- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
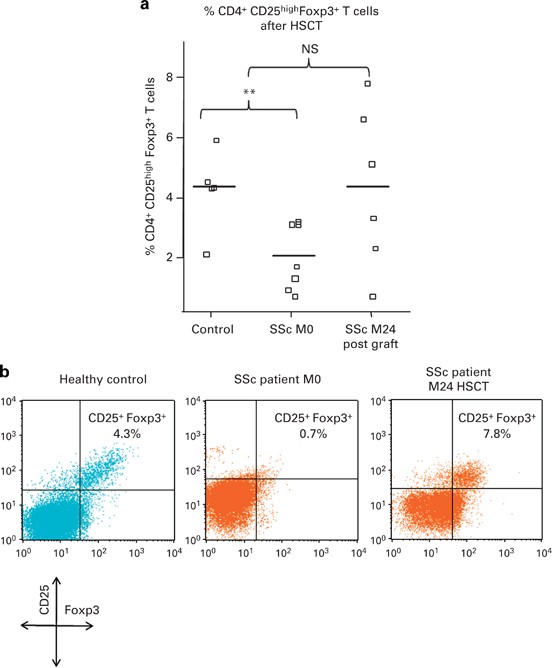
ABSTRACT A set of triterpene A-ring hydroxymethylene-amino-derivatives was synthesized and their antiviral activity was studied. The synthesized compounds were tested for their potential
inhibition of SARS-CoV-2 pseudovirus in BHK-21-hACE2 cells and influenza A/PuertoRico/8/34 (H1N1) virus in MDCK cell culture. Compounds 6, 8 and 19 showed significant anti-SARS-CoV-2
pseudovirus activity with EC50 value of 3.20-11.13 µM, which is comparable to the positive control amodiaquine (EC50 3.17 µM). Among them, 28-_O_-imidazolyl-azepano-betulin 6 and
C3-hydroxymethylene-amino-glycyrrhetol-11,13-diene 19 were identified as the lead compounds with SI values of 7 and 10. The binding mode of compound 6 into the RBD domain of SARS-CoV-2 spike
glycoprotein (PDB code: 7DK3) by docking and molecular dynamics simulation was investigated. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn
more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS
OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURAL, DYNAMIC BEHAVIOUR, IN-VITRO AND
COMPUTATIONAL INVESTIGATIONS OF SCHIFF’S BASES OF 1,3-DIPHENYL UREA DERIVATIVES AGAINST SARS-COV-2 SPIKE PROTEIN Article Open access 01 June 2024 DISCOVERING NATURAL PRODUCTS AS POTENTIAL
INHIBITORS OF SARS-COV-2 SPIKE PROTEINS Article Open access 02 January 2025 IDENTIFICATION OF ANTIVIRAL PHYTOCHEMICALS AS A POTENTIAL SARS-COV-2 MAIN PROTEASE (MPRO) INHIBITOR USING DOCKING
AND MOLECULAR DYNAMICS SIMULATIONS Article Open access 13 October 2021 REFERENCES * World Health Organization: Coronavirus disease (COVID-19) situation report.
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 21 Dec 2022. * Banerjee R, Perera L, Tillekeratne LMV. Potential SARS-CoV-2 main protease
inhibitors. Drug Discov Today. 2021;26:804–16. https://doi.org/10.1016/j.drudis.2020.12.005 Article CAS PubMed Google Scholar * Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural
products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–16. https://doi.org/10.1038/s41573-020-00114-z Article CAS PubMed PubMed Central Google Scholar
* Battineni JK, Koneti PK, Bakshi V, Boggula N. Triterpenoids: a review. Int J Pharm Pharm Sci. 2018;2:91–96. Google Scholar * Hou W, Liu B, Xu H. Celastrol: progresses in
structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur J Med Chem. 2020;189:112081. https://doi.org/10.1016/j.ejmech.2020.112081 Article CAS PubMed
Google Scholar * Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev. 2019;18:929–51.
https://doi.org/10.1007/s11101-019-09623-1 Article CAS Google Scholar * Hussain H, Green IR, Ali I, Khan IA, Ali Z, Al-Sadi AM, Ahmed I. Ursolic acid derivatives for pharmaceutical use: a
patent review (2012–2016). Expert Opin Ther Pat. 2017;27:1061–72. https://doi.org/10.1080/13543776.2017.1344219 Article CAS PubMed Google Scholar * Xiao S, Tian Z, Wang Y, Si L, Zhang
L, Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med Res Rev. 2018;38:951–76. https://doi.org/10.1002/med.21484
Article PubMed PubMed Central Google Scholar * Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, Park KH, Rho MC, Lee WS. SARS-CoV 3CLpro inhibitory effects of quinone-methide
triterpenes from Tripterygium regelii. Bioorg Med Chem Lett. 2010;20:1873–6. https://doi.org/10.1016/j.bmcl.2010.01.152 Article CAS PubMed PubMed Central Google Scholar * Cinatl J,
Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–6.
