- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Patients with triple-negative breast cancers (TNBC) are at a high risk for a recurrent or metastatic disease, and the molecular mechanisms associated with this risk are unclear.
Proteoglycan serglycin (SRGN) proteins are involved in tumor metastasis, but their role in TNBC has not yet been elucidated. This study investigates the _SRGN_ gene expression and how it
regulates TGFβ2 and the downstream signaling of TGFβ2 in TNBC cells and tissues. Our results show that SRGN mRNA and protein expression levels were significantly higher in TNBC cell lines
and tumor tissues than that in non-TNBC cells and tissues. We inhibited SRGN expression and protein secretion using shRNA and we observed this inhibited the invasive motility of TNBC cancer
cells _in vitro_ and metastasis of TNBC cancer cells _in vivo._ SRGN protein treatment increased the expression and secretion of transforming growth factor-β2 (TGFβ2) by activating
CD44/CREB1 signaling and promoted epithelial-to-mesenchymal transition in TNBC cells. Moreover, TGFβ2 treatment increased the mRNA and protein expression of the _SRGN_ gene by activating
Smad3 to target the SRGN relative promoter domain in TNBC cells. Our findings demonstrate that SRGN interacts with TGFβ2 which regulates TNBC metastasis via the autocrine and paracrine
routes. SRGN could serve as a potential target for development of agents or therapeutics for the TNBC. SIMILAR CONTENT BEING VIEWED BY OTHERS CTGF REGULATES CELL PROLIFERATION, MIGRATION,
AND GLUCOSE METABOLISM THROUGH ACTIVATION OF FAK SIGNALING IN TRIPLE-NEGATIVE BREAST CANCER Article 10 March 2021 PLOD3 CONTRIBUTES TO HER-2 THERAPY RESISTANCE IN GASTRIC CANCER THROUGH
FOXO3/SURVIVIN PATHWAY Article Open access 14 July 2022 NIDOGEN-1 SUPPRESSES CELL PROLIFERATION, MIGRATION, AND GLYCOLYSIS VIA INTEGRIN Β1-MEDIATED HIF-1Α DOWNREGULATION IN TRIPLE-NEGATIVE
BREAST CANCER Article Open access 27 March 2025 INTRODUCTION Breast cancer is one of the most common malignancies for women. Although the treatment of breast cancer has greatly improved over
the past few decades, there are still around 50 million people worldwide that die from breast cancer every year. The persistence and prevalence of breast cancer may be due to its high
genetic heterogeneity, since patients who exhibit the same clinical and histological tumor morphology may have divergent molecular genetic characteristics. These divergences can affect the
treatment and prognosis of breast cancer,1, 2, 3 however, the molecular events involved in the treatment resistance have not yet been fully elucidated. Breast cancer is commonly divided into
four types: Luminal A (ER+, PR+, HER2− and low Ki67 expression), Luminal B (ER+, PR+, HER2−/+, high Ki67 or any level expression), HER2 overexpression (ER−, PR−, HER2+), or basal-like.
Approximately 80% of basal-like breast cancer are ER−, PR− and HER2−, which are called TNBC− (triple-negative breast cancer). TNBC is a very heterogeneous group of cancers that is
aggressive, has a high risk of relapse, has poor prognosis, and often occurs in women under 50 years of age. Due to lack of endocrine therapy and HER2 targeted therapy, the main clinical
treatments of TNBC rely on chemotherapy which causes therapeutic resistance.4 Therefore, it is important to determine the molecular mechanism underpinning metastasis and recurrence of TNBC.
Serglycin (SRGN) is a low molecular weight glycoprotein involving in breast cancer metastasis. SRGN can be secreted from cells and integrated into the extracellular matrix. SRGN consists of
a core protein with 158 amino acids that is attached with various mucopolysaccharides (GAGs). The core protein forms three function regions: signal peptide (amino acid residues 1–27),
N-terminal (amino acid residues 28–76), and C-terminal (amino acids 77–158). The C-terminal of SRGN contains multiple serine and glycine repeat regions which bind to the GAGs.5, 6 Studies
have shown that SRGN is mainly expressed in blood cells, endothelial cells, tumor cells, and embryonic stem cells. SRGN has an important role in the storage and secretion of a variety of
proteases, chemokines and cytokine.7, 8 The effect of SRGN in cancer was first found as a marker to distinguish myeloid leukemia from lymphoid leukemia.9 In multiple myeloma, high expression
of SRGN can inhibit the complement activity, which can help tumor cells to escape from immune surveillance.10 Elevated SRGN expression can be used as a prognostic indicator of liver
cancer.11 Recent studies have shown that SRGN can induce an epithelial–mesenchymal transition (EMT) in nasopharyngeal carcinoma cells and aggressive breast cancer cells, promoting tumor cell
invasion and metastasis.12, 13 Lung metastasis of breast cancer is significantly inhibited in SRGN-deficient mice.14 It is suggested that SRGN may be highly associated with breast cancer
metastasis, possibly through regulating EMT activation. In this study, we investigated the expression and biological functions of _SRGN_ gene in TNBC breast cancer cells and observed which
signal pathways were affected. RESULTS SRGN MRNA AND PROTEIN EXPRESSION INCREASES IN BREAST CANCER CELLS AND TISSUES SRGN has been demonstrated to induce proliferation, migration and
invasion in breast cancer cells,13 but its expression in human breast cancer tissues and differential expression in different cell lines has not been reported. We therefore monitored _SRGN_
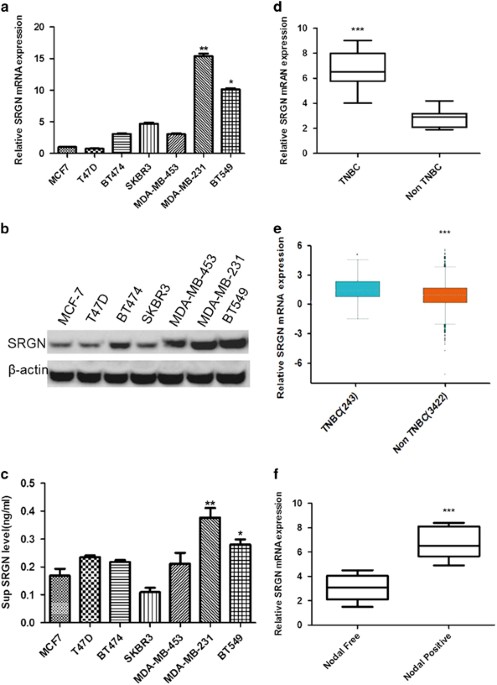
mRNA expression using real-time PCR and measured protein expression by western blot in six breast cancer (BC) cell lines. _SRGN_ mRNA (Figure 1a) and protein (Figure 1b) expression levels
were significantly higher in the TNBC cells (MDA-MB-231 and BT549 cell), than other subtypes of BC cells (MCF7, T47D, BT474 and SKBR3 cells) (_P_<0.05, _P_<0.01 vs MCF cells). We also
measured the SRGN protein level in the supernatant of cultured cells by ELISA and found that the supernatant SRGN level in TNBC was significantly higher than other types of BC cells
(_P_<0.05, _P_<0.01 vs MCF cells, Figure 1c). We further measured mRNA expression of the _SRGN_ gene using RT–PCR in tissues from 106 cases of TNBC and 320 cases of other BC types
(non-TNBC) and we found that TNBC tumors contained significantly higher SRGN mRNA levels compared with other BC types (_P_<0.001, Figure 1d). We searched our observed mRNA expression
pattern of the human _SRGN_ gene against the Breast Cancer Gene-Expression Miner v4.0 database (http://bcgenex.centregauducheau.fr) and again found that mRNA expression of _SRGN_ gene was
significantly higher in basal-like and TNBC tumors than that in non basal-like and non-TNBC tumors (_P_<0.001, Figure 1e). We wanted to understand the association between _SRGN_ gene
expression and breast cancer progression and metastasis, and collected a panel of breast cancer tissue samples and divided them into a positive lymph node metastases group (Node positive)
(_n_=135) and a negative lymph node metastases group (Node negative) (_n_=142). The SRGN mRNA levels were significantly higher in the ‘Nodal positive’ group than that in the ‘Nodal negative’
group (_P_<0.001, Figure 1f). These results suggest that SRGN gene is highly expressed TNBC cells and tissues compared with other BC types and associated with the metastatic phenotype of
BC. SRGN PROMOTES TNBC CELLS MIGRATION, INVASION AND METASTASIS Although one study investigated the biological functions of SRGN in breast cancer cells by overexpression of serglycin,13 the
affection of silencing _SRGN_ gene expression on the proliferation, migration, and invasion of BC cells has not been reported. To determine the biological significance of SRGN, we knocked
down _SRGN_ gene expression by transfecting MDA-MB-231 cells with two shRNAs (shSRGN1# and shSRGN2#) and established stable cell lines with these shRNAs (MDA-MB-231/shSRGN1# and
MDA-MB-231/shSRGN2#) using lentiviral vectors. The _SRGN_ mRNA expression in cells and SRGN protein concentration in the supernatant of cultured cells were measured by real-time PCR and
ELISA, respectively. The SRGN mRNA expression in the cells (Figure 2a) and SRGN protein concentration (Figure 2b) in the supernatant of MDA-MB-231/shSRGN1 (shSRGN1#) and MDA-MB-231/shSRGN2
(shSRGN2#) stable cells were significantly decreased compared with MDA-MB-231 cell stably expressing non-target sequence (shNC) (mRNA: _P_<0.01, _P_<0.001, Protein: _P_<0.05). MTS
analysis showed that silencing of _SRGN_ gene expression had no obvious effect on the viability of MDA-MB-231 cells _in vitro_ (_P_>0.05) (Figure 2c). However, in the xenograft tumor
models, tumors inoculated with MDA-MB-231/shSRGN2# cells (shSRGN2#) grew significantly slower than tumors inoculated with MDA-MB-231/shNC (shNC) (_P_<0.01) (Supplementary Figure 1A).
Tumors were smaller and in MDA-MB-231/hSRGN2# than in MDA-MB-231/shNC (_P_<0.05) (Supplementary Figures 1B–D). The _in vitro_ scratch assay showed that MDA-MB-231/shSRGN1# (shSRGN1#) and
MDA-MB-231/shSRGN2# (shSRGN2#) stable cells migrated significantly slower than MDA-MB-231/shNC stable cells (Figure 2d and e). The Transwell invasion assay showed that significantly less
MDA-MB-231/shSRGN1# (shSRGN1#) and MDA-MB-231/shSRGN2# (shSRGN2#) stable cells invaded to the lower chamber compared with the MDA-MB-231/shNC stable cells (Figure 2f and g). To further
evaluate the effect of SRGN on the metastasis of MDA-MB-231 cells _in vivo_, MDA-MB-231/shSRGN2# and MDA-MB-231/shNC stable cells were injected into mice via tail vein and then lung
metastases were analyzed 5 weeks later. Significantly less nodules were observed in the lung of mice injected MDA-MB-231/shSRGN2# stable cells than mice injected MDA-MB-231/shNC stable cells
(_P_<0.05, Figures 2h–j). These findings suggest that silencing of SRGN gene expression significantly inhibited cell migration and invasion of MDA-MB-231 cells _in vitro_ and tumor
growth and metastasis of xenograft MDA-MB-231 tumors _in vivo_. We also investigated the biological effects of _SRGN_ gene expression in one non-TNBC cell line MCF7 with low endogenous SRGN
expression. The SRGN mRNA (Supplementary Figure 2A) and protein (Supplementary Figure 2B) expression in MCF7 cells stably overexpressing SRGN gene (MCF7-SRGN) was significantly increased
compared with that in control MCF7 sable cells (MCF7-NC). We also observed that the protein level (Supplementary Figure 2C) in the supernatant of cultured MCF7-SRGN stable cells was
significantly increased compared with control (_P_<0.001). The _in vitro_ scratch assay showed that MCF7-SRGN stable cells (MCF7-SRGN) migrated significantly faster than MCF7-NC control
stable cells (Supplementary Figure 2D). The Transwell invasion assay showed that significantly more MCF7-SRGN stable cells (MCF7-SRGN) invaded to the lower chamber compared with the MCF7-NC
stable cells (_P_<0.01, Supplementary Figure 2E,F). These results suggest that SRGN overexpression promotes breast cancer cells invasion and metastasis. SRGN INCREASES TGFΒ2 LEVELS AND
ACTIVATED EMT A previous study demonstrated that SRGN can induce an epithelial–mesenchymal transition (EMT) in nasopharyngeal carcinoma cells12 and the cytokine transforming growth factor β
(TGFβ) is known to trigger the EMT process.15 In addition, the E-cadherin to N-cadherin switch is a biomarker for EMT. The increased vimentin expression is associated with a migratory
phenotype, and up-regulated fibronectin expression is observed in EMT.16 We wanted to know whether SRGN activates EMT in BC cells through regulating _TGFβ2_ gene expression. Western blots
showed that E-cadherin was significantly increased in MDA-MB-231-shSRGN2# cells stably expressing shRNA2# of _SRGN_ gene (shSRGN2#), but was significantly decreased in MCF7 cells
overexpressing _SRGN_ gene compared with their control groups, respectively (Figure 3a). In contrast, the protein expression of vimentin, N-cadherin, and fibronectin was significantly
decreased in MDA-MB-231-shSRGN2# stable cells (shSRGN2#), but was significantly increased in MCF7-SRGN stable cells overexpressing _SRGN_ gene (SRGN) compared with their own control groups
(Figure 3a). These findings suggest that SRGN activates EMT. Real-time PCR showed that _TGFβ2_ mRNA was significantly decreased in MDA-MB-231-shSRGN2# stable cells (MDA231 shSRGN2#) compared
with MDA-MB-231-shNC stable cells (MDA231 shNC) (_P_<0.05), but significantly increased in MCF7-SRGN stable cells compared with MCF7-NC stable cells (MCF7-NC) (_P_<0.05) (Figure 3b).
No significant differences in _TGFβ1_ mRNA expression were observed between MDA231 shSRGN2# and MDA231 shNC, and between MCF7-SRGN and MCF7-NC stable cells (_P_>0.05) (Figure 3b). Western
blots showed that TGFβ2 and pCREB1 protein expression was decreased in MDA-MB-231-shSRGN2# stable cells (shSRGN2#) compared with that in MDA-MB-231-shNC stable cells (shNC), but increased
in MCF7-SRGN stable cells compared with that in MCF7-shNC stable cells (shNC) (Figure 3c). No obvious differences in TGFβ1 and CREB1 protein expression were observed between MDA231 shSRGN2#
and MDA231 shNC, and between MCF7-SRGN and MCF7-NC stable cells (_P_>0.05) (Figure 3c). The concentration of TGFβ1 and TGFβ2 protein in the supernatant of cultured cells was measured by
ELISA (Figure 3d). TGFβ2, but not TGFβ1 concentration was significantly decreased in the supernatant of cultured MDA-MB-231-shSRGN2# stable cells (MDA231 shSRGN2#) compared with that in the
supernatant of cultured MDA-MB-231-shNC stable cells (MDA231 shNC) (_P_<0.05), but significantly increased in the supernatant of cultured MCF7-SRGN stable cells compared with that in the
supernatant of cultured MCF7-NC stable cells (_P_<0.05) (Figure 3d). These findings suggest that SRGN positively regulated TGFβ2, but not TGFβ1 protein expression and increased CREB1
phosphorylation, but not CERB1 protein expression. TGFβ2 can promote the expression of CREB1 in tumor cells23 and CD44 is a classic marker of breast cancer stem cells.17 We wanted to know
whether SRGN can regulate CD44 TGFβ2 protein expression and CREB1 phosphorylation. We therefore tested CERB1 protein expression and phosphorylation in MDA-MB-231 cells to observe the effects
of exogenous SRGN on CD44 and TGFβ2 protein expression. The exogenouse SRGN was the supernatant collected from the cultured MCF7-SRGN stable cells. The CD44 gene expression was silenced by
a siRNA of the CD44 gene (siRCD44). MDA-MB-231 cells were treated with the supernatant (sup) collected from the cultured MCF7-SRGN stable cells, with or without 50 and 100 nm siRCD44 for 48
h. Western blots showed that the supernatant from cultured MCF7-SRGN stable cells significantly increased CD44 and TGFβ2 protein expression, CREB1 protein phosphorylation, but not CREB1
protein expression in MDA-MB-231 cells (Figure 3e). In contrast, 50 nm and 100 nm siRCD44 can block the effects of the supernatant from cultured MCF7-SRGN stable cells on CD44 and TGFβ2
protein expression, and CREB1 protein phosphorylation and even significantly decreased these proteins’ levels compared with that in the untreated cells (−/−) (Figure 3e). ELISA assay showed
that the supernatant from cultured MCF7-SRGN stable cells significantly increased, but siRCD44 significantly decreased TGFβ2 protein concentration in the supernatant of cultured MDA-MB-231
cells (_P_<0.05, _P_<0.01) (Figure 3f). Co-immunoprecipitation analysis showed that SRGN directly bound to TGFβ2 in MDA-MB-231 cells (Figure 3g). These results indicate that exogenous
SRGN can stimulate CD44 and TGFβ2 protein expression, and CREB1 phosphorylation in MDA-MD-231 cells, and TGFβ2 protein secretion from MDA-MD-231 cells. SRGN may interact with TGFβ2 in
MDA-MD-231 cells. TGFΒ2 REGULATES SRGN EXPRESSION We want to know whether TGFβ2 has a feedback regulation on SRGN and treated MDA-MB-231, BT549, MCF7, T47D, BT474, and SKBR3 cells with 0, 15
and 25 ng/ml of TGFβ2 for 48 h. Real-time PCR showed that TGFβ2 treatment significantly increased SRGN mRNA expression in TNBC cell lines BT549 and MDA-MB-231 compared with other cell types
(Figure 4a). ELISA assay showed that TGFβ2 significantly increased SRGN concentration in the supernatant of cultured BT549 and MDA-MB-231 cells compared with other cell types (Figure 4b).
TGFβ receptor inhibitor SD208 was used to further confirm the regulatory role of TGFβ2 signaling on SRGN protein. SD208 treatment dose-dependently down-regulate SRGN protein levels in whole
cells (Figure 4c) and cell membrane (Figure 4d) in MDA-MB-231 cells. Similar to TGFβ2, TGFβ1 also significantly increased the mRNA and protein expression of _SRGN_ gene as well as secretion
of SRGN protein in TNBC cell lines BT549 and MDA-MB-231 (Supplementary Figure 3). These findings suggest that both TGFβ1 and TGFβ2 can regulate SRGN gene expression in TNBC cells. We want to
know how the TGFbeta regulates _SRGN_ gene expression in TNBC cells and performed a bioinformatic assay, which predicted that SMAD3 may have five binding sites in the _SRGN_ promoter. We
therefore tested which binding site has a key role in regulating _SRGN_ gene expression. Western blots showed that TGFβ2-stimulated SRGN protein expression correlated with the increases in
Smad3 protein phosphorylation in MDA-MB-231 cells (Figure 4e). The bioinformatic analysis predicted five potential SMAD2/3 binding sites (GTCT/AGAC) in the SRGN promoter region (−2000 to
+200). To validate it, five different 5'deletion sequences containing different Smad3 binding sites were cloned into the promoter- luciferase reporter vector pGL4. The constructs were
transfected into 293 T cells. Results from this deletion analysis showed that the Smad3 binding sites 1, 4, 5 were essential for the promoter activity upon treatment with 10 ng/ml TGFβ2
(Figure 4f). The pLuc vector containing the SRGN promoter with mutated 1, 4 or 5-binding site of Smad3 (Samd3-1-mut, Samd3-4-mut, Samd3-5-mut) or wild-type Samd3 binding site (Samd3-1-wt,
Samd3-4-wt, Samd3-5-wt) was co-transfected with the pRL-TK Renilla luciferase vector with or without the pCMV-SMAD3 vector (pCMV-Samd3) or control vector (pCMV-control) into 293 T cells. The
luciferase activity assay showed that pCMV-SMAD3 vector transfection significantly increased luciferase activity in cells transfected with pLuc vector containing wild-type Samd3 binding
site compared with transfection with pCMV-control vector (_P_<0.05) (Figure 4g). Also, in cells transfected with pCMV-Smad3 vector, the luciferase activity was significantly decreased
when co-transfected with pLuc vector containing wild-type Samd3 binding site compared with co-transfected with pLuc vector containing mutated Samd3 binding site (Figure 4g). To further
verify the binding sites of SMAD2/3 in SRGN promoter region, we performed CHIP-PCR, which also confirmed that SMAD2/3 could bind to site 1,4,5 in SRGN promoter region (Figure 4h and i). The
pLuc vector containing the SRGN promoter (pLuc-SRGN promoter) and pLuc control vector (pLuc Control) were transfected into MDA-MB-231 cells which expresses endogenously TGFβ2. Result showed
that pLuc-SRGN promoter transfection significantly increased luciferase activity in MDA-MB-231 cells compare to the pLuc Control transfection (_P_<0.05), but 20-μM of SD208 inhibitor
partially reversed the effect of pLuc-SRGN promoter transfection (_P_<0.05) (Figure 4j). Addition of exogenous TGFβ2 further dose-dependently increased the luciferase activity in
MDA-MB-231 cells (Figure 4j). These results suggest the existence of Smad3 binding site in SRGN promoter and TGFβ2 can regulate SRGN protein expression through increasing Smad3 activation.
SRGN AND TGFΒ2 EXPRESSION ARE POSITIVELY ASSOCIATED IN TNBC TISSUES AND SERUM To further validate the association between SRGN and TGFβ2 protein expression in breast cancer patients, we
detected the SRGN and TGFβ2 protein expression in 320 non-TNBC tumor tissues (110 Luminal A, 108 Luminal B, and 102 HER2) and 106 TNBC tumor tissues by immunohistochemical staining. SRGN
(Figures 5a and b) and TGFβ2 (Figures 5a and c) protein expression in TNBC tumor tissues was significantly higher than that in non-TNBC tumor tissues [Luminal A (_P_<0.001), Luminal B
(_P_<0.05), and HER2 (_P_<0.01)]. The expression of SRGN and TGFβ2 protein in breast cancer tumors with (Nodal Positive, _n_=135) and without (Nodal Free, _n_=142) lymph node
metastases was also detected by immunohistochemical staining (Figure 5d). Significantly higher scores of positive SRGN (Figure 5e) and TGFβ2 (Figure 5f) staining was observed in breast
cancer tumors with lymph node metastases (Nodal Positive) compared with breast cancer tumors without lymph node metastases (Nodal Free) (_P_<0.01). The scores of SRGN staining positively
correlated with the scores of TGFβ2 staining in TNBC tissues (Figure 5g), and was significantly higher in TNBC tissues with lymph node metastasis (Nodal Positive) than in TNBC tissues
without lymph node metastasis (Nodal Free) (Figure 5h). We measured the SRGN concentration in the serum of patients by ELISA. The concentration of serum SRGN was significantly higher in
patients with TNBC (TNBC, Figure 5i), in breast cancer patients with lymph node metastasis (Nodal Positive, Figure 5j), and in TNBC patients with lymph node metastasis (Nodal Positive,
Figure 5k) than that in patients without TNBC [Luminal A (_P_<0.01), Luminal B (_P_<0.01), and HER2 (_P_<0.001)] (Figure 5i), in BC patients without lymph node metastasis (Nodal
Free) (_P_<0.01) (Figure 5j), and TNBC patients without lymph node metastasis (Nodal Free) (_P_<0.05) (Figure 5k), respectively. These data suggest that SRGN up-regulation correlates
with TGFβ2 in triple-negative breast cancers which may lead to eventually metastasis. DISCUSSION TNBC is difficult to treat due to its high metastasis rate, its susceptibility to relapse,
and the lack of information regarding its specific targets. This study found that glycoprotein SRGN is highly expressed in the TNBC tumor cells. SRGN enhances TGFβ2 expression and matured
TGFβ2 extracellular secretion and subsequently activates EMT through binding CD44 and phosphorylation of CREB1. At the same time, TGFβ2 is highly expressed in TNBC and regulates SRGN
transcriptional expression by binding to TGFβ receptor, which subsequently activates the downstream SMAD2/3 and positively regulates SRGN expression (Figure 6). Therefore, activation of the
SRGN-TGFβ2 loop in TNBC is essential for maintaining the high metastatic potential of TNBC. SRGN is a small molecule glycoprotein mainly distributing in the cell and cell membrane, but it
can also be secreted and integrated into the extracellular matrix. Studies have shown that SRGN promotes cell migration by binding to its receptor in CD44 in blood cells.18 CD44 is thought
to be a classic marker of breast cancer stem cells and has an important role in tumor stem cell adhesion, invasion, metastasis, apoptosis resistance, chemoresistance, and EMT formation.17
For example, the binding of CD44 to its classical ligand, hyaluronic acid (HA), activates protein kinase C, which in turn activates transcription factor SMAD2/3, and subsequently has an
important role in EMT formation.19, 20 Here, we observe that SRGN can promote the expression of TGFβ2 in TNBC cells through binding to its CD44 ligand, which subsequently activates CREB1.
TGFβ2 is known activator of EMT which can explain the effect of SRGN in the formation of EMT. In addition, SRGN can also mediate the maturation and secretion of MMP9 precursors.21 This
suggests that SRGN may also promote metastasis and EMT though regulating MMP9 and other metal matrix protease molecules. Moreover, TGFβ family members have multiple disulfide bonds, and one
of them is mainly involved in the intermolecular linkage.22 This explains our finding that immunoprecipitation results in SRGN directly bound to TGFβ2 through the disulfide bond, which can
promote the partial secretion and maturation of TGFβ2. A previous study showed that the 5′-UTR of SRGN contains multiple (−80) ETS and (−70) CRE sites which mainly regulate the expression of
SRGN, though different stimulus conditions can regulate different sites.23 In the present study, we found that TGFβ2 regulates the expression of SRGN mainly through the (−745) SMAD3 site.
Some studies have shown that TGFβ2 can promote the expression of CREB1 in tumor cells through activating the TGFβ receptor and form a self-activating loop.24, 25 In addition to our findings
on the SRGN-TGFβ2 loop, we find that TGFβ2, but not TGFβ1, may have a crucial role in TNBC. Therefore, we postulate that TGFβ2 may be an ideal target for future targeting therapy. In
addition, our study demonstrates that SRGN does not affect cell growth _in vitro_, but decreases the ability of tumor formation _in vivo_ (Supplementary Figure 1), suggesting that tumor
microenvironment has a great impact on SRGN tumorigenesis. Previous studies demonstrated that SRGN is mainly involved in immune response, such as promoting the TNFα secretion from
macrophages, and inhibiting classical and non-classical complement activation pathways.8 This implies that SRGN may have an important role in tumor immune escape, which may diminish the
effect of antibody-dependent complement killing of the current tumor targeted therapy. In addition, TGFβ2 also functions as an important immunosuppressive factor in the formation of tumor
immunosuppressive microenvironment.26 In this study, the SRGN-TGFβ2 loop may be an important mechanism for TNBC tumor cells to escape immune surveillance. This is worthy of further study. In
summary, our finding of a SRGN-TGFβ2-positive feedback loop highlights a new target for the therapy of refractory and high recurrence of TNBC breast cancer. MATERIALS AND METHODS REAGENTS
Antibodies for E-cadherin, N-cadherin, vimentin, fibronectin, CREB1, pCREB1, pSMAD2/3, and CD44 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies for TGFβ1, TGFβ2,
and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody for SRGN and anti-Rabbit FITC second antibody were purchased from Abcam (Cambridge, MA, USA). The
recombinant human TGFβ1 and TGFβ2 protein was obtained from R&D Systems (Minneapolis, MN, USA). The TGFβ receptor I kinase inhibitor SD208 was from Selleckchem (Houston, TX, USA) and
MTS (3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) was from Promega (Madison, WI, USA). siRNA of CD44 (siRCD44) was purchased from RiboBio
(Guangzhou, China) and transformed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). SPECIMENS The tumor tissues of 426 breast cancers were collected from January 2008 to 2014 at the
Affiliated Tumor Hospital of Guangzhou Medical University. The serum of 163 breast cancer patients was collected between 2013 and 2015. The information on the immunohistological staining of
ER, PR, HER2, Ki67, and clinical classifications of breast cancer patients were recorded. The tissue and serum samples were divided into Luminal A, Luminal B, HER2 positive, and TNBC group.
Fresh tumor tissues were collected from 135 breast cancer patients with lymph node metastasis and 142 patients without metastasis from 2012 to 2015 to detect RNA. This study was approved by
the Ethics Committee of the Affiliated Cancer Hospital, Guangzhou Medical University and informed consent was waived. CELL CULTURE AND PROLIFERATION ASSAY Breast cancer cell lines MCF7,
T47D, BT474, SKBR3, MDA-MB-231 and BT549, and HEK293T human embryonic kidney cell line were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco's Modified
Eagle's (DMEM) medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco) at 37 °C, 5% CO2 (Thermo, Waltham, MA, USA). Cell growth curves were plotted by using the
cellular viability values assessed by the MTS method using CellTiter 96 Aqueous One Solution Cell Proliferation Assay System (Promega). ESTABLISHMENT OF STABLE CELL LINES The plenshSRGN-GFP
lentiviral vector overexpressing shRNA1 (5′-gaactacttccaggtgaatcc-3′) and shRNA2 (5′-ggaacaggattaccaactagt-3′) to silence _SRGN_ gene expression, pEZ-SRGN lentiviral vector overexpressing
_SRGN_ gene, and control lentiviral vectors (NC) were purchased from Genecopoeia (Guangzhou, China). MDA-MB-231 cells at 70% confluence were transfected with plenshSRGN-GFP lentiviral vector
and control vector using BLOCK-iT Lentiviral Pol II miR RNAi system (Invitrogen) by following the user manual. Twenty-four hours after transfection, fresh medium containing 2 μg/ml of
puromycin (Invitrogen) was added to cells and refreshed every three days for three weeks. Single colonies were then selected, identified, and continuously cultured. This procedure produced
two SRGN shRNA stable expression cell lines and a control stable cell line, which we call MDA-MB-231/shSRGN1#, MDA-MB-231/shSRGN2#, and MDA-MB-231/shNC, respectively. MCF7 cells were
transfected with pEZ-SRGN lentiviral vector to overexpress _SRGN_ gene and transfected with pEZ-NC lentiviral to express the blank vector as control as described above. Stable cells were
selected using 1.5 μg/ml of puromycin. The produced MCF7 cells stably expressing SRGN were named MCF-SRGN. The control MCF7 stable cell line was called MCF7-NC. CELL LYSIS AND WESTERN BLOT
Cultured cells were washed twice before lysis with ice-cold 1 × PBS (phosphate-buffered saline). Cells were then lysed using RIPA (radioimmunoprecipitation assay) buffer supplemented with
proteases and phosphatases inhibitors (Sigma-Aldrich, Sigma, St Louis, MO, USA). Protein concentration in lysates was determined by a Pierce BCA protein assay kit (Thermo Scientific,
Waltham, MA, USA). Equal amount of protein between samples was separated by electrophorese on SDS–PAGE gel and transferred to polyvinylidene difluoride membranes (Merck Millipore, USA).
Membranes were incubated with 5% non-fat milk in TBS (Tris-buffered saline) buffer for an hour at room temperature and then incubated with primary antibodies overnight at 4 °C. The membranes
were washed three times with 1 × TBS buffer before incubation with LICOR 680 nm or 800 nm fluorescent secondary antibodies for one hour. After washing with 1 × TBS buffer, the membranes
were scanned on a LICOR Odyssey system. The acquired images were analyzed with Image Studio Version 4.0 software according to manufacturer’s instructions. REAL-TIME PCR Total RNA was
isolated from breast cancer cells and tumor tissues using TRIzol regents by following the manufacturer’s instructions (Invitrogen). cDNA was generated using SuperScriptIII cDNA kit
(Invitrogen). The real-time PCR was performed using ABI7500 Fast Real-Time PCR System. SRGN was amplified using forward primer: 5′-TCCAACAAGATCCCCCGTCT-3′ and reverse primer:
5′-TTCCGTTAGGAAGCCACTCC-3′). The beta-actin was amplified using forward primer: 5′-AGCACAGAGCCTCGCCTTT-3′ and reverse primer: 5′-ATCATCATCCATGGTGAGCTGG-3′ as an internal control. Data were
analyzed using 2−ΔΔCT method. ELISA (ENZYME-LINKED IMMUNOSORBENT ASSAY) The SRGN concentration in the serum of breast cancer patients and the supernatant of serum-free cultured cells was
measured for 48 h using SRGN ELISA Kit (CUSABIO, China). The concentrations of TGFβ1 and TGFβ2 in the supernatants of cell culture were measured using Human TGF-β1 Quantikine ELISA Kit
(PDB110B) and Human TGF-β2 Quantikine ELISA Kit (PDB250) (R&D Systems), respectively according to the manufacturer's instructions. _IN VITRO_ MIGRATION AND INVASION ASSAYS An _in
vitro_ scratch assay and Transwell assays were used to evaluate the migration and invasion abilities of the tumor cells, respectively. Briefly, cells were grown at monolayer in 12-well
plates (2 × 105) for overnight. An artificial scratch was created in cells, and cell debris was removed by washing with 1 × PBS. Cell migration was photographed and the width of the wound
was measured. Cell invasion was evaluated by a Transwell system. The polycarbonate filters (8-μm pore size) were pre-coated with Matrigel Matrix (BD Biosciences, Franklin Lakes, NJ, USA),
and reconstituted at 37 °C for 30 min. Cells (1 × 105) were suspended in 150 μl of serum-free RPMI 1640 medium and added into the upper chamber, while 600 μl of complete medium was added to
the lower chamber. The cells that migrated through the matrigel and adhered onto the lower chamber after 24 h of incubation were fixed with 4% paraformaldehyde for 20 min, and then stained
with Mayer’s hematoxylin (Sigma-Aldrich, USA), and counted under microscope (five fields per chamber). IMMUNOPRECIPITATION ASSAYS MDA-MB-231 cells were washed twice with ice-cold 1 × PBS,
and then lysed with RIPA buffer supplemented with protease inhibitors. Protein concentrations were determined as described above. One milligram of lysates was incubated with protein
G-agarose and anti-SRGN or TGFβ2 antibody for 4 h at 4 °C. Beads were washed 3 times with immunoprecipitation (IP) buffer (150 mm NaCl, 25 mm Tris-HCl pH 7.5, 0.1% Nonidet P-40), and
proteins were eluted with 1.5 × SDS–PAGE sample buffer. Samples were analyzed by Western blot with anti-SRGN or anti-TGFβ2 antibody. FLOW CYTOMETRY Cultured MDA-MB-231 cells were treated
with or without different concentrations of SD208 inhibitor for 48 h. The suspended cells (1 × 106) were stained with SRGN (1:50) antibody. After 30 min incubation on ice, the cells were
washed with staining buffer (BD Biosciences) and then incubated with FITC-labeled second antibody for 30 min on ice in the dark. After washing, the cells were resuspended in staining buffer.
Data were acquired using a BD CantonII Flow Cytometer (BD Bioscience) and analyzed using FlowJo software (Ashland, OR, USA). LUCIFERASE REPORT ASSAY To determine whether SMAD3 regulates the
promoter activity of SRGN, a region of 2 kb (kilobases) upstream of the first exon of SRGN was cloned into the pLuc-reporter vector upstream of the luciferase gene (Genechem, Shanghai,
China). Bioinformatic assay predicted that SMAD3 may have five binding sites (−2000 to +200, −1630 to +200, −1400 to +200, −1040 to +200, −280 to +200) in the SRGN promoter. Therefore, five
pGL4 reporter vectors were constructed to contain 1 to 5 proposed SMAD3 binding sites. At the same time, three mutation vectors were constructed using the TaKaRa MutanBEST Kit according to
the user manual. The mutation primers included: Site 1: forward primer: 5′-GCCggtaccGGGGGAGCGGTAGGGATAGAC-3′ and reverse primer: 5′-GCCagatctGGCTTAATGCACGTGCCCC-3′; Site 4: forward primer:
5′-GCCggtaccCTACTAAAAATACAAAATTAGTCCAGCGC-3′ and reverse primer: 5′-GCCagatctGACGTGTCACCATGTTGGCCA-3′; and Site 5: forward primer: 5′-GCCggtaccTCAGGAGTCTTGTTCCCCAGC-3′ and reverse primer:
5′-GCCagatctTACCTTGAACTGAGGATTCCAGAAC-3′. Briefly, cells were seeded in 96-well plates and co-transfected with the pLuc vector and the pRL-TK Renilla luciferase vector with or without the
pCMV-SMAD3 vector using Lipofectamine 2000 (Invitrogen). After 48 h, luciferase activity was determined using a Dual-Luciferase Reporter Assay System (Promega). The firefly luciferase
activity was calculated as the mean±s.d. after normalization with the Renilla luciferase activity. CHIP-PCR The ChIP assay was performed using the EZ-CHIP chromatin immunoprecipitation kit
(Merck Millipore, Germany). Briefly, 1% formaldehyde was added to the cultured cells to cross-link the chromatin proteins to DNA. After incubation for 10 min at room temperature, the cells
were washed and then scraped off with ice-cold PBS containing protease inhibitors. Cells were pelleted, resuspended, and subjected to sonication to get about 200–1000 base pairs of DNA.
After removing cell debris, the samples were diluted 10-fold in ChIP dilution buffer containing protease inhibitors. Five-μg of anti-H3 antibody (positive control was provided with the kit),
or anti-pSMAD2/3 antibody (Cell Signaling Technology) were added to the chromatin solution and incubated overnight at 4 °C with rotation. Protein G-agarose was then added and incubated at 4
°C for two hours. The protein/DNA complexes were eluted with ChIP elution buffer. DNA was released form protein/DNA complexes by incubation with 5M NaCl at 65 °C for 4 h. The DNA was
purified and 50 μl of DNA was obtained for each treatment. 0.2 μl of DNA from each group was used as a template for PCR. Primers for the SRGN promoter containing putative SMAD3 binding sites
included forward primer: 5′-AAACTCCTCCCTCCTATCAA-3′, reverse primer: 5′-AGCCTATCATACATCCTTGC-3′ for site 1; forward primer: 5′-TGTATTTATTGTATAACTTT-3′ and reverse:
5′-TGCCTGTAGTCCCAGCTACT-3′ for site 2; forward primer: 5′-CTCGCCACCA CGCCCGGCTA-3′ and reverse primer: 5′-GGCTCCATATTAAAGTTATA-3′ for site 3; forward primer: 5′-GTTTGCTGGGCACGGTGGCT-3′ and
reverse primer: 5′-CCTGAGTAGCTGGGATTACA-3′ for site 4; forward primer: 5′-AAGAAGTTGGCGTGCAGCTG-3′ and reverse primer: 5′-ATGGACCACAGGGCTTACAG-3′ for site 5. The primers amplify human GAPDH
gene used forward primer: 5′-TACTAGCGGTTTTACGGGCG-3′ and reverse prime: 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′. The PCR conditions were as follows: one cycle at 95 °C, 5 min; 32 cycles at 95 °C, 20
s, 60 °C, 30 s, and 72 °C, 30 s; and then one cycle at 72 °C for 7 min. PCR samples were electrophoresed on 2% agarose gels and stained with ethidium bromide. In addition, real-time PCR was
carried out according to standard protocols using an ABI7500 with SYBR Green detection (Applied Biosystems). HISTOLOGICAL ANALYSIS Tissues were fixed in 10% neutral buffered formalin for 48
h and then transferred to 70% ethanol before embedding. Tissues were sectioned at 4-μm, mounted on DakoFlex slides (Dako, Denmark), and stained with haematoxylin & eosin. All reagents
used for immunohistochemistry were obtained from Beyotime Institute of Biotechnology (Beijing, China). Sections (4-μm) were deparaffinized in xylene. Endogenous peroxidase was blocked with
3% hydrogen peroxide in deionized water for 20 min. Antigen was retrieved in citrate buffer (10 mm, pH 6.0) at 95 °C for 30 min. Sections were stained with SRGN (1:100) and TGFβ2 (1:100)
antibody for 1 h at 37 °C, followed by biotinylated secondary antibody for 30 min, reaction with horseradish peroxidase for 30 min, and visualization with hydrogen peroxide-activated diamino
benzidine. After washing with 1 × PBS, sections were counter-stained with hematoxylin, dehydrated using ethanol, cleared with xylene, and mounted in mounting medium. Sections treated
without primary antibodies were used as negative controls. The investigators who performed histological analysis were blinded to the group allocation during the experiment and when assessing
the outcome. ANIMAL EXPERIMENTS The immunocompetent female BALB/c mice (10–11 weeks old) were purchased from Guangdong Animal Center (China) and randomly grouped. The mice were first
injected with 1 × 105 of MDA-MB-231/shSRGN or MDA-MB-231/shNC stable cells via tail vein (_N_=8). After 5 weeks, the rats were killed to observe pulmonary metastases. In addition, 1 × 106 of
MDA-MB-231/shSRGN or MDA-MB-231/shNC stable cells were injected subcutaneously into the right posterior of the mice to observe tumor growth. Tumor volume was calculated using _V_=_a_2 ×
_b_/2 (_a_=width, _b_=length). Tumor growth and metastasis were observed by two investigators blinding to the experimental design through recording GFP signals using a small animal live-body
imager (Bruker _in vivo_ Fx pro, USA). Briefly, mice were anesthetized (inhalation) and placed on the imaging platform. The fluorescent signal was detected with a filter of 520 nm and an
excitation of 480 nm. Tumor size and location was observed. All animal experiments were approved by the Experimental Animal Ethics Committee of Guangzhou Medical University. STATISTICS
Statistical analyses were performed using a GraphPad Prism version 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). A two-tailed Fisher’s exact test was used to determine if the
frequency distribution were statistically significant. Comparison of treatments was performed using one-way analysis of variance with Newman–Keuls post-test or a paired two-way Student’s
_t_-test. Differences were considered statistically significant at values of _P_<0.05. REFERENCES * Zardavas D, Piccart M . Neoadjuvant therapy for breast cancer. _Annu Rev Med_ 2015; 66:
31–48. Article CAS Google Scholar * Zardavas D, Irrthum A, Swanton C, Piccart M . Clinical management of breast cancer heterogeneity. _Nat Rev Clin Oncol_ 2015; 12: 381–394. Article CAS
Google Scholar * Rimawi MF, Schiff R, Osborne CK . Targeting HER2 for the treatment of breast cancer. _Annu Rev Med_ 2015; 66: 111–128. Article CAS Google Scholar * Dietze EC, Sistrunk
C, Miranda-Carboni G, O'Regan R, Seewaldt VL . Triple-negative breast cancer in African-American women: disparities versus biology. _Nat Rev Cancer_ 2015; 15: 248–254. Article CAS
Google Scholar * Scully OJ, Chua PJ, Harve KS, Bay BH, Yip GW . Serglycin in health and diseases. _Anat Rec_ 2012; 295: 1415–1420. Article CAS Google Scholar * Kolset SO, Tveit H .
Serglycin—structure and biology. _Cell Mol Life Sci_ 2008; 65: 1073–1085. Article CAS Google Scholar * Kolseth IB, Reine TM, Vuong TT, Meen AJ, Fan Q, Jenssen TG _et al_. Serglycin is
part of the secretory repertoire of LPS-activated monocytes. _Immun Inflamm Dis_ 2015; 3: 23–31. Article CAS Google Scholar * Kolset SO, Pejler G . Serglycin: a structural and functional
chameleon with wide impact on immune cells. _J Immunol_ 2011; 187: 4927–4933. Article CAS Google Scholar * Niemann CU, Kjeldsen L, Ralfkiaer E, Jensen MK, Borregaard N . Serglycin
proteoglycan in hematologic malignancies: a marker of acute myeloid leukemia. _Leukemia_ 2007; 21: 2406–2410. Article CAS Google Scholar * Skliris A, Happonen KE, Terpos E, Labropoulou V,
Borset M, Heinegard D _et al_. Serglycin inhibits the classical and lectin pathways of complement via its glycosaminoglycan chains: implications for multiple myeloma. _Eur J Immunol_ 2011;
41: 437–449. Article CAS Google Scholar * He L, Zhou X, Qu C, Tang Y, Zhang Q, Hong J . Serglycin (SRGN) overexpression predicts poor prognosis in hepatocellular carcinoma patients. _Med
Oncol_ 2013; 30: 707. Article Google Scholar * Li XJ, Ong CK, Cao Y, Xiang YQ, Shao JY, Ooi A _et al_. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes
metastasis. _Cancer Res_ 2011; 71: 3162–3172. Article CAS Google Scholar * Korpetinou A, Skandalis SS, Moustakas A, Happonen KE, Tveit H, Prydz K _et al_. Serglycin is implicated in the
promotion of aggressive phenotype of breast cancer cells. _PLoS ONE_ 2013; 8: e78157. Article CAS Google Scholar * Roy A, Femel J, Huijbers EJ, Spillmann D, Larsson E, Ringvall M _et al_.
Targeting serglycin prevents metastasis in murine mammary carcinoma. _PLoS ONE_ 2016; 11: e0156151. Article Google Scholar * Gregory PA, Bracken CP, Bert AG, Goodall GJ . MicroRNAs as
regulators of epithelial-mesenchymal transition. _Cell Cycle_ 2008; 7: 3112–3118. Article CAS Google Scholar * Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ . Biomarkers of
epithelial-mesenchymal transition in squamous cell carcinoma. _J Dent Res_ 2013; 92: 114–121. Article CAS Google Scholar * Xu Y, Stamenkovic I, Yu Q . CD44 attenuates activation of the
hippo signaling pathway and is a prime therapeutic target for glioblastoma. _Cancer Res_ 2010; 70: 2455–2464. Article CAS Google Scholar * Goodison S, Urquidi V, Tarin D . CD44 cell
adhesion molecules. _Mol Pathol_ 1999; 52: 189–196. Article CAS Google Scholar * Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY _et al_. The epithelial-mesenchymal transition
generates cells with properties of stem cells. _Cell_ 2008; 133: 704–715. Article CAS Google Scholar * Zoller M . CD44: can a cancer-initiating cell profit from an abundantly expressed
molecule? Nature reviews. _Cancer_ 2011; 11: 254–267. PubMed Google Scholar * Malla N, Berg E, Theocharis AD, Svineng G, Uhlin-Hansen L, Winberg JO . _In vitro_ reconstitution of complexes
between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. _FEBS J_ 2013; 280: 2870–2887. Article CAS Google Scholar * Lawrence DA . Latent-TGF-beta: an
overview. _Mol Cell Biochem_ 2001; 219: 163–170. Article CAS Google Scholar * Schick BP, Petrushina I, Brodbeck KC, Castronuevo P . Promoter regulatory elements and DNase I-hypersensitive
sites involved in serglycin proteoglycan gene expression in human erythroleukemia, CHRF 288-11, and HL-60 cells. _J Biol Chem_ 2001; 276: 24726–24735. Article CAS Google Scholar * Rodon
L, Gonzalez-Junca A, Inda Mdel M, Sala-Hojman A, Martinez-Saez E, Seoane J . Active CREB1 promotes a malignant TGFbeta2 autocrine loop in glioblastoma. _Cancer Discov_ 2014; 4: 1230–1241.
Article CAS Google Scholar * Kim S, Lee J, Jeon M, Nam SJ, Lee JE . Elevated TGF-beta1 and -beta2 expression accelerates the epithelial to mesenchymal transition in triple-negative breast
cancer cells. _Cytokine_ 2015; 75: 151–158. Article CAS Google Scholar * Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D _et al_. Immunosuppressive plasma cells
impede T-cell-dependent immunogenic chemotherapy. _Nature_ 2015; 521: 94–98. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by grants from the
National Natural Science Foundation (No. 81302291; No. 81272450; No. 81402196) of China and Guangzhou Municipal University Science and Technology project (No. 1201430498) AUTHOR INFORMATION
Author notes * Z Zhang and Y Deng: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Affiliated Cancer Hospital & Institute of Guangzhou Medical University,
Guangzhou, Guangdong, China Z Zhang, Y Deng, G Zheng, X Jia, K Luo, Q Qiu, Ni Qiu, J Yin, M Lu, H Liu, Y Gu & Z He * Guangzhou Institute of Snake Venom Research, School of Pharmaceutical
Sciences, Guangzhou Medical University, Guangzhou, Guangdong, China Y Xiong Authors * Z Zhang View author publications You can also search for this author inPubMed Google Scholar * Y Deng
View author publications You can also search for this author inPubMed Google Scholar * G Zheng View author publications You can also search for this author inPubMed Google Scholar * X Jia
View author publications You can also search for this author inPubMed Google Scholar * Y Xiong View author publications You can also search for this author inPubMed Google Scholar * K Luo
View author publications You can also search for this author inPubMed Google Scholar * Q Qiu View author publications You can also search for this author inPubMed Google Scholar * Ni Qiu
View author publications You can also search for this author inPubMed Google Scholar * J Yin View author publications You can also search for this author inPubMed Google Scholar * M Lu View
author publications You can also search for this author inPubMed Google Scholar * H Liu View author publications You can also search for this author inPubMed Google Scholar * Y Gu View
author publications You can also search for this author inPubMed Google Scholar * Z He View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHORS Correspondence to Y Xiong or Z He. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies
this paper on the Oncogenesis website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 62 KB) SUPPLEMENTARY FIGURE 2 (JPG 140 KB) SUPPLEMENTARY FIGURE 3 (JPG 72 KB) RIGHTS AND
PERMISSIONS _Oncogenesis_ is an open-access journal published by _Nature Publishing Group_. This work is licensed under a Creative Commons Attribution 4.0 International License. The images
or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the
Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Z., Deng, Y., Zheng, G. _et al._ SRGN-TGFβ2 regulatory loop confers invasion
and metastasis in triple-negative breast cancer. _Oncogenesis_ 6, e360 (2017). https://doi.org/10.1038/oncsis.2017.53 Download citation * Received: 28 November 2016 * Revised: 30 January
2017 * Accepted: 19 May 2017 * Published: 10 July 2017 * Issue Date: July 2017 * DOI: https://doi.org/10.1038/oncsis.2017.53 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative






