- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A long duration of treatment and emerging drug resistance pose significant challenges for global tuberculosis (TB) eradication efforts. Therefore, there is an urgent need to develop
novel strategies to shorten TB treatment regimens and to treat drug-resistant TB. Using an albumin-fusion strategy, we created a novel albumin-fused granulocyte-macrophage
colony-stimulating factor (albGM-CSF) molecule that harnesses albumin’s long half-life and targeting abilities to enhance the biostability of GM-CSF and direct it to the lymph nodes, where
the effects of GM-CSF can increase dendritic cell populations crucial for eliciting a potent immune response. In this study, we demonstrate that albGM-CSF serves as a novel immunotherapy for
chronic _Mycobacterium tuberculosis_ (_Mtb_) infections by enhancing GM-CSF biostability in serum. Specifically, albumin is very safe, stable, and has a long half-life, thereby enhancing
the biostability of GM-CSF. In the lungs and draining lymph nodes, albGM-CSF is able to increase the numbers of dendritic cells, which are crucial for the activation of naive T cells and for
eliciting potent immune responses. Subcutaneous administration of albGM-CSF alone reduced the mean lung bacillary burden in mice with chronic tuberculosis infection. While GM-CSF
administration was associated with IL-1β release from _Mtb_-infected dendritic cells and macrophages, higher IL-1β levels were observed in albGM-CSF-treated mice with chronic tuberculosis
infection than in mice receiving GM-CSF. Albumin fusion with GM-CSF represents a promising strategy for the control of chronic lung tuberculosis infections and serves as a novel therapeutic
vaccination platform for other infectious diseases and malignancies. SIMILAR CONTENT BEING VIEWED BY OTHERS SUBUNIT VACCINE PROTECTS AGAINST A CLINICAL ISOLATE OF _MYCOBACTERIUM AVIUM_ IN
WILD TYPE AND IMMUNOCOMPROMISED MOUSE MODELS Article Open access 27 April 2021 VACCINATION WITH MINCLE AGONIST UM-1098 AND MYCOBACTERIAL ANTIGENS INDUCES PROTECTIVE TH1 AND TH17 RESPONSES
Article Open access 06 June 2024 NOVEL FUSION PROTEIN REA INDUCES ROBUST PRIME PROTECTION AGAINST TUBERCULOSIS IN MICE Article Open access 31 January 2025 INTRODUCTION Tuberculosis (TB) is
currently the most common cause of death by a single infectious agent worldwide.1 Efforts have been made to implement a 6-month “short-course” combination regimen for the treatment of
drug-susceptible TB. Although this regimen has been shown to be efficacious, it requires proper provision and direct supervision, which can be taxing for health care systems, especially in
TB-endemic regions. Inadequate treatment of TB leads to excess morbidity and mortality, continued transmission, and emergence of drug resistance.2 Therefore, novel strategies are needed to
shorten the duration of curative TB treatment. In addition to novel antimicrobial agents, host-directed therapies represent attractive strategies to combat disease due to drug-susceptible
and drug-resistant _Mycobacterium tuberculosis_ (_Mtb_).3 In particular, host-directed therapies may reverse TB-related lung inflammation and/or augment innate and adaptive immune responses
to accelerate mycobacterial clearance during anti-TB treatment.4 Effective immunity against TB depends on antigen presentation by MHC class I or class II molecules, which occurs in the
draining lymph nodes (dLNs) at the site of infection.5 Dendritic cells (DCs) are key antigen-presenting cells (APCs) that activate naive T cells by upregulating chemokine receptors and
costimulatory molecules.5,6 Mature DCs are characterized by higher expression of surface MHC class II molecules and the integrin-αX chain, as well as CD11c and other costimulatory
molecules.7 Adoptive transfer of antigen-pulsed DCs has been shown to significantly improve vaccination efficacy relative to control treatment, indicating that adequate antigen presentation
in the lungs is one of the key factors for controlling _Mtb_ infection.8 Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor critical for DC
generation, proliferation, and maturation.9,10,11 Other myeloid lineage cells, including monocytes, macrophages, neutrophils, and eosinophils, are also activated by GM-CSF.9 Coadministration
of GM-CSF during vaccination increases antigen-specific IFNγ-secreting T cells and enhances protection against various infectious agents.12,13,14,15,16,17,18 Conversely, deficiency of
GM-CSF is associated with reduced T-cell responses after vaccination.19 Mice vaccinated with a bacillus Calmette–Guérin vaccine including cells expressing murine GM-CSF were found to have
enhanced DC maturation in dLNs and increased protection against disseminated TB.16 In addition to its promotion of the maturation of DCs, GM-CSF is one of the key cytokines that promotes the
differentiation of M1 macrophages, which are key effectors in controlling intracellular pathogens through the release of proinflammatory cytokines.20,21,22 GM-CSF enhances the ability of
human macrophages to inhibit _Mtb_ growth ex vivo.23 GM-CSF−/− mice are highly susceptible to _Mtb_ infection, and anti-GM-CSF autoantibodies increase the risk of cryptococcal meningitis and
pulmonary TB in patients.10,24,25,26 GM-CSF secreted by T cells has been shown to offer protection against _Mtb_ infection in murine models.25 A greater proportion of GM-CSF+
multifunctional CD4+ T cells are present in latently infected individuals than in those with active TB.27 Furthermore, GM-CSF secretion was significantly reduced when CD3+ T cells were
cocultured with myeloid-derived suppressor cells in patients with active TB.28 These observations indicate that GM-CSF plays an important role in innate immunity and initiating adaptive
immunity, indicating the potential utility of this cytokine in anti-TB immunotherapy. Indeed, GM-CSF enhances the bactericidal activity of anti-TB drugs in both mouse models and in
humans.29,30,31 However, the observed synergy of GM-CSF is relatively limited due to its side effects, short half-life of ~7 h, and reduced penetration into the lungs.32 Therefore, an
alternative strategy is required to improve the bioavailability of GM-CSF in the lungs, which are the primary site of TB. A fusion strategy using the fragment crystallizable region (Fc
region) has been used to improve the biostability and half-life of proteins, as well as for mucosal targeting.33,34,35 However, one limitation of this approach is the potential development
of autoantibodies directed against the Fc region.33,34 Similar to immunoglobulin, albumin has an extended serum half-life of 3 weeks due to its size and its ability to undergo neonatal Fc
receptor (FcRn)-mediated recycling, thus preventing intracellular degradation.33 Since albumin is very safe and stable and has a very long half-life, it has been frequently used for drug
delivery. Currently, there are six albumin-based drugs that are commercially available, with many more being tested in clinical trials.36 Labeled human albumin has been used for LN
identification and imaging, suggesting that albumin can traffic to LNs.37 We reasoned that these favorable properties of albumin may be exploited by fusing this protein to GM-CSF to enhance
the serum levels of GM-CSF and augment its effect in the lungs and LNs, thus achieving organ-targeting vaccination. Specifically, we determined whether murine albumin conjugation was able to
increase the effects of GM-CSF in mice. In our proof-of-concept studies, we show that this albumin-fusion strategy (albGM-CSF) enhances the serum levels of GM-CSF, leading to increased DC
populations, cytokine secretion, and CD4+ T-cell activation, thus improving the control of chronic TB in mice. MATERIALS AND METHODS DNA CONSTRUCTS AND PROTEIN EXPRESSION To generate
albumin-fused GM-CSF (albGM-CSF), mouse albumin was first amplified with PCR using the cDNA template of mouse albumin (AAH49971, transOMIC Technologies, Huntsville, AL, USA) and a set of
primers, 5′-AAATCTAGAGCCACCATGAAGTGGGTAACCTTT-3′ and 5′-TTTGAATTCGGCTAAGGCGTCTTTGCATC-3′. The amplified product was then cloned into the XbaI/EcoRI sites of a pcDNA3 vector (Invitrogen
Corp., Carlsbad, CA, USA). Next, for the generation of pcDNA3-AlbGM-CSF, mouse GM-CSF was first amplified via PCR with a cDNA template of the mouse GM-CSF (NM_009969.4) gene synthesized from
Genscript (Piscataway, NJ, USA) and the following primers: 5′-TTTGAATTCGCACCCACCCGCTCACCCAT-3′ and 5′-AAACTTAAGTCATTTTTGGACTGGTTTTTTG-3′. The amplified product was then cloned into the
EcoRI/Afl II sites of pcDNA3-Alb. For the generation of pcDNA3-albGLuc, Gaussia luciferase (GLuc) was first amplified via PCR with a cDNA template of phGLuc (gifted from Dr John Schiller,
NIH) and the primers 5′-AAAGAATTCATGGGAGTCAAAGTTCTGTTTG-3′ and 5′-TTTAAGCTTTTAGTCACCACCGGCCCCCTTG-3′. The amplified product was then cloned into the EcoRI/HindIII sites of pcDNA3-Alb. For
the generation of pET28a-GLuc, GLuc was first amplified via PCR with a cDNA template of phGLuc and the following primers: 5′-AAAGAATTCGAGGCCAAGCCCACCGAGAAC-3′ and
5′-TTTCTCGAGGTCACCACCGGCCCCCTTGA-3′. The amplified product was then cloned into the EcoRI/XhoI sites of the PET28a vector (Novagen Inc., Madison, WI, USA). All plasmid constructs were
confirmed by DNA sequencing. The AlbGM-CSF and albumin-GLuc (albGLuc) proteins were expressed using the Expi293F Expression System Kit (Thermo Fisher Scientific, Waltham, MA, USA) according
to the manufacturer’s instructions. Expi293F cells were transfected with albGM-CSF and alb-GLuc, and the transfection efficiency was determined by the expression levels of the target
protein. Proteins were purified by a HiTrap albumin column (GE Healthcare Life Sciences, Marlborough, MA, USA). GLuc was expressed in _E. coli_ BL21 (Rosetta cells; Novagen) and purified by
Ni+ affinity chromatography (Ni-NTA agarose, Qiagen Sciences, Germantown, MD, USA) according to the manufacturer’s protocol). Mouse GM-CSF was purchased from Genscript. IN VIVO GLUC
ACTIVITIES Eight-to-ten-week-old female C57BL/6J (_n_ = 3–4) (NCI, Frederick, MD, USA) or eight-to-ten-week-old FcRn-knock-out (KO) mice (_n_ = 3–4) (B6.129X1-_Fcgrt_tm1Dcr/DcrJ, Jackson
Laboratory, Bar Harbor, ME, USA) received retro-orbital injections with either Gaussia luciferase (GLuc) (20 μg) or albGLuc (2.8 μg) in 20 μL of phosphate-buffered saline (PBS) following
anesthesia by ketamine/xylazine intraperitoneal injection. Seventy-two hours after injection, mice were euthanized, and the serum, inguinal LNs, and lungs were removed and disrupted by
bead-beating. GLuc activity was measured with coelenterazine-H (Regis) by a GloMax Luminometer (Promega, Madison, WI, USA). The total luminescence was normalized to tissue weight. MICE The
mice were housed in the Oncology Center Animal Facility at the Johns Hopkins Medical Institutes (Baltimore, MD, USA). All animal procedures were performed according to the approved protocols
and in accordance with the recommendations for the proper use and care of laboratory animals. To ensure that animal discomfort, distress, pain, and injury were kept to a minimum, a maximum
of five mice were housed in the same cage. All animals were maintained and all experiments were performed according to the protocols approved by the Institutional Animal Care and Use
Committee at the Johns Hopkins University School of Medicine. LUNG INJURY MODEL Female C57BL/6J mice (6–8 weeks old; _n_ = 3–4) received 20 μg of lipopolysaccharide (LPS) from _E. coli_
O26:B6 (Sigma-Aldrich, St. Louis, MO, USA) via the intranasal route. The control group received PBS. One day later, both groups of mice received either GLuc (20 μg) or albGLuc (2.8 µg) by
intranasal injection. To measure luciferase activity, sera from each group were collected the following day. Luciferase expression was taken to indicate transcytosis activity in the airway,
as previously described.38 MYCOBACTERIA AND GROWTH CONDITIONS Wild-type _Mtb_ H37Rv was grown in Middlebrook 7H9 broth (Difco, Sparks, MD, USA) supplemented with 10% oleic
acid-albumin-dextrose-catalase (Difco), 0.1% glycerol, and 0.05% Tween-80 at 37 °C in a roller bottle.39 BONE MARROW-DERIVED MACROPHAGE (BMDM) AND BONE MARROW-DERIVED DC (BMDC) CELL ASSAYS
Bone marrow was harvested from C57BL/6J mice, as previously described.40 To compare the efficacy of GM-CSF and albGM-CSF, bone marrow cells were incubated with either GM-CSF (0.625 µM) or
albGM-CSF (0.625 µM) at 37 °C in 5% CO2 and RPMI media with 10% fetal bovine serum (FBS) for 7 days (Sigma-Aldrich). Antigen-containing supplemented media were replenished on day 3. The
cells were collected to analyze the percentage of CD11c+ cells and CD11c+MHCII+ cells. For _Mtb_ infection studies, bone marrow cells were incubated with GM-CSF (10 ng/mL, Genscript) to
obtain BMDCs or with macrophage colony-stimulating factor (10 ng/mL, Genscript) to obtain BMDMs. A total of 2 × 105 BMDMs or 5 × 105 BMDCs were plated in a 24-well plate 1 day prior to
infection. H37Rv was used to infect BMDCs at an MOI of 1:2.5 (1.25 × 106 bacteria) for 1 day or BMDMs (5 × 105 bacteria) for 2 days in 1 mL of complete RPMI medium (Gibco Laboratories,
Gaithersburg, MD, USA) with 10% FBS (Sigma-Aldrich) and 0.625 µM GM-CSF or albGM-CSF. ENZYME-LINKED IMMUNOSORBENT ASSAYS (ELISAS) FOR GM-CSF OR IL-1Β The levels of GM-CSF in sera and IL-1β
in cell culture media were determined by ELISAs with mouse GM-CSF or IL-1β DuoSet ELISA kits from R&D Systems (Minneapolis, MN, USA). The IL-1β levels of lung lysates were normalized to
the lysate protein concentration using a Qubit protein assay kit (Thermo Fisher Scientific). INTRACELLULAR CYTOKINE STAINS AND FLOW CYTOMETRY ANALYSES At predetermined time points, the mice
were euthanized, and cells from LNs and spleens were collected, as previously described.41,42 To determine ESAT6 antigen-specific CD4+ or TB10 antigen-specific CD8+ T-cell responses,
splenocytes were incubated with ESAT6 ((MTEQQW NFAGIEAAA) or TB10 (IMYNYPAM) peptides (Genscript) and GolgiPlug (BD Biosciences, San Jose, CA, USA) overnight.43 After incubation, the cells
were washed once with FACScan buffer and then stained with a PE-conjugated monoclonal rat anti-mouse CD4 antibody (BD Biosciences) and/or an APC-conjugated monoclonal rat anti-mouse CD8
antibody (eBioscience, Inc., San Diego, CA, USA). Cells were permeabilized using the Cytofix/Cytoperm kit (BD Biosciences). Intracellular IFN-γ was stained using a FITC-conjugated rat
anti-mouse IFN-γ antibody. Flow cytometry was performed on a FACSCalibur instrument, and the results were analyzed with FlowJo software (Supplementary Fig. 1). To collect pneumocytes, the
lungs were perfused with 1 mL of normal saline by direct injection into the right ventricle of the heart at necropsy. A section of the lung was used for cytometry analysis, and the tissue
samples were incubated at 37 °C for 1 h with intermittent agitation in RPMI medium (Gibco Laboratories) containing collagenase D (1 mg/mL, Sigma-Aldrich), DNAase (0.25 mg/mL, Sigma-Aldrich),
and hyaluronidase type V (1 mg/mL, Sigma-Aldrich). The cells were then filtered through a 70-μm nylon filter mesh to remove undigested tissue fragments and washed with complete RPMI medium.
To identify surface markers of DCs from lungs, spleens, or LNs, PE-conjugated anti-mouse MHCII, FITC-conjugated anti-mouse CD11c, APC-conjugated anti-mouse CD103, APC-conjugated anti-mouse
CD11b, and APC-conjugated anti-mouse DEC205 (eBioscience, Inc.) antibodies were used to stain cells from each of these tissues (Supplementary Figs. 2 and 3). After washing with FACScan
buffer, the cells were counted with a FACSCalibur and analyzed using FlowJo software (Supplementary Figs. 2 and 3). AEROSOL INFECTION OF MICE WITH _MTB_ Female 6–8-week-old C57BL/6J mice
were aerosol-infected with ~100 bacilli of wild-type _Mtb_ H37Rv. After 1 month of infection, groups of mice received human-equivalent doses of isoniazid (10 mg/kg) by esophageal gavage once
daily (5 days/week). In the experimental groups, mice received 0.375 μM GM-CSF or albGM-CSF in 100 µL of PBS by retro-orbital or subcutaneous injection at predetermined time points. Mice
were euthanized on days 30, 60, and 90 after aerosol challenge, lungs were homogenized, and the cells were plated for colony-forming unit assays to evaluate the potential synergistic effect
of each therapeutic vaccine.44 STATISTICAL ANALYSES Mean differences between groups were compared using one-way analysis of variance with Tukey–Kramer post hoc analyses (MedCalc software,
Ostend, Belgium). If the data did not pass through the normality test by the D’Agostino–Pearson test, the differences of groups were compared by the Kruskal–Wallis test with post hoc
analyses. Data from at least three biological replicates were used to calculate means and standard errors of the mean (SEMs) for graphing purposes. To compare differences between
experimental and control groups, statistical analyses employed the Mann–Whitney test for sample sizes less than 4 or an unpaired Student’s _t_ test for sample sizes >4, and a _p_ value of
< 0.05 was considered statistically significant. RESULTS ALBUMIN FUSION INCREASES PROTEIN BIOSTABILITY AND TARGETS THE PROTEIN TO THE LUNGS AND LNS Previous studies have reported that
fusing albumin to various proteins can be used to image LNs.37 In the current study, we used murine albumin-fused GLuc (albGLuc) to determine the tissue distribution and serum levels of
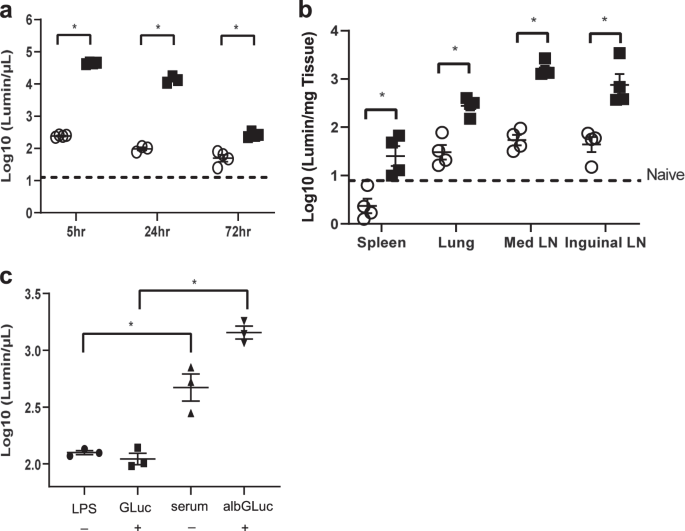
GLuc. Separate groups of female C57BL/6J mice received retro-orbital injections with either GLuc or albGLuc. In the experiments to analyze the enzymatic activity of the protein in different
tissues, both albGLuc (0.028 µg/µL) and GLuc (0.2 µg/µL) had similar luminescence activities prior to injection (1.514 × 1010 vs. 3.981 × 1010 luminescence/mL, respectively). After injection
of albGLuc or GLuc, the serum luciferase activity of the albGLuc group was 100-fold higher than that of the GLuc group 24 h after injection, and this significant difference in serum
luciferase activity was maintained for at least 72 h after injection (Fig. 1a). The luciferase activity was at least fivefold higher in the inguinal and mediastinal LNs of mice injected with
albGLuc than in mice injected with GLuc alone after 72 h of injection (Fig. 1b). The luciferase activity of the lungs was significantly (tenfold) higher in the albGLuc group than in the
GLuc group after 72 h of injection. Therefore, the albumin-fusion strategy appears to significantly increase the levels of target protein in the serum, with enhanced delivery to the LNs and
lungs. FcRn is required for the recycling and exocytosis of albumin-fused proteins.33,45 However, there is limited information about the activity of FcRn in chronic pulmonary TB. To
understand the role of FcRn in transcytosis during pulmonary inflammation, we delivered albGLuc to mice by intranasal injection under inflammatory conditions and subsequently measured serum
luciferase activities through the airway epithelium as an index of protein transcytosis. In mice with lung injuries induced by the intranasal injection of LPS 24 h before albGLuc injection,
serum GLuc activity was found to be significantly higher in the group receiving intravenous injection of albGLuc than in the group receiving GLuc alone (Fig. 1c). Based on these
observations, we conclude that the albumin-fusion strategy offers a promising approach for delivering target proteins to acutely and chronically inflamed mouse lungs. ALBGM-CSF INCREASES THE
NUMBER OF DCS IN THE DLNS AND LUNGS Studies have shown that GM-CSF enhances the intracellular killing of bacteria in vitro and that it is a key cytokine secreted by invariant natural killer
(iNK) T cells to control intracellular _Mtb_ growth.46,47 To test whether a murine albumin-fusion strategy can enhance _Mtb_ control during chronic infection, we generated albGM-CSF, which
was expressed in Expi293F cells and purified by an albumin-binding column (Fig. 2a, b). We used GM-CSF and albGM-CSF to stimulate mouse bone marrow-derived cells ex vivo for 7 days. Both
GM-CSF and albGM-CSF induced the differentiation of bone marrow cells into mature DCs without significant differences between the two groups (Fig. 2c). It has been shown that GM-CSF can
directly inhibit _Mtb_ growth in macrophages.47 To test whether albGM-CSF has the same effects, we incubated _Mtb_-infected macrophages with either GM-CSF or albGM-CSF (Fig. 2d). Both
albGM-CSF and GM-CSF significantly decreased _Mtb_ growth in macrophages. To determine whether albumin fusion enhances the stability of GM-CSF in serum, mice received either GM-CSF or
albGM-CSF by subcutaneous injection. One day after injection, the level of serum GM-CSF was significantly higher in mice receiving albGM-CSF than in those receiving GM-CSF (Fig. 3a). To
investigate whether albGM-CSF could also induce DC maturation in vivo, either albGM-CSF or GM-CSF was subcutaneously injected into C57BL/6J mice. The number of DCs detected in the dLNs of
mice 5 days after injection was significantly increased in mice that received albGM-CSF relative to those that received GM-CSF or no treatment (Fig. 3b). Similarly, the lungs of mice
injected with albGM-CSF contained significantly higher numbers of DCs and mature DCs than those of control mice (Fig. 3c). Therefore, albumin fusion of GM-CSF increases the number of DCs in
the LNs and lungs of mice, providing a novel strategy to enhance immunity against _Mtb_ infection. As previously mentioned, FcRn is required for immunoglobulin G (IgG) recycling and
transport to LNs and prolongs the half-life of both albumin and IgG.48,49,50 To determine whether FcRn is required for albGM-CSF to mediate the enhancement of DC maturation in the LNs or
lungs, FcRn-KO mice and C57BL/6J mice were subcutaneously injected with either albGM-CSF or GM-CSF. Four days after injection, the numbers of DCs observed in the dLNs of C57BL/6J mice
receiving albGM-CSF were significantly increased compared with those observed in mice receiving GM-CSF alone (Fig. 3b), but there was no such difference between albGM-CSF or GM-CSF
treatments in the FcRn-KO mice (Fig. 3d). Interestingly, the number of DCs in the lungs of FcRn-KO mice was still significantly higher than that in the albGM-CSF-treated group, indicating an
alternative mechanism mediating the recruitment of DCs to the lungs by albGM-CSF. Therefore, albGM-CSF-mediated DC recruitment to dLNs is dependent on the presence of FcRn (Fig. 3e).
ALBUMIN FUSION ENHANCES GM-CSF-MEDIATED CONTROL OF CHRONIC _MTB_ INFECTION IN MICE To determine whether albumin fusion increases the immunity induced by GM-CSF during chronic TB in mice, 4
weeks after aerosol infection with _Mtb_, female C57BL/6J mice were intravenously injected once with albGM-CSF or GM-CSF. One group of mice was treated daily (5 days/week) with isoniazid by
esophageal gavage.42 The control group received no treatment. Four weeks after treatment initiation, the mice were euthanized to determine the lung bacterial burden. The lungs of the
albGM-CSF-treated group had a significantly lower mean bacterial burden than those of the untreated and GM-CSF-treated groups (_p_ < 0.01; Fig. 4a). Although GM-CSF alone appeared to
reduce the mean lung bacterial burden relative to no treatment, consistent with prior reports,46,47 this difference was not statistically significant. To determine whether the anti-TB
activity of albGM-CSF was dependent on the intravenous route of administration, female C57BL/6J mice were first infected with _Mtb_ by an aerosol route, and 4 weeks later, they were
subcutaneously injected with either albGM-CSF or GM-CSF once every week for 4 weeks (Fig. 4b). As in the case of intravenous injection, the mean lung bacillary counts were significantly
lower in mice receiving subcutaneous injection of albGM-CSF than in those receiving no treatment or GM-CSF. GM-CSF treatment did not significantly change the bacillary burden in the lungs
compared with no treatment. There was no significant difference in the gross pathology and lung weights between the albGM-CSF-treated, GM-CSF-treated, and untreated groups (data not shown).
Therefore, albGM-CSF, delivered via either the intravenous or subcutaneous route, offers superior therapeutic efficacy against chronic TB relative to GM-CSF or no treatment (Fig. 4a, b).
Given the recognized role of hematopoietic growth factors in adaptive immune responses,12,16 we next harvested intrapulmonary lymphocytes from chronically infected mice subcutaneously
injected with albGM-CSF or GM-CSF and stimulated them with peptides from the immunodominant antigens ESAT6 and TB10.4 to measure antigen-specific CD4+ and CD8+ T cells by intracellular
cytokine staining. The lungs of mice receiving albGM-CSF, despite harboring a lower bacillary burden, contained significantly more ESAT6-specific CD4+ and TB10.4-specific CD8+ T cells than
those of the untreated group (Fig. 4c, d). Compared with those of the GM-CSF-treated group, the lungs of the albGM-CSF-treated group showed significantly higher numbers of TB10.4-specific
CD8+ T cells. At the same time, the isoniazid-treated group, which had a significantly lower mean lung bacillary burden, had significantly fewer ESAT6-specific CD4+ and TB10.4-specific CD8+
T cells than the no-treatment control group. This finding is further supported by previous studies.51,52 Taken together, our data highlight that subcutaneous albGM-CSF plays a potential
immunotherapeutic role through induction of antigen-specific T cells in the lungs. GM-CSF INCREASES THE RELEASE OF THE PROINFLAMMATORY CYTOKINE IL-1Β EX VIVO AND IN VIVO Macrophages and DCs
express the GM-CSF receptor, whereas T cells do not,53 and both can be infected by _Mtb_.54,55,56,57 GM-CSF enhances LPS-induced IL-1β secretion from DCs and macrophages by promoting NF-κB
signaling.58,59 IL-1β plays a key role in the control of intracellular _Mtb_ infection.47 To determine whether GM-CSF can enhance IL-1β secretion during _Mtb_ infection, murine BMDMs were
infected with _Mtb_ H37Rv ex vivo and exposed to 0.625 µM albGM-CSF or GM-CSF. Both GM-CSF and albGM-CSF exposure enhanced IL-1β secretion from BMDMs after overnight infection (Fig. 5a).
Next, murine BMDCs were infected with _Mtb_ and exposed to 0.625 µM albGM-CSF or GM-CSF. Both groups showed higher IL-1β secretion (Fig. 5b). Finally, we sought to determine whether
albGM-CSF injection in vivo could enhance IL-1β secretion in _Mtb_-infected mouse lungs. As expected, mice chronically infected with _Mtb_ receiving albGM-CSF had significantly higher IL-1β
levels in the lungs after normalization with total protein lysate than those receiving GM-CSF (Fig. 5c). Taken together, our findings demonstrate that GM-CSF enhances IL-1β secretion from
macrophages and DCs and that administration of albGM-CSF to _Mtb_-infected mice induces more IL-1β in the lungs. DISCUSSION Host-directed therapy for TB has generated considerable enthusiasm
as a potential approach to improve or reverse TB-induced lung damage, as well as shorten the treatment course for drug-susceptible and drug-resistant disease. However, several important
challenges remain before such therapies can be used in the clinical setting, including the need to ensure their bioavailability at the primary site of infection, the lungs.3 Prior work has
shown that iNK T cells control intracellular _Mtb_ infection by secreting the antimicrobial cytokine GM-CSF.47 In the current study, we used an albumin-fusion strategy to enhance delivery of
GM-CSF to the lungs and dLNs. We found that albGM-CSF increased DCs in the dLNs and yielded an antitubercular effect ex vivo and in mouse lungs. FcRn, a cell membrane-bound receptor, is
expressed in endothelial cells60 and professional APCs61 in the intestines, lungs, and kidneys.48 Endocytosis and transcytosis through FcRn are believed to play a key role in IgG recycling
and transport to LNs.48 Albumin binds to FcRn, which may increase its half-life.49,50 During TB, FcRn is required for IgG homeostasis and CD103+ DCs in the lungs.62 FcRn-targeting vaccines
have shown promising immunological responses, including antibody- and antigen-specific CD4+ and CD8+ T-cell responses.35 It has been shown that the half-life of GM-CSF is less than 24 h in
humans after subcutaneous injection.63 By intravenous injection, the half-life of GM-CSF in mice is 6–8.6 h.32 In our study, we found that an albumin-fusion strategy significantly increased
serum levels at 24 h, and our albumin-fusion strategy requires FcRn to target GM-CSF to LNs. The administration of albGM-CSF still increased the DC population in the lungs of FcRn-KO mice,
indicating that an alternative mechanism may play an important role in the metabolism of albumin in the lungs. This effect may be related to the increase in molecular weight, which is above
the threshold of kidney clearance.64,65 Further studies will be needed to elucidate the underlying mechanism(s) and pharmacodynamics of albGM-CSF under different administration routes.
GM-CSF promotes bone marrow cell proliferation and the expansion of macrophages and granulocytes.66 GM-CSF can enhance intracellular bacterial killing in vitro46,47 and is an important
mediator for controlling intracellular _Mtb_ infection via iNK T cells or keratinocyte growth factor.46,47 GM-CSF-deficient mice infected with _Mtb_ had accelerated mortality and more
extensive pulmonary necrosis than control _Mtb_-infected mice, and these findings were related to impaired Th1 responses in the former mice.67 During chronic TB infection in mice, GM-CSF
expression is decreased relative to that during acute infection, and intratracheal administration of adenovirus encoding GM-CSF enhances _Mtb_ control in acute and chronic infection.30 It
has been shown that GM-CSF-producing T cells play an important role in controlling chronic TB.25 The utility of GM-CSF in controlling chronic TB has been shown in animal models,29 although
limited efficacy was observed in a clinical trial,31 perhaps due to the suboptimal route and frequency of administration. In the current study, we used an albumin-fusion strategy to improve
the bioavailability and targeting of GM-CSF to the lungs and dLNs of mice. During pulmonary TB, there is significantly increased transcytosis, supporting the potential role of this approach
for targeting protein or drugs to the lungs. The ability of GM-CSF to differentiate bone marrow cells into DCs in vitro is well characterized,9,11 as is its role in enhancing antigen
presentation to T cells, thus supporting its potential utility as an adjuvant for TB vaccination.16,20,68,69 Its dual functions in both innate and adaptive immunity make GM-CSF a unique
candidate in treating TB. Macrophages and DCs express the GM-CSF receptor, whereas T cells do not.53 We have shown that both GM-CSF and albGM-CSF can enhance bacterial control during
macrophage infection ex vivo (Fig. 2d). This effect can directly decrease the bacterial burden in the lungs. Furthermore, it has been shown that GM-CSF can enhance LPS-induced IL-1β
secretion from DCs and macrophages by activating NF-κB signaling.58,59 IL-1β is a key cytokine contributing to the control of _Mtb_ growth during chronic infection.70,71 Here, we showed that
IL-1β levels were significantly higher when _Mtb_-infected DCs or macrophages were treated with GM-CSF or albGM-CSF ex vivo than when they received no treatment. In a mouse model of chronic
TB, albGM-CSF-treated mice displayed significantly higher levels of IL-1β in the lungs than untreated mice or mice treated with GM-CSF. In addition to IL-1β, it has been shown that GM-CSF
can enhance tumor necrosis factor α and IL-6 secretion from LPS-stimulated DCs.58 Another important cytokine, IL-17, is associated with GM-CSF.72,73 IL-17-secreting CD4+ T cells are
important for protection against TB,73,74 but Th17 responses are not essential for the control of disease when Th1 responses are intact.75 The role of IL-17 during chronic TB infection is
uncertain75 and may relate to outcomes of chronic infections, such as granuloma formation.76,77 It is possible that there are other cytokines or mechanisms that contribute to the
bactericidal effects of GM-CSF. Further in vivo studies to block the effects of IL-1β or other mediators, e.g., using neutralizing antibodies, will be required to determine the precise
mechanism(s) by which GM-CSF exerts its antimycobacterial effects. Finally, more work is required in preclinical models to evaluate the potential utility of albGM-CSF as an adjunctive
therapy in combination with the standard first-line regimen with the goals of shortening the duration of treatment for drug-susceptible and drug-resistant TB and improving TB-induced lung
pathology. CONCLUSIONS In the current study, we used an albumin-fusion strategy to enhance the delivery of GM-CSF to the lungs and dLNs of mice. We found that albGM-CSF acted as an in situ
vaccine, increasing the number of DCs in the dLNs and lungs and yielding a tuberculocidal effect in the lungs. Further studies are needed to evaluate the potential utility of albGM-CSF as an
adjunctive therapy in combination with the standard first-line regimen to shorten the duration of treatment for drug-susceptible and drug-resistant TB and to improve TB-induced lung
pathology. In addition to its potential utility for chronic pulmonary infections, this albumin-fusion strategy may represent a promising approach for developing novel adjuvants in cancer
prevention and therapeutic vaccines. REFERENCES * WHO. _Global Health Observat_ory (_GHO_) _Data: Tuberculosis_ (_TB_) (WHO, 2018). https://www.who.int/gho/tb/en/. * Fauci, A. S.
Multidrug-resistant and extensively drug-resistant tuberculosis: the National Institute of Allergy and Infectious Diseases Research agenda and recommendations for priority research. _J.
Infect. Dis._ 197, 1493–1498 (2008). Article PubMed Google Scholar * Wallis, R. S. & Hafner, R. Advancing host-directed therapy for tuberculosis. _Nat. Rev. Immunol._ 15, 255–263
(2015). Article CAS PubMed Google Scholar * Khan, N., Vidyarthi, A., Javed, S. & Agrewala, J. N. Innate Immunity holding the flanks until reinforced by adaptive immunity against
_Mycobacterium tuberculosis_ infection. _Front. Microbiol._ 7, 328 (2016). PubMed PubMed Central Google Scholar * Bhatt, K., Hickman, S. P. & Salgame, P. Cutting edge: a new approach
to modeling early lung immunity in murine tuberculosis. _J. Immunol._ 172, 2748–2751 (2004). Article CAS PubMed Google Scholar * Banchereau, J. & Steinman, R. M. Dendritic cells and
the control of immunity. _Nature_ 392, 245–252 (1998). Article CAS PubMed Google Scholar * Shortman, K. & Liu, Y. J. Mouse and human dendritic cell subtypes. _Nat. Rev. Immunol._ 2,
151–161 (2002). Article CAS PubMed Google Scholar * Griffiths, K. L. et al. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine
efficacy. _Nat. Commun._ 7, 13894 (2016). Article CAS PubMed PubMed Central Google Scholar * Gasson, J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor.
_Blood_ 77, 1131–1145 (1991). Article CAS PubMed Google Scholar * Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with
granulocyte/macrophage colony-stimulating factor. _J. Exp. Med._ 176, 1693–1702 (1992). Article CAS PubMed Google Scholar * Becher, B., Tugues, S. & Greter, M. GM-CSF: from growth
factor to central mediator of tissue inflammation. _Immunity_ 45, 963–973 (2016). Article CAS PubMed Google Scholar * Weiss, W. R. et al. A plasmid encoding murine granulocyte-macrophage
colony-stimulating factor increases protection conferred by a malaria DNA vaccine. _J. Immunol._ 161, 2325–2332 (1998). CAS PubMed Google Scholar * Siddiqui, A. A. et al. Induction of
protective immunity against _Schistosoma mansoni_ via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating
factor and interleukin-4. _Infect. Immun._ 71, 3844–3851 (2003). Article CAS PubMed PubMed Central Google Scholar * Qiu, J. T. et al. Novel codon-optimized GM-CSF gene as an adjuvant to
enhance the immunity of a DNA vaccine against HIV-1 Gag. _Vaccine_ 25, 253–263 (2007). Article CAS PubMed Google Scholar * Parker, J. N. et al. Genetically engineered herpes simplex
viruses that express IL-12 or GM-CSF as vaccine candidates. _Vaccine_ 24, 1644–1652 (2006). Article CAS PubMed Google Scholar * Ryan, A. A. et al. Improved protection against
disseminated tuberculosis by _Mycobacterium bovis_ bacillus Calmette-Guerin secreting murine GM-CSF is associated with expansion and activation of APCs. _J. Immunol._ 179, 8418–8424 (2007).
Article CAS PubMed Google Scholar * Fensterle, J., Grode, L., Hess, J., Kaufmann, S. H. & Effective, D. N. A. vaccination against listeriosis by prime/boost inoculation with the gene
gun. _J. Immunol._ 163, 4510–4518 (1999). CAS PubMed Google Scholar * Kim, J. J. et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector
with a DNA immunogen. _J. Immunol._ 158, 816–826 (1997). CAS PubMed Google Scholar * Wada, H., Noguchi, Y., Marino, M. W., Dunn, A. R. & Old, L. J. T cell functions in
granulocyte/macrophage colony-stimulating factor deficient mice. _Proc. Natl Acad. Sci. USA_ 94, 12557–12561 (1997). Article CAS PubMed PubMed Central Google Scholar * Ruan, J., Duan,
Y., Li, F. & Wang, Z. Enhanced synergistic anti-Lewis lung carcinoma effect of a DNA vaccine harboring a MUC1-VEGFR2 fusion gene used with GM-CSF as an adjuvant. _Clin. Exp. Pharmacol.
Physiol._ 44, 71–78 (2017). Article CAS PubMed Google Scholar * Ginhoux, F., Schultze, J. L., Murray, P. J., Ochando, J. & Biswas, S. K. New insights into the multidimensional
concept of macrophage ontogeny, activation and function. _Nat. Immunol._ 17, 34–40 (2016). Article CAS PubMed Google Scholar * Sica, A., Erreni, M., Allavena, P. & Porta, C.
Macrophage polarization in pathology. _Cell. Mol. Life Sci._ 72, 4111–4126 (2015). Article CAS PubMed Google Scholar * Denis, M. & Ghadirian, E. Granulocyte-macrophage
colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. _Immunol. Lett._ 24, 203–206 (1990). Article CAS PubMed Google Scholar * Szeliga, J. et al.
Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. _Tuberculosis_ 88, 7–20 (2008). Article CAS PubMed Google Scholar * Rothchild, A. C. et al.
Role of granulocyte-macrophage colony-stimulating factor production by T cells during _Mycobacterium tuberculosis_ infection. _mBio_ 8, e01514–e01517 (2017). Article CAS PubMed PubMed
Central Google Scholar * Rosen, L. B. et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. _J. Immunol._ 190, 3959–3966 (2013). Article CAS PubMed Google Scholar
* Mueller, H. et al. _Mycobacterium tuberculosis_-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. _Cytokine_ 43, 143–148 (2008). Article CAS
PubMed Google Scholar * du Plessis, N. et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent _Mycobacterium tuberculosis_ infection
suppresses T-cell function. _Am. J. Respir. Crit. Care Med._ 188, 724–732 (2013). Article PubMed CAS Google Scholar * Zhang, Y. et al. Immunotherapy using IL-2 and GM-CSF is a potential
treatment for multidrug-resistant _Mycobacterium tuberculosis_. _Sci. China Life Sci._ 55, 800–806 (2012). Article CAS PubMed Google Scholar * Francisco-Cruz, A., Mata-Espinosa, D.,
Estrada-Parra, S., Xing, Z. & Hernandez-Pando, R. Immunotherapeutic effects of recombinant adenovirus encoding granulocyte-macrophage colony-stimulating factor in experimental pulmonary
tuberculosis. _Clin. Exp. Immunol._ 171, 283–97 (2013). Article CAS PubMed PubMed Central Google Scholar * Pedral-Sampaio, D. B. et al. Use of Rhu-GM-CSF in pulmonary tuberculosis
patients: results of a randomized clinical trial. _Braz. J. Infect. Dis._ 7, 245–252 (2003). Article CAS PubMed Google Scholar * Burgess, A. W. & Metcalf, D. Serum half-life and
organ distribution of radiolabeled colony stimulating factor in mice. _Exp. Hematol._ 5, 456–464 (1977). CAS PubMed Google Scholar * Strohl, W. R. Fusion proteins for half-life extension
of biologics as a strategy to make biobetters. _BioDrugs_ 29, 215–239 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, L. Pharmacokinetics of monoclonal antibodies and
Fc-fusion proteins. _Protein Cell_ 9, 15–32 (2017). Article PubMed PubMed Central CAS Google Scholar * Ye, L., Zeng, R., Bai, Y., Roopenian, D. C. & Zhu, X. Efficient mucosal
vaccination mediated by the neonatal Fc receptor. _Nat. Biotechnol._ 29, 158–163 (2011). Article CAS PubMed PubMed Central Google Scholar * Larsen, M. T., Kuhlmann, M., Hvam, M. L.
& Howard, K. A. Albumin-based drug delivery: harnessing nature to cure disease. _Mol. Cell. Ther._ 4, 3 (2016). Article PubMed PubMed Central Google Scholar * Wang, Y. et al. In vivo
albumin labeling and lymphatic imaging. _Proc. Natl Acad. Sci. USA_ 112, 208–213 (2015). Article PubMed CAS Google Scholar * Palaniyandi, S. et al. Inhibition of CD23-mediated IgE
transcytosis suppresses the initiation and development of allergic airway inflammation. _Mucosal Immunol._ 8, 1262–1274 (2015). Article CAS PubMed PubMed Central Google Scholar * Dutta,
N. K. et al. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. _Antimicrob. Agents Chemother._ 56, 3726–3731 (2012). Article CAS PubMed PubMed
Central Google Scholar * Peng, S. et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life.
_Hum. Gene Ther._ 16, 584–593 (2005). Article CAS PubMed Google Scholar * Peng, S. et al. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the absence of
CD4+ T cells. _Cell Biosci._ 4, 11 (2014). Article PubMed PubMed Central CAS Google Scholar * Chuang, Y. M. et al. The stringent response factors PPX1 and PPK2 play an important role in
_Mycobacterium tuberculosis_ metabolism, biofilm formation, and sensitivity to isoniazid in vivo. _Antimicrob. Agents Chemother._ 60, 6460–6470 (2016). Article CAS PubMed PubMed Central
Google Scholar * Brandt, L., Oettinger, T., Holm, A., Andersen, A. B. & Andersen, P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to
_Mycobacterium tuberculosis_. _J. Immunol._ 157, 3527–3533 (1996). CAS PubMed Google Scholar * Chuang, Y. M., Belchis, D. A. & Karakousis, P. C. The polyphosphate kinase gene ppk2 is
required for _Mycobacterium tuberculosis_ inorganic polyphosphate regulation and virulence. _mBio_ 4, e00039–13 (2013). Article CAS PubMed PubMed Central Google Scholar * Sleep, D.
Albumin and its application in drug delivery. _Expert Opin. Drug Deliv._ 12, 793–812 (2015). Article CAS PubMed Google Scholar * Pasula, R., Azad, A. K., Gardner, J. C., Schlesinger, L.
S. & McCormack, F. X. Keratinocyte growth factor administration attenuates murine pulmonary _Mycobacterium tuberculosis_ infection through granulocyte-macrophage colony-stimulating
factor (GM-CSF)-dependent macrophage activation and phagolysosome fusion. _J. Biol. Chem._ 290, 7151–7159 (2015). Article CAS PubMed PubMed Central Google Scholar * Rothchild, A. C.,
Jayaraman, P., Nunes-Alves, C. & Behar, S. M. iNKT cell production of GM-CSF controls _Mycobacterium tuberculosis_. _PLoS Pathog._ 10, e1003805 (2014). Article PubMed PubMed Central
CAS Google Scholar * Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. _Nat. Rev. Immunol._ 7, 715–725 (2007). Article CAS PubMed Google Scholar *
Chaudhury, C. et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. _J. Exp. Med._ 197, 315–322 (2003). Article CAS PubMed
PubMed Central Google Scholar * Chaudhury, C., Brooks, C. L., Carter, D. C., Robinson, J. M. & Anderson, C. L. Albumin binding to FcRn: distinct from the FcRn-IgG interaction.
_Biochemistry_ 45, 4983–4990 (2006). Article CAS PubMed Google Scholar * Kamath, A., Woodworth, J. S. & Behar, S. M. Antigen-specific CD8+ T cells and the development of central
memory during _Mycobacterium tuberculosis_ infection. _J. Immunol._ 177, 6361–6369 (2006). Article CAS PubMed Google Scholar * Hoang, T. et al. ESAT-6 (EsxA) and TB10.4 (EsxH) based
vaccines for pre- and post-exposure tuberculosis vaccination. _PloS ONE_ 8, e80579 (2013). Article PubMed PubMed Central CAS Google Scholar * Rosas, M., Gordon, S. & Taylor, P. R.
Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. _Eur. J. Immunol._ 37, 2518–2528 (2007). Article CAS PubMed PubMed Central Google Scholar *
Henderson, R. A., Watkins, S. C. & Flynn, J. L. Activation of human dendritic cells following infection with _Mycobacterium tuberculosis_. _J. Immunol._ 159, 635–643 (1997). CAS PubMed
Google Scholar * Prendergast, K. A. & Kirman, J. R. Dendritic cell subsets in mycobacterial infection: control of bacterial growth and T cell responses. _Tuberculosis_ 93, 115–122
(2013). Article CAS PubMed Google Scholar * Jiao, X. et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. _J. Immunol._ 168,
1294–1301 (2002). Article CAS PubMed Google Scholar * Bodnar, K. A., Serbina, N. V. & Flynn, J. L. Fate of _Mycobacterium tuberculosis_ within murine dendritic cells. _Infect.
Immun._ 69, 800–809 (2001). Article CAS PubMed PubMed Central Google Scholar * Khameneh, H. J., Isa, S. A., Min, L., Nih, F. W. & Ruedl, C. GM-CSF signalling boosts dramatically
IL-1 production. _PloS ONE_ 6, e23025 (2011). Article CAS PubMed PubMed Central Google Scholar * Ahn, S. et al. GM-CSF and IL-4 produced by NKT cells inversely regulate IL-1beta
production by macrophages. _Immunol. Lett._ 182, 50–56 (2017). Article CAS PubMed Google Scholar * Ward, E. S., Zhou, J., Ghetie, V. & Ober, R. J. Evidence to support the cellular
mechanism involved in serum IgG homeostasis in humans. _Int. Immunol._ 15, 187–95 (2003). Article CAS PubMed Google Scholar * Akilesh, S., Christianson, G. J., Roopenian, D. C. &
Shaw, A. S. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. _J. Immunol._ 179, 4580–4588 (2007). Article CAS PubMed Google Scholar *
Vogelzang, A. et al. Neonatal Fc receptor regulation of lung immunoglobulin and CD103+ dendritic cells confers transient susceptibility to tuberculosis. _Infect. Immun._ 84, 2914–2921
(2016). Article CAS PubMed PubMed Central Google Scholar * Cebon, J. et al. Pharmacokinetics of human granulocyte-macrophage colony-stimulating factor using a sensitive immunoassay.
_Blood_ 72, 1340–1347 (1988). Article CAS PubMed Google Scholar * Andersen, J. T., Daba, M. B., Berntzen, G., Michaelsen, T. E. & Sandlie, I. Cross-species binding analyses of mouse
and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. _J. Biol. Chem._ 285, 4826–4836 (2010). Article PubMed CAS Google Scholar * Andersen, J.
T. et al. Single-chain variable fragment albumin fusions bind the neonatal Fc receptor (FcRn) in a species-dependent manner: implications for in vivo half-life evaluation of albumin fusion
therapeutics. _J. Biol. Chem._ 288, 24277–24285 (2013). Article CAS PubMed PubMed Central Google Scholar * Burgess, A. W., Camakaris, J. & Metcalf, D. Purification and properties of
colony-stimulating factor from mouse lung-conditioned medium. _J. Biol. Chem._ 252, 1998–2003 (1977). Article CAS PubMed Google Scholar * Gonzalez-Juarrero, M. et al. Disruption of
granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control _Mycobacterium tuberculosis_ infection. _J. Leukoc. Biol._ 77,
914–922 (2005). Article CAS PubMed Google Scholar * Nambiar, J. K., Ryan, A. A., Kong, C. U., Britton, W. J. & Triccas, J. A. Modulation of pulmonary DC function by vaccine-encoded
GM-CSF enhances protective immunity against _Mycobacterium tuberculosis_ infection. _Eur. J. Immunol._ 40, 153–161 (2010). Article CAS PubMed Google Scholar * Dou, J. et al.
Investigation of immunogenic effect of the BCG priming and Ag85A- GM-CSF boosting in Balb/c mice model. _Immunobiology_ 215, 133–142 (2010). Article CAS PubMed Google Scholar * Carvalho,
N. B. et al. Impaired TNF, IL-1beta, and IL-17 production and increased susceptibility to _Mycobacterium tuberculosis_ infection in HTLV-1 infected individuals. _Tuberculosis_ 108, 35–40
(2018). Article CAS PubMed Google Scholar * Zhang, Y., Doerfler, M., Lee, T. C., Guillemin, B. & Rom, W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis
factor-alpha by _Mycobacterium tuberculosis_ components. _J. Clin. Investig._ 91, 2076–2083 (1993). Article CAS PubMed PubMed Central Google Scholar * Mourik, B. C., Lubberts, E., de
Steenwinkel, J. E. M., Ottenhoff, T. H. M. & Leenen, P. J. M. Interactions between type 1 interferons and the Th17 response in tuberculosis: lessons learned from autoimmune diseases.
_Front. Immunol._ 8, 294 (2017). Article PubMed PubMed Central CAS Google Scholar * Laan, M. et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17
and TNF-alpha. _Eur. Respir. J._ 21, 387–393 (2003). Article CAS PubMed Google Scholar * Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell
responses after vaccination and during _Mycobacterium tuberculosis_ challenge. _Nat. Immunol._ 8, 369–377 (2007). Article CAS PubMed Google Scholar * Khader, S. A. et al. IL-23
compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is
available. _J. Immunol._ 175, 788–795 (2005). Article CAS PubMed Google Scholar * Erdmann, H. et al. The increased protection and pathology in _Mycobacterium tuberculosis_-infected
IL-27R-alpha-deficient mice is supported by IL-17A and is associated with the IL-17A-induced expansion of multifunctional T cells. _Mucosal Immunol._ 11, 1168–80 (2018). Article CAS PubMed
Google Scholar * Okamoto Yoshida, Y. et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. _J. Immunol._ 184, 4414–4422 (2010).
Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Ms Lucy Wangaruro and Ms Sheridia Daniels for administrative support and Dr T. C. Wu for
critical review and helpful discussion. FUNDING The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National
Institutes of Health under award number R21AI22922. AUTHOR INFORMATION Author notes * Yu-Min Chuang Present address: Section of Infectious Diseases, Department of Internal Medicine, Yale
University School of Medicine, New Haven, CT, USA AUTHORS AND AFFILIATIONS * Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Yu-Min Chuang, Liangmei
He, Ya-Chea Tsai, Max A. Cheng, Emily Farmer & Chien-Fu Hung * Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA Michael L. Pinn & Petros C.
Karakousis Authors * Yu-Min Chuang View author publications You can also search for this author inPubMed Google Scholar * Liangmei He View author publications You can also search for this
author inPubMed Google Scholar * Michael L. Pinn View author publications You can also search for this author inPubMed Google Scholar * Ya-Chea Tsai View author publications You can also
search for this author inPubMed Google Scholar * Max A. Cheng View author publications You can also search for this author inPubMed Google Scholar * Emily Farmer View author publications You
can also search for this author inPubMed Google Scholar * Petros C. Karakousis View author publications You can also search for this author inPubMed Google Scholar * Chien-Fu Hung View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.-M.C. contributed to the design of the study, performance of the experiments, and writing of
the manuscript; L.H. contributed to the performance of the experiments; M.L.P. contributed to the performance of the experiments; Y.-C.T. contributed to the performance of the experiments;
M.A.C. contributed to the writing and preparation of the manuscript; E.F. contributed to the writing and preparation of the manuscript; P.C.K. contributed to the design of the study and
writing of the manuscript; and C.-F.H. contributed to the design of the study. CORRESPONDING AUTHOR Correspondence to Chien-Fu Hung. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE The housing and handling of mice follow guidelines established by the Johns Hopkins Medical Institutions Animal
Care and Use Committee and the National Institutes of Health. The Oncology Center Animal Facility has full-time veterinary support through the Department of Comparative Medicine. Animals are
monitored daily for infection and other illnesses by trained animal technicians. The criteria include infection, failure to thrive, perceived pain, respiratory distress, etc. Research
support services include training classes, and the capacity for full postmortem analysis is available on request. Only trained laboratory personnel and animal technicians were allowed to
handle laboratory animals. All individuals handling mice were registered to protocols of the Johns Hopkins Animal Care and Use Committee. SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURE 1
SUPPLEMENTAL FIGURE 2 SUPPLEMENTAL FIGURE 3 SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chuang, YM., He, L., Pinn, M.L. _et al._ Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy
against chronic tuberculosis. _Cell Mol Immunol_ 18, 2393–2401 (2021). https://doi.org/10.1038/s41423-020-0439-2 Download citation * Received: 07 January 2020 * Accepted: 20 March 2020 *
Published: 07 May 2020 * Issue Date: October 2021 * DOI: https://doi.org/10.1038/s41423-020-0439-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * Tuberculosis * albumin * GM-CSF * therapeutic vaccine * fusion protein