https://doi.org/10.1016/S0140-6736(03)13615-X Article CAS PubMed PubMed Central Google Scholar * Wu CY, Jan JT, Ma SH, Kuo C-J, Juan H-F, Cheng Y-SE, Hsu H-H, Huang H-C, Wu D, Brik A,
Liang F-S, Liu R-S, Fang J-M, Chen S-T, Liang P-H, Wong C-H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci USA. 2004;101:10012–7.
https://doi.org/10.1073/pnas.0403596101 Article CAS PubMed PubMed Central Google Scholar * Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, Doerr HW, Cinatl J
Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–9. https://doi.org/10.1021/jm0493008 Article CAS PubMed Google Scholar * Wen
CC, Kuo YH, Jan JT, Liang PH, Wang SY, Liu HG, Lee CK, Chang ST, Kuo CJ, Lee SS, Hou CC, Hsiao PW, Chien SC, Shyur LF, Yang NS. Specific plant terpenoids and lignoids possess potent
antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50:4087–95. https://doi.org/10.1021/jm070295s Article CAS PubMed Google Scholar * Stevaert B,
Krasniqi B, Van Loy B, Nguyen T, Thomas J, Vandeput J, Jochmans D, Thiel V, Dijkman R, Dehaen W, Voet A, Naesens L. Betulonic acid derivatives interfering with human coronavirus 229E
replication via the nsp15 endoribonuclease. J Med Chem. 2021;64:5632–44. https://doi.org/10.1021/acs.jmedchem.0c02124 Article CAS PubMed Google Scholar * Krasilnikov IV, Kudriavtsev AV,
Vakhrusheva AV, Frolova ME, Ivanov AV, Stukova MA, Romanovskaya-Romanko EA, Vasilyev KA, Mushenkova NV, Isaev AA. Design and immunological properties of the novel subunit virus-like vaccine
against SARS-CoV-2. Vaccines. 2020;10:69. https://doi.org/10.3390/vaccines10010069 Article CAS Google Scholar * Yarovaya OI, Shcherbakov DN, Borisevich SS, Sokolova AS, Gureev MA,
Khamitov EM, Rudometova NB, Zybkina AV, Mordvinova ED, Zaykovskaya AV, Rogachev AD, Pyankov OV, Maksyutov RA, Salakhutdinov NF. Borneol ester derivatives as entry inhibitors of a wide
spectrum of SARS-CoV-2 viruses. Viruses. 2022;14:1295. https://doi.org/10.3390/v14061295 Article CAS PubMed PubMed Central Google Scholar * Kadela-Tomanek M, Jastrzebska M, Marciniec K,
Chrobak E, Bebenek E, Boryczka S. Lipophilicity, pharmacokinetic properties, and molecular docking study on SARS-CoV-2 target for betulin triazole derivatives with attached 1,4-quinone.
Pharmaceutics. 2021;13:781. https://doi.org/10.3390/pharmaceutics13060781 Article CAS PubMed PubMed Central Google Scholar * Tretyakova EV, Ma X, Kazakova OB, Shtro AA, Petukhova GD,
Smirnova AA, Xu H, Xiao S. Abietic, maleopimaric and quinopimaric dipeptide Ugi-4CR derivatives and their potency against influenza A and SARS-CoV-2. Nat Prod Res. Published online: 17 Aug
2022. https://doi.org/10.1080/14786419.2022.2112040 * Tret’yakova EV, Ma X, Kazakova OB, Shtro AA, Petukhova GD, Klabukov AM, Dyatlov DS, Smirnova AA, Xu H, Xiao S. Synthesis and Evaluation
of diterpenic mannich bases as antiviral agents against influenza A and SARS-CoV-2. Phytochem Lett 2022;51:91–96. https://doi.org/10.1016/j.phytol.2022.07.010 Article CAS PubMed PubMed
Central Google Scholar * Szostak M, Yao L, Aube J. Proximity effects in nucleophilic addition reactions to medium-bridged twisted lactams: remarkably stable tetrahedral intermediates. J Am
Chem Soc. 2010;132:2078–84. https://doi.org/10.1021/ja909792h Article CAS PubMed PubMed Central Google Scholar * Evans DA, Borg G, Scheidt KA. Remarkably stable tetrahedral
intermediates: carbinols from nucleophilic additions to N-acylpyrroles. Angew Chem Int Ed. 2002;41:3188–91. 10.1002/1521-3773(20020902)41:17<3188::AID-ANIE3188>3.0.CO;2-H Article CAS
Google Scholar * Iwasawa T, Hooley RJ, Rebek J. Stabilization of labile carbonyl addition intermediates by a synthetic receptor. Science 2007;317:493–6.
https://doi.org/10.1126/science.11432 Article CAS PubMed Google Scholar * Hooley RJ, Restorp P, Iwasawa T, Rebek J. Cavitands with introverted functionality stabilize tetrahedral
intermediates. J Am Chem Soc. 2007;129:15639–43. https://doi.org/10.1021/ja0756366 Article CAS PubMed Google Scholar * Subik P, Welc B, Wisz B, Wolowiec S. Stable hemiaminals attached to
PAMAM dendrimers. Tetrahedron Lett. 2009;50:6512–4. https://doi.org/10.1016/j.tetlet.2009.09.031 Article CAS Google Scholar * Barys M, Ciunik Z, Drabent K, Kwiecien A. Stable hemiaminals
containing a triazole ring. N J Chem. 2010;34:2605–11. https://doi.org/10.1039/C0NJ00346H Article CAS Google Scholar * Scott MS, Lucas AC, Luckhurst CA, Prodger JC, Dixon DJ.
Ligand-mediated enantioselective addition of lithium carbazolates to aldehydes. Org Biomol Chem. 2006;4:1313–27. https://doi.org/10.1039/B515356E Article CAS PubMed Google Scholar *
Viuff AH, Besenbacher LM, Kamori A, Jensen MT, Kilian M, Kato A, Jensen HH. Stable analogues of nojirimycin—synthesis and biological evaluation of nojiristegine and manno-nojiristegine. Org
Biomol Chem. 2015;13:9637–58. https://doi.org/10.1039/C5OB01281C Article CAS PubMed Google Scholar * Giniyatullina GV, Flekhter OB, Baikova IP, Starikova ZA, Tolstikov GA. Effective
synthesis of methyl 3β-amino-3-deoxybetulinate. Chem Nat Comp. 2008;44:603–5. https://doi.org/10.1007/s10600-008-9138-4 Article CAS Google Scholar * Smirnova A, Petrova A, Lobov E,
Minnibaeva T, Tran Thi Phoung L, Tran Van M, Myint Khine M, Esaulkova I, Slita A, Zarubaev V, Kazakova O. Azepanodipterocarpol is potential candidate for inhibits influenza H1N1 type among
other lupane, oleanane, and dammarane A-ring amino-triterpenoids. J Antibiot. 2022;75:258–67. https://doi.org/10.1038/s41429-022-00514-w Article CAS Google Scholar * Santos RC, Salvador
JAR, Marín S, Cascante M. Novel semisynthetic derivatives of betulin and betulinic acid with cytotoxic activity. Bioorg Med Chem. 2009;17:6241–50. https://doi.org/10.1016/j.bmc.2009.07.050
Article CAS PubMed Google Scholar * Kazakova O, Rubanik L, Lobov A, Poleshchuk N, Baikova I, Kapustina Y, Petrova A, Korzun T, Lopatina T, Fedorova A, Rybalova T, Polovianenko D, Mioc M,
Șoica C. Synthesis of erythrodiol C-ring derivatives and their activity against Chlamydia trachomatis. Steroids. 2021;175:108912. https://doi.org/10.1016/j.steroids.2021.108912 Article CAS
PubMed Google Scholar * Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Moller R, Hoagland D, Oishi K, Horiuchi S, Uhl S, Blanco-Melo D, Albrecht RA, Liu W-C, Jordan T, Nilsson-Payant BE,
Golynker I, Frere J, Logue J, Haupt R, McGrath M, Weston S, Zhang T, Plebani R, Soong M, Nurani A, Min Kim S, Zhu DY, Benam KH, Goyal G, Gilpin SE, Prantil-Baun R, Gygi SP, Powers RK,
Carlson KE, Frieman M, tenOever BR, Ingber DE. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng. 2021;5:815–29.
https://doi.org/10.1038/s41551-021-00718-9 Article CAS PubMed PubMed Central Google Scholar * Smee DF, Hurst BL, Evans WJ, Clyde N, Wright S, Peterson C, Jung KH, Day CW. Evaluation of
cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J Virol Methods. 2017;246:51–57. https://doi.org/10.1016/j.jviromet.2017.03.012
Article CAS PubMed PubMed Central Google Scholar * Lopatina TV, Medvedeva NI, Baikova IP, Iskhakov AS, Kazakova OB. Synthesis and cytotoxicity of O- and N-acylderivatives of
azepanobetulin. Russ J Bioorg Chem. 2019;45:292–301. https://doi.org/10.1134/S106816201904006X Article CAS Google Scholar * Kazakova OB, Rubanik LV, Smirnova IE, Savinova OV, Petrova AV,
Poleschuk NN, Khusnutdinova EF, Boreko EI, Kapustina YM. Synthesis and in vitro activity of oleanane type derivatives against Chlamydia trachomatis. Org Commun. 2019;12:169–75.
https://doi.org/10.25135/acg.oc.66.19.07.1352 Article CAS Google Scholar * Kazakova OB, Brunel JM, Khusnutdinova EF, Negrel S, Giniyatullina GV, Lopatina TV, Petrova AV. A-ring modified
triterpenoids and their spermidine-aldimines with strong antibacterial activity. Molbank. 2019;3:M1078. https://doi.org/10.3390/M1078 Article Google Scholar * Medvedeva NI, Kazakova OB,
Lopatina TV, Smirnova IE, Giniyatullina GV, Baikova IP, Kataev VE. Synthesis and antimycobacterial activity of triterpeniс A-ring azepanes. Eur J Med Chem. 2018;143:464–72.
https://doi.org/10.1016/j.ejmech.2017.11.035 Article CAS PubMed Google Scholar * Kazakova O, Smirnova I, Lopatina T, Giniyatullina G, Petrova A, Khusnutdinova E, Csuk R, Serbian I,
Loesche A. Synthesis and cholinesterase inhibiting potential of A-ring azepano- and 3-amino-3,4-seco-triterpenoids. Bioorg Chem. 2020;101:104001. https://doi.org/10.1016/j.bioorg.2020.104001
Article CAS PubMed Google Scholar * Ma C, Nakamura N, Hattori M. Chemical modification of oleanene type triterpenes and their inhibitory activity against HIV-1 protease dimerization.
Chem Pharm Bul. 2000;48:1681–8. https://doi.org/10.1248/cpb.48.1681 Article CAS Google Scholar * Jain SM, Atal CK. Synthesis of amino derivatives of ursolic acid. Ind J Chem., Section B
25B. 1986;4:427-8. * Schrödinger Release 2018-4: LigPrep, Schrödinger, LLC, New York, NY; 2018. * Schrödinger Release 2018-4: induced fit docking protocol; glide, Schrödinger, LLC, New York,
NY, 2018; Prime, Schrödinger, LLC, New York, NY; 2018. * Case DA, Darden TA, Cheatham TE, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Wang B, Hayik S,
Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh M-J, Cui G, Roe DR, Mathews DH, Seetin MG,
Sagui C, Babin V, Luchko T, Gusarov S, Kovalenko A, Kollman PA. Amber 14 reference manual, University of California, San Francisco, 2014. Download references FUNDING This research was funded
by RFBR and NSFC according to the research project № 20-53-55001 and the International Cooperation and Exchange Program of China (NSFC-RFBR, No. 82161148006). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Ufa Institute of Chemistry UFRC RAS, pr. Oktyabrya 71, 450054, Ufa, Russia Oxana Kazakova, Elena Tretyakova & Irina Smirnova * State Key Laboratory of Natural and
Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, 100191, China Xinyuan Ma, Hongwei Jin, Demin Zhou & Sulong Xiao * Institute of Chemical Biology, Shenzhen
Bay Laboratory, Shenzhen, 518132, China Xinyuan Ma, Demin Zhou & Sulong Xiao * Department of Virology, St. Petersburg Pasteur Institute of Epidemiology and Microbiology, Experimental
Virology Laboratory, 14 Mira St., St. Petersburg, 197001, Russia Alexander Slita, Ekaterina Sinegubova & Vladimir Zarubaev Authors * Oxana Kazakova View author publications You can also
search for this author inPubMed Google Scholar * Xinyuan Ma View author publications You can also search for this author inPubMed Google Scholar * Elena Tretyakova View author publications
You can also search for this author inPubMed Google Scholar * Irina Smirnova View author publications You can also search for this author inPubMed Google Scholar * Alexander Slita View
author publications You can also search for this author inPubMed Google Scholar * Ekaterina Sinegubova View author publications You can also search for this author inPubMed Google Scholar *
Vladimir Zarubaev View author publications You can also search for this author inPubMed Google Scholar * Hongwei Jin View author publications You can also search for this author inPubMed
Google Scholar * Demin Zhou View author publications You can also search for this author inPubMed Google Scholar * Sulong Xiao View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Oxana Kazakova or Sulong Xiao. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
MATERIALS RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or
other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kazakova, O., Ma, X., Tretyakova, E. _et al._ Evaluation of A-ring hydroxymethylene-amino- triterpenoids as inhibitors of SARS-CoV-2 spike
pseudovirus and influenza H1N1. _J Antibiot_ 77, 39–49 (2024). https://doi.org/10.1038/s41429-023-00677-0 Download citation * Received: 13 March 2023 * Revised: 24 October 2023 * Accepted:
28 October 2023 * Published: 24 November 2023 * Issue Date: January 2024 * DOI: https://doi.org/10.1038/s41429-023-00677-0 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative