- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Abnormal activation of telomerase occurs in most cancer types, which facilitates escaping from cell senescence. As the key component of telomerase, telomerase reverse transcriptase
(TERT) is regulated by various regulation pathways. _TERT_ gene changing in its promoter and phosphorylation respectively leads to TERT ectopic expression at the transcription and protein
levels. The co-interacting factors play an important role in the regulation of TERT in different cancer types. In this review, we focus on the regulators of TERT and these downstream
functions in cancer regulation. Determining the specific regulatory mechanism will help to facilitate the development of a cancer treatment strategy that targets telomerase and cancer cell
senescence. SIMILAR CONTENT BEING VIEWED BY OTHERS TARGETING TELOMERASE FOR CANCER THERAPY Article 30 July 2020 TEMPLATE ACTIVATING FACTOR-I EPIGENETICALLY REGULATES THE _TERT_ TRANSCRIPTION
IN HUMAN CANCER CELLS Article Open access 06 September 2021 TELOXANTRON INHIBITS THE PROCESSIVITY OF TELOMERASE WITH PREFERENTIAL DNA DAMAGE ON TELOMERES Article Open access 28 November
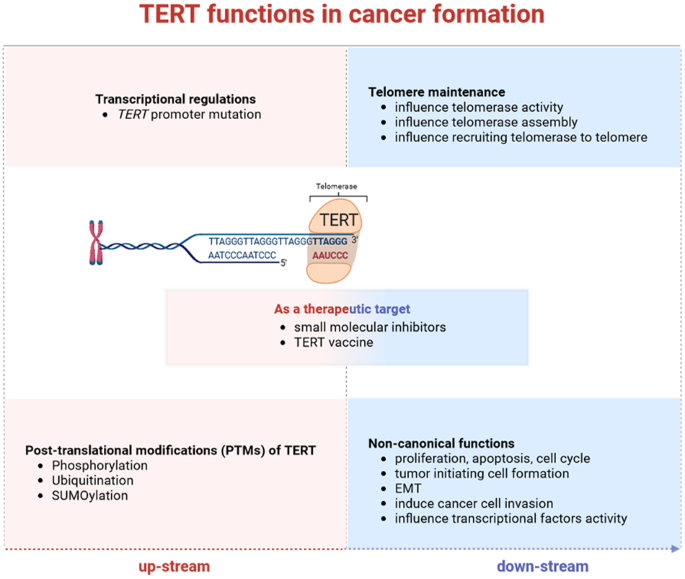
2022 FACTS * As the most important catalytic subunit component of telomerase, TERT is regulated by transcriptional factors and PTM-related activation. * Telomere maintenance by regulating
telomerase activity and assembly with telomere-binding proteins, as well as recruiting telomerase to telomere, are regulated by TERT in telomerase-positive cancer cells. * The non-canonical
biological function of TERT and its influence on different components of the tumor microenvironment in cancer cells, including angiogenesis, vascularization, EMT, proliferation, inflammatory
factors, and immune response, are influenced by TERT. * Small molecular inhibitors of TERT and TERT vaccine are under research as potential therapeutic targets. INTRODUCTION Telomeres, the
repetitive ends of chromosomes, comprise ca. 10–15 kb of tandem double-stranded 5′-TTAGGG-3′ repeats. Based on recent research by the ICGC/TCGA Pan-Cancer Analysis of Whole Genomes (PCAWG)
Consortium, while telomere sequences comprise long stretches of 5′-TTAGGG-3′ (G-type) repeat arrays, there is increasing evidence that telomeric-associated sequences include variants—most
commonly 5′-TGAGGG-3′ (G-type), 5′-TCAGGG-3′ (C-type), and 5′-TTGGGG-3′ (J-type) repeats, toward the proximal, subtelomeric regions [1]. These DNA sequences are coated with shelterin, a
specialized protein complex that plays a fundamental role in regulating telomere protection [2,3,4]. Telomere structure and function serve to maintain chromosomal integrity and regulate cell
division. In most cancers, germline and stem cells, long-term proliferation requires telomerase activity to overcome the telomere shortening, which occurs with cell division. This
shortening eventually arrests cellular growth, potentially providing an initial proliferative barrier to tumor formation in humans [5]. In adults, most stem cells exhibit telomerase
activity, although at substantially lower levels. Although such reduced levels are sufficient for slowing down telomere shortening and increasing the replicative lifespan [6], they cannot
prevent replicative senescence. Telomeres maintain DNA by acting on its 3′ overhang via the telomerase RNA component (TERC or TR) template; the telomerase adds single-stranded 5′-TTAGGG-3′
repeats to the lagging strand of the newly synthesized DNA. Finally, new primer RNA is added, and this is subsequently filled in with DNA polymerase alpha. In cancer, abnormal telomerase
activation causes cancer cells to overcome cell growth arrest, resulting in cell immortalization [7]. Telomerase reverse transcriptase (TERT) is a key component of telomerase. Human TERT was
first cloned in 1997 [5, 8,9,10], eight years after telomerase was first identified in HeLa cells [11]. The catalytic component of the telomerase holoenzyme complex comprises single
molecules of TERT, WRAP53 (also known as TCAB1), two molecules of H/ACA ribonucleoprotein complex subunits DKC1, NOP10, NHP2, and GAR1, and a telomerase RNA template [12,13,14]. While TERT
is rarely detected in normal human cells, except in embryonic cells, germ cells, and stem cells, TERC is universally expressed as a lncRNA in normal cells [5, 12, 15,16,17]. However, when
acting as part of the telomerase holoenzyme [18], TERT can regulate telomere elongation, ultimately participating in tumor formation in a telomere-dependent or -independent manner. Mutant
TERT promoters, commonly detected in various types of cancer, induce TERT overexpression by recruiting transcription factors that do not normally regulate TERT gene expression and promoting
abnormal telomerase activation [19,20,21,22,23]. Functionally, TERT affects cancer formation mostly by maintaining telomere length and enabling cells to avert cell senescence [24]. TERT has
other non-canonical activity independently of its telomere-lengthening function, in particular, affecting the DNA repair response [25], gene transcription [24], and ubiquitin-proteasomal
function [26, 27]. In this review, we focus on the regulators of TERT and the specific regulatory in cancer. These contents will help to facilitate the development of the cancer treatment
strategy that targets telomerase and cancer cell senescence. STRUCTURAL PROPERTIES OF TERT TERT contains 1132 amino acids and comprises 4 telomerase-specific motifs, including the telomerase
essential N-terminal (TEN) domain, the TERT RNA-binding domain (TRBD), the reverse transcriptase (RT) domain, and the C-terminal extension (CTE) [8, 28,29,30]. A new TERT domain, TRAP, was
recently characterized via cryoelectron microscopy mapping of the _Tetrahymena_ telomerase; TRAP is a large insertion of RT fingers [31, 32]. TERT and telomerase RNA (hTR) are composed of
telomerase catalytic core of telomerase; TERT is associated with the pseudoknot/template (PK/t) domain and conserved regions 4 and 5 (CR4/5) of hTR. A H/ACA ribonucleoprotein (RNP) lobe is
essential for telomerase biogenesis, which forms a telomerase holoenzyme with a TERT-related catalytic core [33]. Telomerase access to and activity at telomeres for telomere maintenance is
tightly regulated. In normal conditions, a six-numbered protein complex, called shelterin, is composed of POT1, TPP1, TIN2, TRF1, TRF2, and RAP1 and protects the telomeric DNA ends. As a
main component of shelterin, TPP1 has been implicated in telomerase recruitment to telomeres. Structurally, the TEN domain of TERT provides an anchor site for binding to telomeric DNA, while
this binding initiates the primer-template interaction for telomere elongation. Furthermore, the TEN domain provides binding sites for TPP1 and contributes indirectly to telomerase activity
by forming a complex with TRAP that stabilizes the active fold of TRAP, which participates directly in telomere repeat-addition processivity [30, 32]. TRBD interacts with the RT thumb
subdomain, resulting in a closed ring-like tertiary structure with a large cavity at its center that binds to the primer-template duplex [34]. The fingers and palms of the TERT’s RT domain
interact with the RNA backbone to place the template in the active site [35]. The RT motifs secure the hTR to the protein to maintain ribonucleoprotein stability and provide an active site
for catalysis [36]. The CTE and TRBD domains of TERT interact simultaneously with the CR4/5 domain of hTR. Although the hTR binding sites are not active TERT sites, this interaction ensures
the correct positioning of the TERT domain following TPP1 binding to the TEN domain, which allows the anchor site to engage the DNA substrate [37]. Moreover, TPP1, POT1, and TIN2 form a
complex within Shelterin. POT1 binds to both the DNA substrate and TEN domain of TERT, forming a gate in front of the active telomerase site, which stabilizes DNA binding [38]. As a result,
TPP1 and POT1 provide more space to accommodate the G-quadruplex structures formed by the telomeric-DNA product, reduce DNA dissociation during repeat-synthesis, and cooperatively increase
telomerase repeat-addition processivity [5, 16, 37, 38]. EFFECTS ON TERT IN CANCER FORMATION TRANSCRIPTIONAL REGULATION It is suggested that the transcription of hTERT is the dominant step
in the regulation of telomerase activity. Recurrent mutations and chromosomal rearrangements in TERT promoters are frequent in human cancers. Alteration in the _TERT_ gene includes genomic
gains/amplifications, promoter rearrangements, and “hotspot” promoter mutations at a position relative to the transcription start site [39]. These alterations have been reported in multiple
cancer types, especially _TERT_ promoter mutations, which are associated with increased _TERT_ expression [20, 21, 40,41,42]. In a pan-cancer study of somatic TERT promoter mutations and
amplification, Sounak et al. found that among 30,733 specimens, almost 11.3% of cases with _TERT_ promoter mutation were accompanied by 2.3% _TERT_ amplification and 86.3% with no TERT
amplification or promoter mutation [40]. It is indicated that TERT overexpression, caused by promoter mutation, is attributed to telomerase abnormal activation in cancer, although only a
small portion of cancer patients are recognized as having TERT mutation. hTERT promoter mutations are recognized in melanomas, while different types of cancer, including astrocytic glioma
[43], sebaceous neoplasms [41], thyroid cancer [44], bladder cancers [45], and chondrosarcoma [46] have also been detected. The promoter mutation −124 C > T has been reported in almost
half of patients with chondrosarcoma [46]; together, −124 C > T and −146 C > T are potentially important biomarkers in malignant melanoma linked to poor prognosis [42]. In 2020,
members of PCAWG characterized the genomic footprint of the telomere mechanism and reported C228T or C250T promoter mutations in different cancer types [1], consistent with findings obtained
in thyroid cancer and sebaceous neoplasms [41, 44]. However, TERT promoter mutations are not frequently detected in leukemias and colorectal cancers [47, 48]. Therefore, it has been
hypothesized that in cancers originating in tissues with low self-renewal rates and limited ability to maintain tissue homeostasis, hTERT promoter mutations occur more often than those in
cancer that already display high telomerase expression [47, 49]. Allelic DNA methylation within the _TERT_ promoter further contributes to the regulation of TERT expression in cancer.
Differential allelic expression occurs in almost 50% of cancer cell lines [50], which is more common than in human normal tissues. Hypermethylation of the _TERT_ hypermethylated oncological
region enhances TERT expression when coupled with a hypomethylated proximal core promoter. However, this impact is abolished when the core _TERT_ promoter is hypermethylated, which is often
the case when _TERT_ promoter mutations are present [51, 52]. Mechanistically, mutant _TERT_ promoters enable “enhancer hijacking” near the _TERT_ transcription start site [1, 53], which
eventually leads to telomerase activation by abrogating transcription silencing of _TERT_ [47]. In glioblastoma multiforme (GBM) cells, the NF-κB signal pathway selectively induces TERT
expression with mutant TERT promoters, which is due to the p52-binding sites formation after specific mutation in TERT promoters [54]. In BRAFV600E-driven human cancer cells, including
melanoma cell line A375, thyroid cancer cell lines BCPAP and 8305 C, and breast cancer cell line MDA-MB-231, MAPK/ERK signaling affects the binding capacity of GABP to mutant TERT promoter
and eventually results in TERT activation. Specifically, the downstream factor Sp1 is phosphorylated, resulting in HDAC1 dissociation from the zinc finger DNA-binding domain of Sp1 and
constantly promotes GABPA binding to the mutant _TERT_ promoter [55]. Similarly, in BRAFV600E papillary thyroid cancer (PTC) cells, MAPK pathway activation by the BRAFV600E mutation
upregulates E-twenty six (ETS) transcription factors, including _ETV1_, _ETV4_, _ETV5_, and _ELF3_, and increases TERT expression by binding to the ETS-binding site which generated by the
TERT promoter mutations [56]. Phosphorylation of FOS, another downstream factor of _BRAF V600E_, promotes FOS binding to the GABPB 5′ UTR, constantly activating GABPB and promoting its
binding to the mutant _TERT_ promoter in PTC cells and melanoma cells [57, 58]. In summary, these studies demonstrate that _BRAF V600E_ and _TERT_ promoter mutations cooperatively upregulate
_TERT_ expression via the activation of the MAPK signal pathway dependent on FOS and GABP. POST-TRANSLATIONAL MODIFICATIONS (PTMS) OF TERT Following translation, PTM occurs via the addition
of various chemical groups to one or more amino acid residues. PTM, which is especially common in cancer, can change the protein’s physicochemical properties by altering its spatial
conformation and activity state, subcellular localization, and protein–protein interactions [59]. PTMs increase the biochemical diversity of TERT functions, thereby affecting telomerase
regulation in cancer cells. TERT phosphorylation is the most important type, which plays an important role in regulating telomerase holoenzyme integrity. Thr249 [60], Ser824 [61], Ser227
[61], Ser457 [62], and Tyr707 [63] are known sites of phosphorylation by different kinases; the specific impacts are listed in Table 1. In the human melanoma SK-MEL 28 cell line, Akt (also
known as protein kinase B) phosphorylates TERT at serine 824 and 277, enhancing telomerase activity [61]. Akt inactivation via the phosphatidylinositol-Akt kinase pathway inhibitor
wortmannin diminishes TERT phosphorylation and inhibits human telomerase activity [61]. In H1299 cells, phosphorylation at serine 227 by Akt is required for efficient nuclear translocation
of hTERT and cellular immortalization [64]. Genistein, an isoflavone abundant in soy, has multiple molecular effects on cancer propagation [65]. In prostate cancer cells, genistein reduces
telomerase activity by inhibiting Akt, thereby dephosphorylating TERT [66, 67]. The dietary component of genistein is a potential therapeutic molecule within a TERT-targeting cancer
treatment strategy. Protein kinase C (PKC), a serine/threonine kinase, participates directly or indirectly in various signal transduction pathways via target-protein phosphorylation [68].
PKC phosphorylation of TERT promotes the association between hsp90 and TERT, thus maintaining telomerase holoenzyme integrity [69]. The PKC activator (e.g., phorbol myristate acetate)
enhances telomerase activity, whereas the PKC inhibitors (BIC, H-7, and bisindolylmaleimide I) inhibit it [69,70,71]. PKC isoenzymes _α_, β, δ, ε, and ζ are overexpressed in cancer patients
and differentially correlated with high telomerase activity in different cancer types. In head and neck cancer cells, OEC-M1, PKC α, β, δ, ε, and ζ are involved in telomerase regulation
through phosphorylation [69]. In nasopharyngeal carcinoma cells, among these isoenzymes, only PKC-ζ participates in activating telomerase activity [72]. In human breast cancer cell PMC42,
PKC-α is implicated in TERT phosphorylation [73], while protein phosphatase 2 A (PP2A) directly dephosphorylates TERT, affecting telomerase assembly and disrupting its activity on the
contrary [74]. These findings reveal the various functions of different PKC isoenzymes. CDK1, a cell-cyclin-dependent serine/threonine kinase, plays an important role in telomere maintenance
[75, 76]. During mitosis, CDK1 phosphorylates TERT at threonine 249, and this posttranslational modification is essential for tumor formation in pancreatic cancer and hepatocellular
carcinoma [60]. hTERT phosphorylation at threonine 249 is positively associated with aggressive cancer phenotypes with high proliferative activity, high pathological grade, and severe
nuclear atypia [77]. Phosphorylated hTERT expression is not associated with telomere length, suggesting that this phosphorylation might influence tumor formation dependent on RdRP activity
rather than telomerase activity in the HeLa cell line. Under oxidative stress, the Scr kinase family phosphorylates TERT at tyrosine 707, triggering TERT export from the nucleus to
mitochondria in a GTPase Ran-dependent manner [63, 78]. In addition, H2O2 treatment downregulates mitochondrial TERT via phosphorylation by Src kinase in human embryonic kidney cells [79],
which indicated that Scr kinase-related TERT phosphorylation could result in the translocation of TERT protein, which might influence telomerase activity both in cancer cells and human
embryonic kidney cells. Dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 2 (Dyrk2), also a cyclin-dependent kinase [80], binds directly to TERT, phosphorylating it at Ser457
[62], following which the Dyrk2–EDVP E3 ligase complex ubiquitinates TERT for degradation, downregulating it [62]. Hypoxia increases telomerase activity, possibly by activating the
mitogen-activated protein kinase (MAPK) signal. In the A2780 and HT-29 cell lines, MAPK inhibitors diminish the upregulation of telomerase activity but do not influence basal telomerase
activity [81]. In many cancers, inhibiting MAPK pathway effectors inhibits TERT mRNA expression [82]. However, evidence showing that MAPK directly phosphorylates TERT remains lacking. c-Abl,
a crucial prototypic non-receptor tyrosine kinase, is commonly activated by DNA damage and implicated in various cellular processes. c-Abl binds with and phosphorylates DNA-damage response
proteins such as P73, and activates p53, often inducing cell death [83, 84]. Following exposure to ionizing radiation, c-Abl is activated by DNA damage and tyrosine phosphorylation of TERT
increases; this is abolished by c-Abl deletion [85]. Following genotoxic stress, TERT phosphorylation inhibits telomerase activity and negatively regulates telomere length [85]. The
oncogenic counterpart of c-Abl, the BCR-ABL fusion protein, causes certain human leukemias [86]. The translocation of chromosomes 22 and 9 results in the formation of the Philadelphia
chromosome (Ph), the cytogenetic characteristic of chronic myeloid leukemia [87]. In this process, part of the breakpoint cluster region (BCR) gene on chromosome 22 fuses with the ABL gene
on chromosome 9, forming BCR-ABL fusion protein with tyrosine kinase [88]. TERT expression and telomerase activity are higher in BCR-ABL positive cells (the K562 cell line) than BCR-ABL
deficient cells (the HL60 cell line), as a result of TERT phosphorylation at tyrosine residues by BCR-ABL [87]. Gleevec inhibits TERT phosphorylation and downregulates TERT mRNA in BCR-ABL
positive cells by inhibiting BCR-ABL kinase activity [88]. In BCR-ABL positive cells, Gleevec (imatinib), an inhibitor of receptor tyrosine kinase, induces telomerase translocation out of
the nucleolus, indicating that TERT phosphorylation can influence telomerase activity by altering its location in cancer cells, consistent with other findings [64, 89, 90]. Ubiquitination of
TERT, another form of PTM, also occurs in human cancers. Makorin Ring finger protein 1, encoded by _MKRN1_- a ubiquitin ligase, is a TERT-binding protein that regulates TERT degradation
[91, 92]. The proteasome inhibitor MG132 reverses the loss of telomerase activity by promoting MKRN1 accumulation during cell cycle arrest [92]. Sphingosine kinase 2 generates
lysophospholipid sphingosine 1-phosphate, a factor that binds to TERT and inhibits the interaction of TERT with MKRN1 [93]. Human double minute 2 (HDM2), an E3 ubiquitin-protein ligase,
induces ubiquitination of target genes involved in tumorigenesis, including p53 [94]. As with MKRN1, HDM2 binds to TERT and induces TERT polyubiquitination [95]. In U2OS cells, HDM2
polyubiquitinates TERT principally at the N-terminus at five potential sites. Depletion of HDM2 strengthens cellular protective effects against apoptosis by stabilizing TERT [95]. HDM2 is
also required in the p73-dependent relief of p53-mediated TERT suppression on a transcriptional level [96]. Polo-like kinase 1 (Plk1) is a Ser/Thr kinase that participates primarily in
various aspects of mitotic events. Plk1 interacts directly with TERT, independently of its kinase activity, and enhances the stability of TERT by inhibiting its ubiquitin-mediated
degradation by MKRN1 and HDM2, thereby increasing cancer cell telomerase activity [97, 98]. Hsc70-interacting protein (CHIP), which interacts with Hsc/Hsp70, is physically associated with
TERT and downregulates telomerase activity via ubiquitin-mediated degradation, independent of Hsp90 binding. This degradation results in cytoplasmic accumulation of TERT, inhibiting its
nuclear localization [99]. However, CHIP does not influence telomerase activity because the interaction between TERT and CHIP occurs in the cytoplasm, suggesting that CHIP may be associated
only with intermediate or immature nonfunctional TERT [99]. Dyrk2 can directly phosphorylate TERT, which results in TERT degradation. This degradation is associated with the EDD-DDB1-VprBP
E3 ligase complex, while Dyrk2 acts as a scaffolding protein for ubiquitination substrates [62]. Small ubiquitin-related modifier (SUMO) modification (SUMOylation) is a key regulatory
modification of eukaryotic cells that may alter target protein activity, interactions, or longevity, ultimately influencing physiological processes, especially those in cancer cells
[100,101,102,103,104,105]. SUMO1 directly SUMOylates TERT via CBX4, a SUMO E3 ligase. The SUMOylation of TERT enhances the migration and invasiveness of MCF7 cells [106]. TERT-RELATED
REGULATIONS IN TELOMERE MAINTENANCE TERT is regulated by specific modulators, and these regulations influence the telomerase assembly, trafficking, blocking association, and mainly
telomerase activity. Some positive regulators of telomerase promote telomere elongation by influencing telomerase holoenzyme assembly. In contrast, negative regulators inhibit telomerase
activity, particularly by preventing telomerase from binding with the long telomere-end or with telomere-binding proteins (Table 2 and Fig. 1). FACTORS THAT INFLUENCE TELOMERASE HOLOENZYME
ASSEMBLY Different domains and unique spatial structures provide a structural basis for TERT to assemble telomerase holoenzymes. Therefore, modulators that contribute to the intracellular
distribution of TERT also influence this tightly regulated process. The 70-kDa heat shock protein (HSP70), a ubiquitous molecular chaperone that controls various cellular protein-folding and
remodeling processes [107], transiently binds to TERT in the absence of TR. The 90-kDa heat shock protein (HSP90), accompanied by chaperone p23, binds specifically with TERT and ensures its
correct assembly with the template RNA [108]. HSP90 and p23 load TERT into the Cajal body, generating an enzymatically active telomerase complex. Once an active assembled complex is formed,
the combination of HSP70 and TERT is abolished [108] and Telomerase Cajal body protein 1 (TCAB1) then binds to TERT and facilitates telomerase translocation from the Cajal bodies into the
nucleoplasm, where TPP1 facilitates the recruitment of the complex to the telomere (Fig. 2) [12, 109]. In HeLa cells, Nuclear valosin-containing protein-like (NVL), which was first
recognized as an interactor with TERT in yeast, could interact with TERT and is positively associated with telomerase holoenzyme assembly. Knockdown of endogenous NVL2 downregulates TERT,
reducing telomerase activity [110]. This suggests that NVL2 is essential for telomerase enzymatic activity. FACTORS THAT INFLUENCE THE RECRUITMENT OF TELOMERASE TO THE TELOMERE After
telomerase holoenzyme assembly, the recruitment of telomerase to the telomere is a limiting step to promoting telomere addition. The modulators of TERT also participate in this critical
process. hEST1A and hEST1B are human homologs of the yeast telomerase subunit Est1p, which is a functional factor in telomere maintenance. hEST1A and hEST1B bind to TERT independently of the
RNA subunit, participating in telomere maintenance by activating or recruiting telomerase to the telomere [111, 112]. During the S phase of the cell cycle, telomerase is recruited to the
telomere via the interaction of TPP1 with the TEN domain of TERT; telomerase then extends the G-rich strand of the telomere via TPP1–POT1 complex-promoted translocation of telomerase. In the
late S/G2 phase, the CST complex (comprising CTC1, TEN1, and STN1) restricts the interaction between telomerase and TPP1–POT1, preventing telomerase from accessing the newly synthesized
G-rich telomere strand, thus enabling it to bind to telomeric single-stranded DNA [113,114,115,116,117,118,119]. The PIF1 family helicases, which have multiple roles in eukaryotes,
negatively regulate telomerase activity by preventing it from combining with the long telomere ends [120, 121]. In addition, PIF1 binds directly to TERT and influences proliferation and
apoptosis independent of telomerase activity, although the mechanisms remain unclear in cervical cancer cells [122, 123]. Activating transcription factor 7 (ATF7), the primary factor in
stress-induced telomere shortening, binds to TERT and leads to the release of ATF7 and telomerase from telomeres, eventually resulting in telomere shortening [124]. However, in HeLa cells,
shRNA-induced alteration of ATF7 expression did not affect telomerase activity, which indicated a telomerase-independent telomere length regulation [124]. FACTORS THAT INFLUENCE TELOMERASE
ACTIVITY As the main component subunit of telomerase, the regulations of TERT result in changes in telomerase activity. The oncogene PinX1, first identified in a yeast two-hybrid screen as a
TRF1-binding protein, is a major haploinsufficient tumor suppressor essential for maintaining telomerase activity and chromosome stability [125]. PinX1 binds to the 74 aa C-terminal
fragment of TERT in its telomerase inhibitory domain, inhibiting telomerase activity and thereby inducing telomere shortening [126]. PinX1–TERT binding maintains PinX1-mediated TRF1
stability and influences the TRF1 nucleolar localization, which is important in preserving telomere integrity [127, 128]. MCRS2, a novel isoform of human micro-spherule protein 1 (MCRS1),
interacts with PinX1. MCRS2 binds to TERT, forming a stable complex that inhibits telomerase activity, thereby shortening the telomere [129]. The gene encoding promyelocytic leukemia (PML),
a tumor suppressor primarily expressed in the nucleoplasm, interacts directly or indirectly with TERT in HepG2, U2OS, and HFF cell lines, negatively regulating telomerase activity and
eventually resulting in telomere shortening [130]. TERT-RELATED REGULATIONS FACILITATE ONCOGENESIS INDEPENDENT OF TELOMERASE TERT exhibits non-canonical functions involved in tumor
oncogenesis independent of telomerase activity, which is detailed in the following sections (Table 2 and Fig. 3). FACTORS THAT INFLUENCE TELOMERE MAINTENANCE INDEPENDENT OF TELOMERASE In
human cancer cell lines, TERT physically forms a complex with BRG1, a SWI/SNF-related chromatin remodeling protein, and nucleostemin, a nucleolar GTP-binding protein. The
TERT–BRG1–nucleostemin complex drives tumor-initiating cell formation by regulating BRG1 activity rather than contributing directly to telomere maintenance [131, 132]. FACTORS THAT INFLUENCE
DIFFERENT COMPONENTS OF THE TUMOR MICROENVIRONMENT According to the heterogeneity of different types of cancers, the tumor microenvironment (TME) is composed of neoplastic, stromal,
endothelial, and infiltrating immune cells, and the interactions of these elements determine the pathological process in cancer progression [133, 134]. Previous studies have shown that the
interactions between TERT and other factors, including c-MET [135], nuclear factor κB (NF-κB) p65 [136, 137], Myc [138], and _Sp1_ [139], influence downstream gene expression and signal
transduction. These telomere-independent activities contribute to the regulations of other cell processes in TME, including angiogenesis, vascularization, epithelial-to-mesenchymal
transition (EMT) [135, 140], and inflammation [136, 137], eventually promoting cancer cell proliferation [122]. EFFECTS ON BLOOD VESSELS: ANGIOGENESIS AND VASCULARIZATION In human gastric
tumor samples, TERT expressions are positively associated with VEGF expressions. Mechanistically, _Sp1_, the transcription factor of VEGF, interacts with TERT and facilitates angiogenesis in
cancer via VEGF activation [139]. In hepatocellular carcinoma cells, TERT binding to _Sp1_ stimulates DNMT3B transcription and induces aberrant cancer-specific global DNA methylation and
AKT hyperactivation [141]. The activation of AKT modulates the increased expression of VEGF and activates angiogenesis both in normal tissues and in cancers [142]. The binding of TERT to MYC
in cells with high MYC expression can stabilize MYC protein by inhibiting MYC ubiquitination, thereby activating the downstream targets of MYC, such as _EIF2A_ and _NCL_ [138]. In breast
cancer cells, VEGF expression is increased by c-MYC and eventually induces tumor angiogenesis [143]. TERT also regulates MMP expression, especially MMP9, in an NF-κB-dependent manner [137].
MMP9 knockdown inhibits angiogenesis in prostate cancer cells via the release of angiostatin, which serves as a key regulator of angiogenesis [144]. Besides, in an NSCLC mouse model, TERT
deficiency decreased the positive signal of PanCK/CD31 double staining and CD34 staining, suggesting the involvement of TERT in vascularization [145]. EFFECTS ON MESENCHYMAL TISSUE In A549
and H1299 lung cancer cells, hTERT overexpression upregulates c-MET and promotes the expression of mesenchymal markers, including vimentin and N-cadherin [135]. In the NSCLC mouse model,
TERT deficiency inhibits the expression of c-MET and reduces EMT by influencing the expression of mesenchymal markers, including vimentin and fibronectin [145]. Similarly, a _TERT_ promoter
variant enhances EMT in prostate cancer and promotes the development of castration-resistant prostate cancer [140]. In HPV-immortalized cervical cancer cells, overexpression of TERT alters
EMT characteristics [146]. The authors showed that TERT is a critical factor in the EMT process in cancer progression. EFFECTS ON INFLAMMATORY FACTORS AND IMMUNE RESPONSE The NF-κB family
comprises inducible transcription factors with important roles in cancer progression [147] and acts as a pro-inflammatory transcription factor involved in the regulation of inflammation
[147, 148]. TERT binding to NF-κB (also known as p65) is required for the promotion of NF-κB-dependent gene expression such as IL-6 and TNF-α [136]; these factors are critical for
inflammation in cancer progression. In an NSCLC mouse model, TERT promotes lung inflammation by influencing the expression of _Ifng_, _Tnf_, _Il10_, and _PD-1_, markers of inflammation, and
tumor immunosuppression [145]. TERT also affects the immune system and is considered a self-antigen constitutively expressed in many tumors, with potential therapeutic value for anticancer
immunotherapy [149]. TERT is recognized by the adaptive immune system; in particular, CD4+ and CD8+ T cells recognize TERT as short peptides processed inside the cell before being exported
to and presented at the cell surface by major histocompatibility complex I/II molecules, which induces an adaptive immune response associated with the inhibition of tumor growth
[150,151,152,153,154]. These findings provide the theoretical basis for the discovery and the clinical application of the TERT vaccine in cancer treatment. THE CURRENT AND FUTURE
PHARMACOLOGICAL STRATEGIES TARGETING TERT OR TELOMERASE Owing to their good anti-cancer properties, TERT and telomerase are used as therapeutic targets for telomerase-positive cancer. Small
molecule inhibitors and telomerase-targeted vaccines are the most frequent types in drug development. TMPyP4, RHPS4, BRACO-19, and telomestatin are typical ligands that fold the
single-stranded telomeric DNA (the telomeric substrate) into a four-stranded quadruplex structure, inhibiting telomerase catalytic activity [155]. In humans, TMPyP4 downregulates TERT
transcription [156], inhibiting telomerase activity and TERT expression [157, 158]. However, the suppression proliferative of RHPS4 in glioblastoma stem-like cells is independent of
telomeric dysfunction [159], which indicates a differential influence in cancer treatment. Significantly, a high level of G-rich DNA exists throughout the genome, especially in the promoter
regions of oncogenes. Therefore, G4 ligands have several risks when used in telomerase inhibition and may have a significant off-target effect [160,161,162]. 6-thio-2’-deoxyguanosine
(6-thio-dG, also named THIO) is a nucleoside analog that could induce telomere dysfunction by incorporating into telomeric DNA in telomerase-positive cells [163]. In NSCLC xenografts,
6-thio-dG treatment reduces tumor growth by increasing telomere damage [145]. The therapeutic effect has also been validated in Gliomas [164] and Melanoma [165, 166] both in vivo and in
vitro. Ilgen Mender et al. found that 6-thio-dG mitigates chemotherapy resistance to ECFG inhibitors and is commonly used in chemotherapy combinations (e.g., gemcitabine and cisplatin) in
NSCLC cell lines [167]. In xenografts from neuroblastoma cell lines, pediatric high-risk group-3 medulloblastoma xenografts, and an orthotopic patient-derived xenograft model of diffuse
intrinsic pontine glioma, 6-thio-dG inhibits tumor growth by inducing telomere dysfunction, which showed a promising approach to mitigate therapy-resistant telomerase-positive pediatric
brain tumors [168, 169]. Similarly, 6-thio-dG significantly improves the efficacy of the combination of anti-PD-L1 and anti-VEGF based on CD8+ T cells in Hepatocellular Carcinoma [170],
consistent with earlier research [171]. Directly targeting active TERT sites is a more popular therapeutic strategy than using compounds that inhibit TERT DNA-binding [172, 173].
2-[[(E)-3-naphthalen-2-ylbut-2-enoyl] amino] benzoic acid (BIBR1532) non-competitively binds to the active sites of TERT, inhibiting its telomerase activity [24, 30]. Amin et al. found that
short-term BIBR1532 impairs cellular proliferation in a zebrafish model, which is a potential anticancer treatment strategy [174]. Recent research on new BIBR1532-based analogs, designed to
overcome ineligible pharmacokinetics, showed greater antitumor activity in the Ehrlich carcinoma model [175]. Imetelstat (GRN163L), a specific telomerase inhibitor that directly binds to the
TR component in the catalytic site of the telomerase enzyme, has been used in a clinical study [176]. Imetalstat can induce cell death and improve the treatment efficacy of chemotherapy in
patient-derived xenografts of Acute myeloid leukemia (AML) [177, 178]. A phase II study of lower-risk myelodysplastic patients reported meaningful effects with Imetelstat [179], and a phase
III study of lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents reported that approximately 40% patients achieved transfusion
independency at least 8 weeks [180]. Therefore, almost 91% of patients had grade 3–4 treatment-emergent adverse events [180]. These results indicate that telomerase-targeting therapy has
considerable prospects but warrants further investigations to reduce adverse drug reactions. Telomerases are HLA class-I antigens, hence TERT-derived vaccines have also been considered as an
approach to stimulate immune response in targeting cancers [181]. To date, GV1001 single-use [182] or combined with HR2822 [183], UV1 [184] single-use or combined with Ipilimumab
(checkpoint inhibitor) [185], Vx-001 [186], and Gx-301 [187] have undergone clinical trials in patients with different types of cancer. Based on CD4+/CD8+ T cell recognition, the TERT
vaccine presents a promising therapeutic efficacy in a proportion of cancer, although its safety and universal applicability in different types of cancer requires further research and
verification [153, 188, 189]. CONCLUSIONS AND PERSPECTIVES Telomerase reactivation is essential for cancer progression, but it does not occur during normal cell senescence.
Telomerase-mediated stress responses in cancer result in telomere escape from shortening with every division. As the most important catalytic subunit component of telomerase, TERT is
regulated by various regulation pathways, including transcriptional factors, PTM-related activation, and inhibition by co-interacting proteins. The most common _TERT_ gene changing is
promoter mutant, which results in the binding of the ETS family transcription factor on “enhance hijacking” near the TERT transcription start sites. As for PTMs of TERT protein,
phosphorylation has been extensively studied, and ubiquitination and SUMOylation have been reported in various cancer cells. The phosphorylation of TERT mainly results in telomerase activity
changing by influencing the stability of TERT with the regulation of ubiquitination, maintaining telomerase holoenzyme integrity, and changing the translocation of TERT in cancer cells
between nuclear and cytoplasmic. Besides, a small portion of the phosphorylation of TERT does not influence telomerase activity more than RdRP activity, which indicates that the functions of
TERT in cancers should be considered. The modulators of TERT, which regulate telomerase assembly, trafficking, blocking association, especially telomerase activity have been well-researched
in recent years. Some positive regulators of telomerase promote telomere elongation by influencing telomerase holoenzyme assembly, recruiting telomerase to telomere, and stabilizing TERT
protein. Negative regulators inhibit telomerase activity, particularly by preventing telomerase from binding with the long telomere-end or with telomere-binding proteins. Besides, the
modulators that induce the non-canonical function of TERT that regulates gene expression by some transcription factors, such as c-MET, NF-κB p65, Myc, and Sp1, also participate in cancer
progression. To date, various small molecular drugs, including G4 ligands representative of TMPyP4 and telomerstatin, 6-thio-dG, BIBR1532, Imetelastat, and TERT vaccine, have been
investigated. However, due to its limited effect and undetermined drug safety, further research remains warranted before clinical use. To summarize, the TERT modulators discussed in this
review provide potential targets for cancer treatment. Interventions targeting atypical telomere-related molecules influence cancer-associated cellular processes, exerting synergistic
anticancer effects. However, further research remains warranted to identify novel treatment strategies. REFERENCES * Sieverling L, Hong C, Koser SD, Ginsbach P, Kleinheinz K, Hutter B, et
al. Genomic footprints of activated telomere maintenance mechanisms in cancer. Nat Commun. 2020;11:733. Article CAS PubMed PubMed Central Google Scholar * Amano H, Chaudhury A,
Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab. 2019;29:1274–90.e9. Article CAS
PubMed PubMed Central Google Scholar * Srinivas N, Rachakonda S, Kumar R. Telomeres and telomere length: a general overview. Cancers (Basel). 2020;12:558. Article CAS PubMed Google
Scholar * Amir M, Khan P, Queen A, Dohare R, Alajmi MF, Hussain A, et al. Structural features of nucleoprotein CST/shelterin complex involved in the telomere maintenance and its association
with disease mutations. Cells. 2020;9:359. Article CAS PubMed PubMed Central Google Scholar * Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet.
2019;20:299–309. Article CAS PubMed Google Scholar * Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative
lifespan of HSCs during serial transplantation. Blood. 2003;102:517–20. Article CAS PubMed Google Scholar * Claude E, Decottignies A. Telomere maintenance mechanisms in cancer:
telomerase, ALT or lack thereof. Curr Opin Genet Dev. 2020;60:1–8. Article CAS PubMed Google Scholar * Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al.
Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–9. Article CAS PubMed Google Scholar * Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass
MB, et al. A mammalian telomerase-associated protein. Science. 1997;275:973–7. Article CAS PubMed Google Scholar * Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, et al.
Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–15. Article CAS PubMed PubMed Central Google Scholar * Morin GB. The human
telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9. Article CAS PubMed Google Scholar * Venteicher AS, Abreu EB, Meng Z,
McCann KE, Terns RM, Veenstra TD, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–8. Article CAS PubMed
PubMed Central Google Scholar * Nguyen THD, Tam J, Wu RA, Greber BJ, Toso D, Nogales E, et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature. 2018;557:190–5.
Article CAS PubMed PubMed Central Google Scholar * Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol
Cell Biol. 2010;30:2775–86. Article CAS PubMed PubMed Central Google Scholar * Nguyen THD, Collins K, Nogales E. Telomerase structures and regulation: shedding light on the chromosome
end. Curr Opin Struct Biol. 2019;55:185–93. Article CAS PubMed PubMed Central Google Scholar * Turner KJ, Vasu V, Griffin DK. Telomere biology and human phenotype. Cells. 2019;8:73.
Article CAS PubMed PubMed Central Google Scholar * Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36:D339–43. Article CAS PubMed
Google Scholar * Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–5. Article
CAS PubMed PubMed Central Google Scholar * Heidenreich B, Kumar R. TERT promoter mutations in telomere biology. Mutat Res Rev Mutat Res. 2017;771:15–31. Article CAS PubMed Google
Scholar * Yuan X, Dai M, Xu D. TERT promoter mutations and GABP transcription factors in carcinogenesis: more foes than friends. Cancer Lett. 2020;493:1–9. Article CAS PubMed Google
Scholar * Hafezi F, Jaxel L, Lemaire M, Turner JD, Perez-Bercoff D. TERT promoter mutations increase sense and antisense transcription from the TERT promoter. Biomedicines. 2021;9:1773.
Article CAS PubMed PubMed Central Google Scholar * Bell RJ, Rube HT, Xavier-Magalhães A, Costa BM, Mancini A, Song JS, et al. Understanding TERT promoter mutations: a common path to
immortality. Mol Cancer Res. 2016;14:315–23. Article CAS PubMed PubMed Central Google Scholar * Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter
mutations in familial and sporadic melanoma. Science. 2013;339:959–61. Article CAS PubMed Google Scholar * Dratwa M, Wysoczańska B, Łacina P, Kubik T, Bogunia-Kubik K. TERT-regulation
and roles in cancer formation. Front Immunol. 2020;11:589929. Article CAS PubMed PubMed Central Google Scholar * Masutomi K, Possemato R, Wong JM, Currier JL, Tothova Z, Manola JB, et
al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci USA. 2005;102:8222–7. Article CAS PubMed PubMed Central Google Scholar *
Im E, Yoon JB, Lee HW, Chung KC. Human telomerase reverse transcriptase (hTERT) positively regulates 26S proteasome activity. J Cell Physiol. 2017;232:2083–93. Article CAS PubMed Google
Scholar * Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang JW, et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut.
2017;66:31–42. Article CAS PubMed Google Scholar * Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase.
Science. 1997;276:561–7. Article CAS PubMed Google Scholar * Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7:484–94. Article CAS
PubMed PubMed Central Google Scholar * Liu B, He Y, Wang Y, Song H, Zhou ZH, Feigon J. Structure of active human telomerase with telomere shelterin protein TPP1. Nature. 2022;604:578–83.
Article CAS PubMed PubMed Central Google Scholar * Wang Y, Gallagher-Jones M, Sušac L, Song H, Feigon J. A structurally conserved human and Tetrahymena telomerase catalytic core. Proc
Natl Acad Sci USA. 2020;117:31078–87. Article CAS PubMed PubMed Central Google Scholar * Jiang J, Wang Y, Sušac L, Chan H, Basu R, Zhou ZH, et al. Structure of telomerase with telomeric
DNA. Cell. 2018;173:1179–90.e13. Article CAS PubMed PubMed Central Google Scholar * Egan ED, Collins K. An enhanced H/ACA RNP assembly mechanism for human telomerase RNA. Mol Cell
Biol. 2012;32:2428–39. Article CAS PubMed PubMed Central Google Scholar * Gillis AJ, Schuller AP, Skordalakes E. Structure of the _Tribolium castaneum_ telomerase catalytic subunit
TERT. Nature. 2008;455:633–7. Article CAS PubMed Google Scholar * Mitchell M, Gillis A, Futahashi M, Fujiwara H, Skordalakes E. Structural basis for telomerase catalytic subunit TERT
binding to RNA template and telomeric DNA. Nat Struct Mol Biol. 2010;17:513–8. Article CAS PubMed Google Scholar * Bryan TM, Goodrich KJ, Cech TR. Telomerase RNA bound by protein motifs
specific to telomerase reverse transcriptase. Mol Cell. 2000;6:493–9. Article CAS PubMed Google Scholar * Ghanim GE, Fountain AJ, van Roon AM, Rangan R, Das R, Collins K, et al.
Structure of human telomerase holoenzyme with bound telomeric DNA. Nature. 2021;593:449–53. Article CAS PubMed PubMed Central Google Scholar * Sekne Z, Ghanim GE, van Roon AM, Nguyen
THD. Structural basis of human telomerase recruitment by TPP1-POT1. Science. 2022;375:1173–6. Article CAS PubMed PubMed Central Google Scholar * Gupta S, Won H, Chadalavada K, Nanjangud
GJ, Chen YB, Al-Ahmadie HA, et al. TERT copy number alterations, promoter mutations and rearrangements in adrenocortical carcinomas. Endocr Pathol. 2022;33:304–14. Article CAS PubMed
Google Scholar * Gupta S, Vanderbilt CM, Lin YT, Benhamida JK, Jungbluth AA, Rana S, et al. A pan-cancer study of somatic TERT promoter mutations and amplification in 30,773 tumors profiled
by clinical genomic sequencing. J Mol Diagn. 2021;23:253–63. Article CAS PubMed PubMed Central Google Scholar * Muñoz-Jiménez MT, Blanco L, Ruano Y, Carrillo R, Santos-Briz Á,
Riveiro-Falkenbach E, et al. TERT promoter mutation in sebaceous neoplasms. Virch Arch. 2021;479:551–8. Article Google Scholar * Guo Y, Chen Y, Zhang L, Ma L, Jiang K, Yao G, et al. TERT
promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. Article PubMed PubMed Central Google Scholar * Fujimoto K, Arita H, Satomi K, Yamasaki K, Matsushita Y,
Nakamura T, et al. TERT promoter mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic glioma with molecular features of glioblastoma. Acta Neuropathol.
2021;142:323–38. Article CAS PubMed Google Scholar * Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23:R143–55. Article CAS PubMed PubMed Central
Google Scholar * Weyerer V, Eckstein M, Strissel PL, Wullweber A, Lange F, Tögel L, et al. TERT promoter mutation analysis of whole-organ mapping bladder cancers. Genes (Basel).
2021;12:230. Article CAS PubMed Google Scholar * Zhang Y, Chen Y, Yang C, Seger N, Hesla AC, Tsagkozis P, et al. TERT promoter mutation is an objective clinical marker for disease
progression in chondrosarcoma. Mod Pathol. 2021;34:2020–7. Article CAS PubMed PubMed Central Google Scholar * Chiba K, Johnson JZ, Vogan JM, Wagner T, Boyle JM, Hockemeyer D.
Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife. 2015;4:e07918. Article PubMed PubMed Central Google Scholar * Heidenreich B, Rachakonda PS, Hemminki K,
Kumar R. TERT promoter mutations in cancer development. Curr Opin Genet Dev. 2014;24:30–7. Article CAS PubMed Google Scholar * Ramlee MK, Wang J, Toh WX, Li S. Transcription regulation
of the human telomerase reverse transcriptase (hTERT) gene. Genes. 2016;7:50. Article PubMed PubMed Central Google Scholar * Huang FW, Bielski CM, Rinne ML, Hahn WC, Sellers WR,
Stegmeier F, et al. TERT promoter mutations and monoallelic activation of TERT in cancer. Oncogenesis. 2015;4:e176. Article CAS PubMed PubMed Central Google Scholar * Lee DD, Komosa M,
Sudhaman S, Leão R, Zhang CH, Apolonio JD, et al. Dual role of allele-specific DNA hypermethylation within the TERT promoter in cancer. J Clin Invest. 2021;131:e146915. Article CAS PubMed
PubMed Central Google Scholar * Lee DD, Leão R, Komosa M, Gallo M, Zhang CH, Lipman T, et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J Clin
Invest. 2019;129:223–9. Article PubMed Google Scholar * Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, et al. Systematic analysis of telomere length and somatic alterations
in 31 cancer types. Nat Genet. 2017;49:349–57. Article CAS PubMed PubMed Central Google Scholar * Li Y, Zhou QL, Sun W, Chandrasekharan P, Cheng HS, Ying Z, et al. Non-canonical NF-κB
signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat Cell Biol. 2015;17:1327–38. Article CAS PubMed PubMed Central Google Scholar * Wu Y, Shi L, Zhao Y,
Chen P, Cui R, Ji M, et al. Synergistic activation of mutant TERT promoter by Sp1 and GABPA in BRAF(V600E)-driven human cancers. NPJ Precis Oncol. 2021;5:3. Article CAS PubMed PubMed
Central Google Scholar * Song YS, Yoo SK, Kim HH, Jung G, Oh AR, Cha JY, et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat
Cancer. 2019;26:629–41. Article CAS PubMed Google Scholar * Liu R, Zhang T, Zhu G, Xing M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer.
Nat Commun. 2018;9:579. Article CAS PubMed PubMed Central Google Scholar * Akıncılar SC, Khattar E, Boon PL, Unal B, Fullwood MJ, Tergaonkar V. Long-range chromatin interactions drive
mutant TERT Promoter Activation. Cancer Discov. 2016;6:1276–91. Article PubMed Google Scholar * Liu X, Zhang Y, Wang Y, Yang M, Hong F, Yang S. Protein phosphorylation in cancer: role of
nitric oxide signaling pathway. Biomolecules. 2021;11:1009. Article CAS PubMed PubMed Central Google Scholar * Yasukawa M, Ando Y, Yamashita T, Matsuda Y, Shoji S, Morioka MS, et al.
CDK1 dependent phosphorylation of hTERT contributes to cancer progression. Nat Commun. 2020;11:1557. Article CAS PubMed PubMed Central Google Scholar * Kang SS, Kwon T, Kwon DY, Do SI.
Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–90. Article CAS PubMed Google
Scholar * Jung HY, Wang X, Jun S, Park JI. Dyrk2-associated EDD-DDB1-VprBP E3 ligase inhibits telomerase by TERT degradation. J Biol Chem. 2013;288:7252–62. Article CAS PubMed PubMed
Central Google Scholar * Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase
family-dependent phosphorylation of tyrosine 707. Mol Cell Biol. 2003;23:4598–610. Article CAS PubMed PubMed Central Google Scholar * Chung J, Khadka P, Chung IK. Nuclear import of
hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci. 2012;125:2684–97. CAS PubMed Google Scholar * Mukund V, Mukund D, Sharma V, Mannarapu
M, Alam A. Genistein: its role in metabolic diseases and cancer. Crit Rev Oncol Hematol. 2017;119:13–22. Article PubMed Google Scholar * Li Y, Liu L, Andrews LG, Tollefsbol TO. Genistein
depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer. 2009;125:286–96. Article CAS PubMed PubMed Central Google Scholar * Eitsuka T,
Nakagawa K, Kato S, Ito J, Otoki Y, Takasu S, et al. Modulation of telomerase activity in cancer cells by dietary compounds: a review. Int J Mol Sci. 2018;19:478. Article PubMed PubMed
Central Google Scholar * Kang JH, Toita R, Kim CW, Katayama Y. Protein kinase C (PKC) isozyme-specific substrates and their design. Biotechnol Adv. 2012;30:1662–72. Article CAS PubMed
Google Scholar * Chang JT, Lu YC, Chen YJ, Tseng CP, Chen YL, Fang CW, et al. hTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck
cancer cells. Br J Cancer. 2006;94:870–8. Article CAS PubMed PubMed Central Google Scholar * Ku WC, Cheng AJ, Wang TC. Inhibition of telomerase activity by PKC inhibitors in human
nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun. 1997;241:730–6. Article CAS PubMed Google Scholar * Bodnar AG, Kim NW, Effros RB, Chiu CP. Mechanism of telomerase
induction during T cell activation. Exp Cell Res. 1996;228:58–64. Article CAS PubMed Google Scholar * Yu CC, Lo SC, Wang TC. Telomerase is regulated by protein kinase C-zeta in human
nasopharyngeal cancer cells. Biochem J. 2001;355:459–64. Article CAS PubMed PubMed Central Google Scholar * Li H, Zhao L, Yang Z, Funder JW, Liu JP. Telomerase is controlled by protein
kinase Calpha in human breast cancer cells. J Biol Chem. 1998;273:33436–42. Article CAS PubMed Google Scholar * Li H, Zhao LL, Funder JW, Liu JP. Protein phosphatase 2A inhibits nuclear
telomerase activity in human breast cancer cells. J Biol Chem. 1997;272:16729–32. Article CAS PubMed Google Scholar * Teixeira MT, Gilson E. When CDK1 rides the telomere cycle. Mol Cell.
2006;24:491–2. Article CAS PubMed Google Scholar * Liu CC, Gopalakrishnan V, Poon LF, Yan T, Li S. Cdk1 regulates the temporal recruitment of telomerase and Cdc13-Stn1-Ten1 complex for
telomere replication. Mol Cell Biol. 2014;34:57–70. Article PubMed PubMed Central Google Scholar * Matsuda Y, Yamashita T, Ye J, Yasukawa M, Yamakawa K, Mukai Y, et al. Phosphorylation
of hTERT at threonine 249 is a novel tumor biomarker of aggressive cancer with poor prognosis in multiple organs. J Pathol. 2022;257:172–85. Article CAS PubMed PubMed Central Google
Scholar * Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, et al. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative
stress. J Cell Sci. 2008;121:1046–53. Article CAS PubMed Google Scholar * Büchner N, Zschauer TC, Lukosz M, Altschmied J, Haendeler J. Downregulation of mitochondrial telomerase reverse
transcriptase induced by H2O2 is Src kinase dependent. Exp Gerontol. 2010;45:558–62. Article PubMed Google Scholar * Tandon V, de la Vega L, Banerjee S. Emerging roles of DYRK2 in cancer.
J Biol Chem. 2021;296:100233. Article CAS PubMed PubMed Central Google Scholar * Seimiya H, Tanji M, Oh-hara T, Tomida A, Naasani I, Tsuruo T. Hypoxia up-regulates telomerase activity
via mitogen-activated protein kinase signaling in human solid tumor cells. Biochem Biophys Res Commun. 1999;260:365–70. Article CAS PubMed Google Scholar * Stern JL, Hibshman G, Hu K,
Ferrara SE, Costello JC, Kim W, et al. Mesenchymal and MAPK expression signatures associate with telomerase promoter mutations in multiple cancers. Mol Cancer Res. 2020;18:1050–62. Article
CAS PubMed PubMed Central Google Scholar * Bohio AA, Wang R, Zeng X, Ba X. c‑Abl‑mediated tyrosine phosphorylation of DNA damage response proteins and implications in important cellular
functions (Review). Mol Med Rep. 2020;22:612–9. Article CAS PubMed Google Scholar * Pan B, Yang L, Wang J, Wang Y, Wang J, Zhou X, et al. C-Abl tyrosine kinase mediates neurotoxic prion
peptide-induced neuronal apoptosis via regulating mitochondrial homeostasis. Mol Neurobiol. 2014;49:1102–16. Article CAS PubMed Google Scholar * Kharbanda S, Kumar V, Dhar S, Pandey P,
Chen C, Majumder P, et al. Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr Biol. 2000;10:568–75. Article CAS PubMed Google Scholar * Hantschel O,
Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. Article CAS PubMed Google Scholar * Chai JH, Zhang Y, Tan WH, Chng WJ, Li B,
Wang X. Regulation of hTERT by BCR-ABL at multiple levels in K562 cells. BMC Cancer. 2011;11:512. Article CAS PubMed PubMed Central Google Scholar * Shtivelman E, Lifshitz B, Gale RP,
Roe BA, Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986;47:277–84. Article CAS PubMed Google Scholar * Liu K,
Hodes RJ, Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT
phosphorylation and nuclear translocation. J Immunol. 2001;166:4826–30. Article CAS PubMed Google Scholar * Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of
vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001;21:3336–42. Article CAS PubMed PubMed Central Google Scholar * Kim JH, Park SM, Kang MR, Oh SY, Lee TH,
Muller MT, et al. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev. 2005;19:776–81. Article CAS PubMed PubMed Central Google Scholar
* Salvatico J, Kim JH, Chung IK, Muller MT. Differentiation linked regulation of telomerase activity by Makorin-1. Mol Cell Biochem. 2010;342:241–50. Article CAS PubMed Google Scholar
* Panneer Selvam S, De Palma RM, Oaks JJ, Oleinik N, Peterson YK, Stahelin RV, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by
allosterically mimicking protein phosphorylation. Sci Signal. 2015;8:ra58. Article PubMed Google Scholar * Wang H, Zhao D, Nguyen LX, Wu H, Li L, Dong D, et al. Targeting cell membrane
HDM2: a novel therapeutic approach for acute myeloid leukemia. Leukemia. 2020;34:75–86. Article CAS PubMed Google Scholar * Oh W, Lee EW, Lee D, Yang MR, Ko A, Yoon CH, et al. Hdm2
negatively regulates telomerase activity by functioning as an E3 ligase of hTERT. Oncogene. 2010;29:4101–12. Article CAS PubMed Google Scholar * Toh WH, Kyo S, Sabapathy K. Relief of
p53-mediated telomerase suppression by p73. J Biol Chem. 2005;280:17329–38. Article CAS PubMed Google Scholar * Huang Y, Sun L, Liu N, Wei Q, Jiang L, Tong X, et al. Polo-like kinase 1
(Plk1) up-regulates telomerase activity by affecting human telomerase reverse transcriptase (hTERT) stability. J Biol Chem. 2015;290:18865–73. Article CAS PubMed PubMed Central Google
Scholar * Kishi K, van Vugt MA, Okamoto K, Hayashi Y, Yaffe MB. Functional dynamics of Polo-like kinase 1 at the centrosome. Mol Cell Biol. 2009;29:3134–50. Article CAS PubMed PubMed
Central Google Scholar * Lee JH, Khadka P, Baek SH, Chung IK. CHIP promotes human telomerase reverse transcriptase degradation and negatively regulates telomerase activity. J Biol Chem.
2010;285:42033–45. Article CAS PubMed PubMed Central Google Scholar * Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem.
2013;82:357–85. Article CAS PubMed Google Scholar * Eifler K, Vertegaal ACO. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem Sci. 2015;40:779–93.
Article CAS PubMed PubMed Central Google Scholar * Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol.
2018;52:1081–94. CAS PubMed PubMed Central Google Scholar * Xie M, Yu J, Ge S, Huang J, Fan X. SUMOylation homeostasis in tumorigenesis. Cancer Lett. 2020;469:301–9. Article CAS PubMed
Google Scholar * Yau TY, Molina O, Courey AJ. SUMOylation in development and neurodegeneration. Development. 2020;147:dev175703. Article CAS PubMed PubMed Central Google Scholar *
Sheng Z, Zhu J, Deng YN, Gao S, Liang S. SUMOylation modification-mediated cell death. Open Biol. 2021;11:210050. Article CAS PubMed PubMed Central Google Scholar * Sanyal S, Mondal P,
Sen S, Sengupta Bandyopadhyay S, Das C. SUMO E3 ligase CBX4 regulates hTERT-mediated transcription of CDH1 and promotes breast cancer cell migration and invasion. Biochem J.
2020;477:3803–18. Article CAS PubMed Google Scholar * Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–80. Article CAS
PubMed Google Scholar * Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J Biol Chem.
2001;276:15571–4. Article CAS PubMed Google Scholar * Arndt GM, MacKenzie KL. New prospects for targeting telomerase beyond the telomere. Nat Rev Cancer. 2016;16:508–24. Article CAS
PubMed Google Scholar * Her J, Chung IK. The AAA-ATPase NVL2 is a telomerase component essential for holoenzyme assembly. Biochem Biophys Res Commun. 2012;417:1086–92. Article CAS PubMed
Google Scholar * Snow BE, Erdmann N, Cruickshank J, Goldman H, Gill RM, Robinson MO, et al. Functional conservation of the telomerase protein Est1p in humans. Curr Biol. 2003;13:698–704.
Article CAS PubMed Google Scholar * Reichenbach P, Höss M, Azzalin CM, Nabholz M, Bucher P, Lingner J. A human homolog of yeast Est1 associates with telomerase and uncaps chromosome ends
when overexpressed. Curr Biol. 2003;13:568–74. Article CAS PubMed Google Scholar * Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell
Biol. 2020;21:384–97. Article CAS PubMed PubMed Central Google Scholar * Latrick CM, Cech TR. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding
translocation. EMBO J. 2010;29:924–33. Article CAS PubMed PubMed Central Google Scholar * Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, et al. The POT1-TPP1 telomere complex is
a telomerase processivity factor. Nature. 2007;445:506–10. Article CAS PubMed Google Scholar * Lim CJ, Barbour AT, Zaug AJ, Goodrich KJ, McKay AE, Wuttke DS, et al. The structure of
human CST reveals a decameric assembly bound to telomeric DNA. Science. 2020;368:1081–5. Article CAS PubMed PubMed Central Google Scholar * Miyake Y, Nakamura M, Nabetani A, Shimamura
S, Tamura M, Yonehara S, et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol Cell. 2009;36:193–206.
Article CAS PubMed Google Scholar * Wang F, Stewart JA, Kasbek C, Zhao Y, Wright WE, Price CM. Human CST has independent functions during telomere duplex replication and C-strand
fill-in. Cell Rep. 2012;2:1096–103. Article CAS PubMed PubMed Central Google Scholar * Chen LY, Redon S, Lingner J. The human CST complex is a terminator of telomerase activity. Nature.
2012;488:540–4. Article CAS PubMed Google Scholar * Li JR, Yu TY, Chien IC, Lu CY, Lin JJ, Li HW. Pif1 regulates telomere length by preferentially removing telomerase from long telomere
ends. Nucleic Acids Res. 2014;42:8527–36. Article CAS PubMed PubMed Central Google Scholar * Phillips JA, Chan A, Paeschke K, Zakian VA. The pif1 helicase, a negative regulator of
telomerase, acts preferentially at long telomeres. PLoS Genet. 2015;11:e1005186. Article PubMed PubMed Central Google Scholar * Wang J, Zhu X, Ying P, Zhu Y. PIF1 affects the
proliferation and apoptosis of cervical cancer cells by influencing TERT. Cancer Manag Res. 2020;12:7827–35. Article CAS PubMed PubMed Central Google Scholar * Gagou ME, Ganesh A, Phear
G, Robinson D, Petermann E, Cox A, et al. Human PIF1 helicase supports DNA replication and cell growth under oncogenic-stress. Oncotarget. 2014;5:11381–98. Article PubMed PubMed Central
Google Scholar * Maekawa T, Liu B, Nakai D, Yoshida K, Nakamura KI, Yasukawa M, et al. ATF7 mediates TNF-α-induced telomere shortening. Nucleic Acids Res. 2018;46:4487–504. Article CAS
PubMed PubMed Central Google Scholar * Zhou XZ, Huang P, Shi R, Lee TH, Lu G, Zhang Z, et al. The telomerase inhibitor PinX1 is a major haploinsufficient tumor suppressor essential for
chromosome stability in mice. J Clin Invest. 2011;121:1266–82. Article CAS PubMed PubMed Central Google Scholar * Zhou XZ, Lu KP. The Pin2/TRF1-interacting protein PinX1 is a potent
telomerase inhibitor. Cell. 2001;107:347–59. Article CAS PubMed Google Scholar * Yoo JE, Park YN, Oh BK. PinX1, a telomere repeat-binding factor 1 (TRF1)-interacting protein, maintains
telomere integrity by modulating TRF1 homeostasis, the process in which human telomerase reverse Transcriptase (hTERT) plays dual roles. J Biol Chem. 2014;289:6886–98. Article CAS PubMed
PubMed Central Google Scholar * Soohoo CY, Shi R, Lee TH, Huang P, Lu KP, Zhou XZ. Telomerase inhibitor PinX1 provides a link between TRF1 and telomerase to prevent telomere elongation. J
Biol Chem. 2011;286:3894–906. Article CAS PubMed Google Scholar * Song H, Li Y, Chen G, Xing Z, Zhao J, Yokoyama KK, et al. Human MCRS2, a cell-cycle-dependent protein, associates with
LPTS/PinX1 and reduces the telomere length. Biochem Biophys Res Commun. 2004;316:1116–23. Article CAS PubMed Google Scholar * Oh W, Ghim J, Lee EW, Yang MR, Kim ET, Ahn JH, et al. PML-IV
functions as a negative regulator of telomerase by interacting with TERT. J Cell Sci. 2009;122:2613–22. Article CAS PubMed Google Scholar * Okamoto N, Yasukawa M, Nguyen C, Kasim V,
Maida Y, Possemato R, et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin. Proc Natl Acad Sci USA. 2011;108:20388–93. Article CAS PubMed PubMed
Central Google Scholar * Maida Y, Masutomi K. Telomerase reverse transcriptase moonlights: Therapeutic targets beyond telomerase. Cancer Sci. 2015;106:1486–92. Article CAS PubMed PubMed
Central Google Scholar * Cangelosi D, Morini M, Zanardi N, Sementa AR, Muselli M, Conte M, et al. Hypoxia predicts poor prognosis in neuroblastoma patients and associates with biological
mechanisms involved in telomerase activation and tumor microenvironment reprogramming. Cancers (Basel). 2020;12:2343. Article CAS PubMed Google Scholar * Petrova V,
Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. Article PubMed PubMed Central Google Scholar * Prasad RR, Mishra DK, Kumar
M, Yadava PK. Human telomerase reverse transcriptase promotes the epithelial to mesenchymal transition in lung cancer cells by enhancing c-MET upregulation. Heliyon. 2022;8:e08673. Article
CAS PubMed Google Scholar * Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, et al. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270–81.
Article CAS PubMed Google Scholar * Ding D, Xi P, Zhou J, Wang M, Cong YS. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via
NF-κB-dependent transcription. FASEB J. 2013;27:4375–83. Article CAS PubMed Google Scholar * Koh CM, Khattar E, Leow SC, Liu CY, Muller J, Ang WX, et al. Telomerase regulates MYC-driven
oncogenesis independent of its reverse transcriptase activity. J Clin Invest. 2015;125:2109–22. Article PubMed PubMed Central Google Scholar * Liu N, Ding D, Hao W, Yang F, Wu X, Wang M,
et al. hTERT promotes tumor angiogenesis by activating VEGF via interactions with the Sp1 transcription factor. Nucleic Acids Res. 2016;44:8693–703. Article CAS PubMed PubMed Central
Google Scholar * Dong X, Zhang Q, Hao J, Xie Q, Xu B, Zhang P, et al. Large multicohort study reveals a prostate cancer susceptibility allele at 5p15 regulating TERT via androgen
signaling-orchestrated chromatin binding of E2F1 and MYC. Front Oncol. 2021;11:754206. Article CAS PubMed PubMed Central Google Scholar * Yu J, Yuan X, Sjöholm L, Liu T, Kong F, Ekström
TJ, et al. Telomerase reverse transcriptase regulates DNMT3B expression/aberrant DNA methylation phenotype and AKT activation in hepatocellular carcinoma. Cancer Lett. 2018;434:33–41.
Article CAS PubMed Google Scholar * Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. Article CAS PubMed PubMed Central Google Scholar * Gao
FY, Li XT, Xu K, Wang RT, Guan XX. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun Signal. 2023;21:28. Article PubMed PubMed Central
Google Scholar * Gupta A, Zhou CQ, Chellaiah MA. Osteopontin and MMP9: associations with VEGF expression/secretion and angiogenesis in PC3 prostate cancer cells. Cancers (Basel).
2013;5:617–38. Article CAS PubMed Google Scholar * Piñeiro-Hermida S, Bosso G, Sánchez-Vázquez R, Martínez P, Blasco MA. Telomerase deficiency and dysfunctional telomeres in the lung
tumor microenvironment impair tumor progression in NSCLC mouse models and patient-derived xenografts. Cell Death Differ. 2023;30:1585–600. Article PubMed PubMed Central Google Scholar *
Wang A, Zhou D, Krawczyk E, Li T, Simic V, Lu J, et al. Overexpression of the telomerase holoenzyme induces EMT and tumorigenesis of HPV-immortalized keratinocytes. J Med Virol.
2023;95:e28681. Article CAS PubMed Google Scholar * Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. Article CAS PubMed Google Scholar * Gupta SC,
Kunnumakkara AB, Aggarwal S, Aggarwal BB. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front Immunol. 2018;9:2160. Article PubMed PubMed Central Google
Scholar * Zanetti M. A second chance for telomerase reverse transcriptase in anticancer immunotherapy. Nat Rev Clin Oncol. 2017;14:115–28. Article CAS PubMed Google Scholar * Schroers
R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res.
2002;62:2600–5. CAS PubMed Google Scholar * Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, et al. Human telomerase reverse transcriptase-specific T-helper responses
induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9:4743–55. CAS PubMed Google Scholar * Godet Y, Fabre E, Dosset M, Lamuraglia
M, Levionnois E, Ravel P, et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect
with chemotherapy response. Clin Cancer Res. 2012;18:2943–53. Article CAS PubMed Google Scholar * Dosset M, Godet Y, Vauchy C, Beziaud L, Lone YC, Sedlik C, et al. Universal cancer
peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin Cancer Res. 2012;18:6284–95. Article CAS PubMed Google Scholar * Adotévi O,
Mollier K, Neuveut C, Dosset M, Ravel P, Fridman WH, et al. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8
T-cell immunity in vivo. Blood. 2010;115:3025–32. Article PubMed Google Scholar * Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer
strategy? Nat Rev Drug Discov. 2011;10:261–75. Article CAS PubMed PubMed Central Google Scholar * Grand CL, Han H, Muñoz RM, Weitman S, Von Hoff DD, Hurley LH, et al. The cationic
porphyrin TMPyP4 down-regulates c-MYC and human telomerase reverse transcriptase expression and inhibits tumor growth in vivo. Mol Cancer Ther. 2002;1:565–73. CAS PubMed Google Scholar *
Konieczna N, Romaniuk-Drapała A, Lisiak N, Totoń E, Paszel-Jaworska A, Kaczmarek M, et al. Telomerase Inhibitor TMPyP4 Alters Adhesion and Migration of Breast-Cancer Cells MCF7 and
MDA-MB-231. Int J Mol Sci. 2019;20:2670. Article CAS PubMed PubMed Central Google Scholar * Cheng MJ, Cao YG. TMPYP4 exerted antitumor effects in human cervical cancer cells through
activation of p38 mitogen-activated protein kinase. Biol Res. 2017;50:24. Article PubMed PubMed Central Google Scholar * Berardinelli F, Tanori M, Muoio D, Buccarelli M, di Masi A, Leone
S, et al. G-quadruplex ligand RHPS4 radiosensitizes glioblastoma xenograft in vivo through a differential targeting of bulky differentiated- and stem-cancer cells. J Exp Clin Cancer Res.
2019;38:311. Article CAS PubMed PubMed Central Google Scholar * Tsai R, Fang K, Yang P, Hsieh Y, Chiang I, Chen Y, et al. TERRA regulates DNA G-quadruplex formation and ATRX recruitment
to chromatin. Nucleic Acids Res. 2022;50:12217–34. Article CAS PubMed PubMed Central Google Scholar * Yan C, Chang Y, Gao H, Zhang Q, Peng S, Wang D, et al. G-quadruplex apurinic
site-programmed chiral cyanine assemblies for specifically recognizing guanosine and guanine. Analyst. 2021;146:5866–72. Article CAS PubMed Google Scholar * Shankar U, Mishra SK, Jain N,
Tawani A, Yadav P, Kumar A. Ni(+2) permease system of Helicobacter pylori contains highly conserved G-quadruplex motifs. Infect Genet Evol. 2022;101:105298. Article CAS PubMed Google
Scholar * Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2’-deoxyguanosine. Cancer Discov.
2015;5:82–95. Article CAS PubMed Google Scholar * Yu S, Wei S, Savani M, Lin X, Du K, Mender I, et al. A modified nucleoside 6-Thio-2’-deoxyguanosine exhibits antitumor activity in
gliomas. Clin Cancer Res. 2021;27:6800–14. Article CAS PubMed PubMed Central Google Scholar * Zhang G, Wu LW, Mender I, Barzily-Rokni M, Hammond MR, Ope O, et al. Induction of telomere
dysfunction prolongs disease control of therapy-resistant melanoma. Clin Cancer Res. 2018;24:4771–84. Article CAS PubMed PubMed Central Google Scholar * Reyes-Uribe P, Adrianzen-Ruesta
MP, Deng Z, Echevarria-Vargas I, Mender I, Saheb S, et al. Exploiting TERT dependency as a therapeutic strategy for NRAS-mutant melanoma. Oncogene. 2018;37:4058–72. Article CAS PubMed
PubMed Central Google Scholar * Mender I, LaRanger R, Luitel K, Peyton M, Girard L, Lai TP, et al. Telomerase-mediated strategy for overcoming non-small cell lung cancer targeted therapy
and chemotherapy resistance. Neoplasia. 2018;20:826–37. Article CAS PubMed PubMed Central Google Scholar * Sengupta S, Sobo M, Lee K, Senthil Kumar S, White AR, Mender I, et al. Induced
telomere damage to treat telomerase expressing therapy-resistant pediatric brain tumors. Mol Cancer Ther. 2018;17:1504–14. Article CAS PubMed Google Scholar * Fischer-Mertens J, Otte F,
Roderwieser A, Rosswog C, Kahlert Y, Werr L, et al. Telomerase-targeting compounds Imetelstat and 6-thio-dG act synergistically with chemotherapy in high-risk neuroblastoma models. Cell
Oncol (Dordr). 2022;45:991–1003. Article CAS PubMed Google Scholar * Mender I, Siteni S, Barron S, Flusche AM, Kubota N, Yu C, et al. Activating an adaptive immune response with a
telomerase-mediated telomere targeting therapeutic in hepatocellular carcinoma. Mol Cancer Ther. 2023;22:737–50. Article CAS PubMed PubMed Central Google Scholar * Mender I, Zhang A,
Ren Z, Han C, Deng Y, Siteni S, et al. Telomere stress potentiates STING-dependent anti-tumor immunity. Cancer Cell. 2020;38:400–11.e6. Article CAS PubMed PubMed Central Google Scholar
* Sullivan HJ, Readmond C, Radicella C, Persad V, Fasano TJ, Wu C. Binding of telomestatin, TMPyP4, BSU6037, and BRACO19 to a telomeric G-quadruplex-duplex hybrid probed by all-atom
molecular dynamics simulations with explicit solvent. ACS Omega. 2018;3:14788–806. Article CAS PubMed PubMed Central Google Scholar * Mulholland K, Wu C. Binding of telomestatin to a
telomeric G-quadruplex DNA probed by all-atom molecular dynamics simulations with explicit solvent. J Chem Inf Model. 2016;56:2093–102. Article CAS PubMed Google Scholar * Amin A,
Morello M, Petrara MR, Rizzo B, Argenton F, De Rossi A, et al. Short-term TERT inhibition impairs cellular proliferation via a telomere length-independent mechanism and can be exploited as a
potential anticancer approach. Cancers (Basel). 2023;15:2673. Article CAS PubMed Google Scholar * Al-Karmalawy AA, Nafie MS, Shaldam MA, Elmaaty AA, Antar SA, El-Hamaky AA, et al.
Ligand-based design on the dog-bone-shaped BIBR1532 pharmacophoric features and synthesis of novel analogues as promising telomerase inhibitors with in vitro and in vivo evaluations. J Med
Chem. 2023;66:777–92. Article CAS PubMed Google Scholar * Carloni LE, Wechselberger R, De Vijlder T. Characterization of in vitro G-quadruplex formation of imetelstat telomerase
inhibitor. Nucleic Acid Ther. 2021;31:341–50. Article CAS PubMed Google Scholar * Barwe SP, Huang F, Kolb EA, Gopalakrishnapillai A. Imetelstat induces leukemia stem cell death in
pediatric acute myeloid leukemia patient-derived xenografts. J Clin Med. 2022;11:1923. Article CAS PubMed PubMed Central Google Scholar * Bruedigam C, Porter AH, Song A, Vroeg In de Wei
G, Stoll T, Straube J, et al. Imetelstat-mediated alterations in fatty acid metabolism to induce ferroptosis as a therapeutic strategy for acute myeloid leukemia. Nat Cancer. 2023. *
Steensma DP, Fenaux P, Van Eygen K, Raza A, Santini V, Germing U, et al. Imetelstat achieves meaningful and durable transfusion independence in high transfusion-burden patients with
lower-risk myelodysplastic syndromes in a phase II study. J Clin Oncol. 2021;39:48–56. Article CAS PubMed Google Scholar * Platzbecker U, Santini V, Fenaux P, Sekeres MA, Savona MR,
Madanat YF, et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational,
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;S0140-6736:01724–5. Google Scholar * Kailashiya C, Sharma HB, Kailashiya J. Telomerase based anticancer
immunotherapy and vaccines approaches. Vaccine. 2017;35:5768–75. Article CAS PubMed Google Scholar * Jo JH, Kim YT, Choi HS, Kim HG, Lee HS, Choi YW, et al. Efficacy of GV1001 with
gemcitabine/capecitabine in previously untreated patients with advanced pancreatic ductal adenocarcinoma having high serum eotaxin levels (KG4/2015): an open-label, randomised, Phase 3
trial. Br J Cancer. 2024;130:43–52. Article CAS PubMed Google Scholar * Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, et al. Telomerase peptide
vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–64. Article CAS PubMed Google Scholar * Ellingsen EB, O’Day S,
Mezheyeuski A, Gromadka A, Clancy T, Kristedja TS, et al. Clinical activity of combined telomerase vaccination and pembrolizumab in advanced melanoma: results from a phase I trial. Clin
Cancer Res. 2023;29:3026–36. Article CAS PubMed PubMed Central Google Scholar * Ellingsen EB, Bounova G, Kerzeli I, Anzar I, Simnica D, Aamdal E, et al. Characterization of the T cell
receptor repertoire and melanoma tumor microenvironment upon combined treatment with ipilimumab and hTERT vaccination. J Transl Med. 2022;20:419. Article CAS PubMed PubMed Central Google
Scholar * Gridelli C, Ciuleanu T, Domine M, Szczesna A, Bover I, Cobo M, et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in
patients with stage IV non-small cell lung cancer: final results of a randomised phase 2 clinical trial. Br J Cancer. 2020;122:1461–6. Article CAS PubMed PubMed Central Google Scholar *
Filaci G, Fenoglio D, Nolè F, Zanardi E, Tomasello L, Aglietta M, et al. Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant prostate cancer: a randomized
phase II trial. Cancer Immunol Immunother. 2021;70:3679–92. Article CAS PubMed PubMed Central Google Scholar * Jansons J, Skrastina D, Kurlanda A, Petkov S, Avdoshina D, Kuzmenko Y, et
al. Reciprocal inhibition of immunogenic performance in mice of two potent DNA immunogens targeting HCV-related liver cancer. Microorganisms. 2021;9:1073. Article CAS PubMed PubMed
Central Google Scholar * Negrini S, De Palma R, Filaci G. Anti-cancer immunotherapies targeting telomerase. Cancers (Basel). 2020;12:2260. Article CAS PubMed Google Scholar * Jakob S,
Schroeder P, Lukosz M, et al. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J Biol Chem.
2008;283:33155–61. Article CAS PubMed PubMed Central Google Scholar * Maekawa T, Liu B, Nakai D, et al. ATF7 mediates TNF-α-induced telomere shortening. Nucleic Acids Res.
2018;46:4487–504. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants from the National Natural Science Foundation
of China (Nos. 82020108024 and 81772924), the Health Commission of Jilin Province (No. 2020J033), and the Graduate Innovation Fund of Jilin University (No. 101832020CX260). The funders had
no role in the design of the study and collection, analysis, interpretation of data, decision to publish, or preparation of the paper. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Key
Laboratory of Pathobiology, Ministry of Education, Jilin University, Changchun, 130021, Jilin, China Mingdi Liu, Yuning Zhang, Yongping Jian, Liting Gu, Dan Zhang, Yishu Wang & Zhi-Xiang
Xu * Department of Urology, The First Hospital of Jilin University, Changchun, 130021, Jilin, China Honglan Zhou & Zhi-Xiang Xu Authors * Mingdi Liu View author publications You can
also search for this author inPubMed Google Scholar * Yuning Zhang View author publications You can also search for this author inPubMed Google Scholar * Yongping Jian View author
publications You can also search for this author inPubMed Google Scholar * Liting Gu View author publications You can also search for this author inPubMed Google Scholar * Dan Zhang View
author publications You can also search for this author inPubMed Google Scholar * Honglan Zhou View author publications You can also search for this author inPubMed Google Scholar * Yishu
Wang View author publications You can also search for this author inPubMed Google Scholar * Zhi-Xiang Xu View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS ML developed the initial idea and the application of net benefit theory. ML, YZ, and LG review previous related publications. Draft conceived and designed by ML and YZ. The
paper was revised and approved by YJ, LG, HS, YL, and DZ. All authors read and approved the final paper. CORRESPONDING AUTHORS Correspondence to Honglan Zhou, Yishu Wang or Zhi-Xiang Xu.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. The figures were created with BioRender software, ©biorender.com. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by Professor Anastasis Stephanou RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, M., Zhang, Y., Jian, Y. _et
al._ The regulations of telomerase reverse transcriptase (TERT) in cancer. _Cell Death Dis_ 15, 90 (2024). https://doi.org/10.1038/s41419-024-06454-7 Download citation * Received: 22
September 2023 * Revised: 04 January 2024 * Accepted: 08 January 2024 * Published: 26 January 2024 * DOI: https://doi.org/10.1038/s41419-024-06454-7 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(749x0:751x2)/Gregory-Gourdet--df3a6f9d96834f16b23acdcee4e0d457.jpg)




