicer, J., Asllani,
I. & Wager, T.D. Estimating and testing variance components in a multi-level GLM. _Neuroimage_ 59, 490–501 (2012). Article PubMed Google Scholar * Mazaika, P., Hoeft, F., Glover, G.H.
& Reiss, A.L. Methods and software for fMRI analysis of clinical subjects. _Organ. Hum. Brain Mapp_ 475 (2009). * Hare, T.A., O'Doherty, J., Camerer, C.F., Schultz, W. &
Rangel, A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. _J. Neurosci._ 28, 5623–5630 (2008). Article CAS
PubMed PubMed Central Google Scholar * Pessiglione, M., Seymour, B., Flandin, G., Dolan, R.J. & Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behavior in
humans. _Nature_ 442, 1042–1045 (2006). Article CAS PubMed PubMed Central Google Scholar * Behrens, T.E., Hunt, L.T., Woolrich, M.W. & Rushworth, M.F. Associative learning of social
value. _Nature_ 456, 245–249 (2008). Article CAS PubMed PubMed Central Google Scholar * Niv, Y., Edlund, J.A., Dayan, P. & O'Doherty, J.P. Neural prediction errors reveal a
risk-sensitive reinforcement-learning process in the human brain. _J. Neurosci._ 32, 551–562 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, J., Delgado, M.R. &
Phelps, E.A. How instructed knowledge modulates the neural systems of reward learning. _Proc. Natl. Acad. Sci. USA_ 108, 55–60 (2011). Article PubMed Google Scholar * Maldjian, J.A.,
Laurienti, P.J., Kraft, R.A. & Burdette, J.H. An automated method for neuroanatomic and cytoarchitectonic atlas–based interrogation of fMRI data sets. _Neuroimage_ 19, 1233–1239 (2003).
Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank neurologists P. Greene, R. Alcalay, L. Coté and the nursing staff of the Center for Parkinson's Disease
and Other Movement Disorders at Columbia University Presbyterian Hospital for help with patient recruitment and discussion of the findings, N. Johnston and B. Vail for help with data
collection, M. Sharp, K. Duncan, D. Sulzer, J. Weber and B. Doll for insightful discussion, and M. Pessiglione and G.E. Wimmer for helpful comments on an earlier version of the manuscript.
This study was supported by the Michael J. Fox Foundation and the US National Institutes of Health (R01MH076136). AUTHOR INFORMATION Author notes * Tor D Wager and Daphna Shohamy: These
authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Psychology Department, Columbia University, New York, New York, USA Liane Schmidt, Erin Kendall Braun & Daphna
Shohamy * Psychology Department, University of Colorado at Boulder, Boulder, Colorado, USA Tor D Wager * Kavli Center for Brain Science, Columbia University, New York, New York, USA Daphna
Shohamy Authors * Liane Schmidt View author publications You can also search for this author inPubMed Google Scholar * Erin Kendall Braun View author publications You can also search for
this author inPubMed Google Scholar * Tor D Wager View author publications You can also search for this author inPubMed Google Scholar * Daphna Shohamy View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS D.S. and T.D.W. planned the experiment. L.S., T.D.W. and D.S. developed the experimental design. L.S. and E.K.B. collected data.
L.S. analyzed data. D.S. and T.D.W. supervised and assisted in data analysis. L.S., E.K.B., T.D.W. and D.S. wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Tor D Wager or
Daphna Shohamy. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 EXPERIMENTAL AND
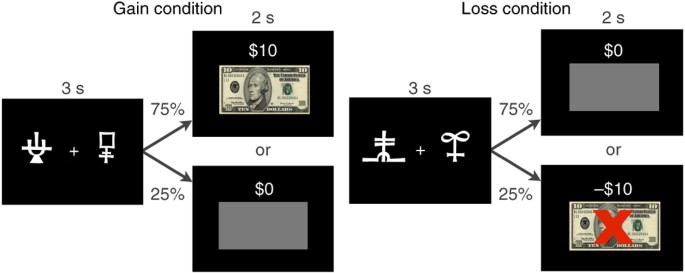
TASK DESIGN. (A) Experimental design. Each patient was scanned 3 times: off drug, on placebo, and on drug. Off drug and placebo scan sessions were counterbalanced across patients (order 1: 8
patients; order 2: 10 patients). Scan sessions lasted for 1 h and were separated by a 1 h break. Placebo and dopaminergic medication were crushed into orange juice and administered 30 min
before the respective scan session. (B) Task structure. The outcome of a trial could be a gain of $10, nothing ($0), or a loss of $10. Two cue pairs, a gain and a loss cue pair, were
randomly intermixed. Within the gain cue pair, optimal choices led to a gain of $10 with a probability of.75 and to nothing ($0) with a probability of.25. Within the loss cue pair, optimal
choices led to nothing ($0) with a probability of.75 and to a loss of $10 with a probability of.25. SUPPLEMENTARY FIGURE 2 PARTIAL CORRELATIONS BETWEEN ON DRUG AND PLACEBO CONTROLLING FOR
THE EFFECTS OF OFF DRUG (_N_ = 18). Partial correlations between on drug and placebo controlling for effects of off drug for reward learning (left) (r = 0.34, p = 0.08) and motor symptoms
(right) (r = 0.59, p = 0.01). On drug and placebo expressed as residuals after confounds due to off drug effects were regressed out. SUPPLEMENTARY FIGURE 3 BOLD RESPONSES TO CHOICES AND
FEEDBACK IN THE VMPFC AND THE VENTRAL STRIATUM (_N_ = 15). (A) Parameter estimates for choices (correct vs. incorrect) from the vmPFC region of interest in the gain and loss condition for
off drug (gray), placebo (blue), and on drug (black) treatments. (B) Parameter estimates for feedback (correct vs. incorrect) from the ventral striatum region of interest in the gain and
loss condition for the off drug (gray), placebo (blue), and on drug (black) treatments. Error bars represent within subject standard errors. SUPPLEMENTARY FIGURE 4 VENTRAL STRIATUM RESPONSES
TO COMPONENTS OF THE PREDICTION ERROR (_N_ = 15). Parameter estimates (betas) from the ventral striatum at time of outcome for the two components of prediction error – expected value and
reward – in the off drug (gray), placebo (blue) and on drug (black) treatments. Error bars represent within subject standard errors. SUPPLEMENTARY FIGURE 5 REACTION TIMES DURING LEARNING
ACROSS CONDITIONS AND TREATMENT. Average reaction time in seconds for the gain (left) and loss condition (right) in the off drug (gray), placebo (blue), and on drug (black) treatments. The
reaction time curves depict how fast patients chose the optimal choice cue. Error bars represent within-subject standard errors. SUPPLEMENTARY FIGURE 6 BEHAVIORAL RESULTS FOR THE SUBGROUP OF
PATIENTS SCANNED WITH FMRI (_N_ = 15). Percentage of observed optimal choices binned across bocks of 8 trials (left), smoothed (middle), and modeled optimal choices (right) in the gain
(top) and loss (bottom) conditions for the off drug (gray), placebo (blue), and on drug (black) treatments. The learning curves depict how often patients chose the 75% rewarding cue (t11 =
5.2, p < 0.001) during the gain condition and the 75% nothing cue (t11 = 4.1, p < 0.01) during the loss condition. Error bars represent within-subject standard errors. SUPPLEMENTARY
FIGURE 7 OBSERVED AND MODELED BEHAVIORAL RESULTS FOR THE SCANNED PATIENTS (N = 15). Percentage of observed behavioral choices (dots) and modeled optimal choices (solid lines) across trials
for off drug (gray), placebo (blue), and on drug (black) treatments. The learning curves depict how often patients chose the 75% rewarding cue (t11 = 5.2, p < 0.001) during the gain
condition and the 75% loss cue (t11 = −4.1, p < 0.01) during the loss condition. The modeled learning curves represent the probabilities of choice on each trial, as predicted by the RL
model. SUPPLEMENTARY FIGURE 8 DIRECT COMPARISONS OF VALUE AND PREDICTION ERROR RESPONSES ACROSS TREATMENTS. Statistical parametric maps (SPMs) are superimposed on the average structural
scan. (A) SPMs are masked for the vmPFC ROI, defined a priori by MNI = [−1, 27, −8]) from Hare et al. 2008, at p < 0.05 uncorrected. (B) Whole brain activations for value at p < 0.05
uncorrected. (C) SPMs are masked for the ventral striatum ROI, defined a priori by MNI = [−10, 12, −8]) from Pessiglione et al. 2006, at p < 0.05 uncorrected. (D) Whole brain activations
for prediction error at p < 0.05 uncorrected. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–8 and Supplementary Tables 1–2 (PDF 795 kb) SUPPLEMENTARY
METHODS CHECKLIST (PDF 530 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schmidt, L., Braun, E., Wager, T. _et al._ Mind matters: placebo enhances
reward learning in Parkinson's disease. _Nat Neurosci_ 17, 1793–1797 (2014). https://doi.org/10.1038/nn.3842 Download citation * Received: 15 April 2014 * Accepted: 16 September 2014 *
Published: 19 October 2014 * Issue Date: December 2014 * DOI: https://doi.org/10.1038/nn.3842 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative