- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
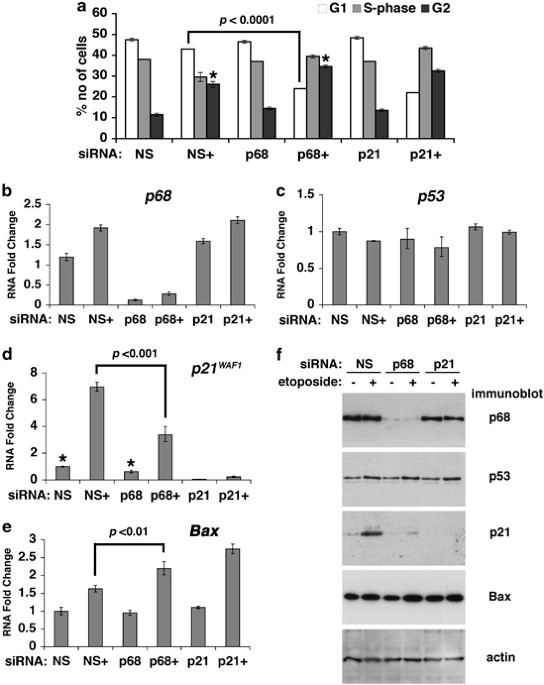
ABSTRACT The RNA helicase p68 (DDX5) is an established co-activator of the p53 tumour suppressor that itself has a pivotal role in orchestrating the cellular response to DNA damage. Although
several factors influence the biological outcome of p53 activation, the mechanisms governing the choice between cell-cycle arrest and apoptosis remain to be elucidated. In the present
study, we show that, while p68 is critical for p53-mediated transactivation of the cell-cycle arrest gene _p21__WAF1/CIP1_, it is dispensable for induction of several pro-apoptotic genes in
response to DNA damage. Moreover, p68 depletion results in a striking inhibition of recruitment of p53 and RNA Pol II to the _p21_ promoter but not to the _Bax_ or _PUMA_ promoters,
providing an explanation for the _selective_ effect on _p21_ induction. Importantly, these findings are mirrored in a novel inducible p68 knockout mouse model in which p68 depletion results
in a selective inhibition of p21 induction in several tissues. Moreover, in the bone marrow, p68 depletion results in an increased sensitivity to γ-irradiation, consistent with an increased
level of apoptosis. These data highlight a novel function of p68 as a modulator of the decision between p53-mediated growth arrest and apoptosis _in vitro_ and _in vivo._ Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print
issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS MRE11 LIBERATES CGAS FROM NUCLEOSOME SEQUESTRATION DURING TUMORIGENESIS Article Open access 10 January 2024 P53 ISOFORMS DIFFERENTIALLY IMPACT ON THE POLΙ DEPENDENT
DNA DAMAGE TOLERANCE PATHWAY Article Open access 13 October 2021 TRIM33 MASKS A NON-TRANSCRIPTIONAL FUNCTION OF E2F4 IN REPLICATION FORK PROGRESSION Article Open access 23 August 2023
REFERENCES * Vousden KH, Prives C . Blinded by the light: the growing complexity of p53. _Cell_ 2009; 137: 413–431. Article CAS Google Scholar * Espinosa JM . Mechanisms of regulatory
diversity within the p53 transcriptional network. _Oncogene_ 2008; 27: 4013–4023. Article CAS Google Scholar * Murray-Zmijewski F, Slee EA, Lu X . A complex barcode underlies the
heterogeneous response of p53 to stress. _Nat Rev Mol Cell Biol_ 2008; 9: 702–712. Article CAS Google Scholar * Linder P . Dead-box proteins: a family affair—active and passive players in
RNP-remodeling. _Nucleic Acids Res_ 2006; 34: 4168–4180. Article CAS Google Scholar * Fuller-Pace FV, Moore HC . RNA helicases p68 and p72: multifunctional proteins with important
implications for cancer development. _Future Oncol_ 2011; 7: 239–251. Article CAS Google Scholar * Endoh H, Maruyama K, Masuhiro Y, Kobayashi Y, Goto M, Tai H _et al_. Purification and
identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. _Mol Cell Biol_ 1999; 19: 5363–5372.
Article CAS Google Scholar * Clark EL, Coulson A, Dalgliesh C, Rajan P, Nicol SM, Fleming S _et al_. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and
is overexpressed in prostate cancer. _Cancer Res_ 2008; 68: 7938–7946. Article CAS Google Scholar * Bates GJ, Nicol SM, Wilson BJ, Jacobs AM, Bourdon JC, Wardrop J _et al_. The DEAD box
protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. _EMBO J_ 2005; 24: 543–553. Article CAS Google Scholar * Metivier R, Penot G, Hubner MR, Reid G, Brand H,
Kos M _et al_. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. _Cell_ 2003; 115: 751–763. Article CAS Google
Scholar * Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L _et al_. GADD45 induction of a G2/M cell cycle checkpoint. _Proc Natl Acad Sci U S A_ 1999; 96: 3706–3711. Article CAS
Google Scholar * Caretti G, Schiltz RL, Dilworth FJ, Di Padova M, Zhao P, Ogryzko V _et al_. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle
differentiation. _Dev Cell_ 2006; 11: 547–560. Article CAS Google Scholar * Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K _et al_. DEAD-box RNA helicase subunits
of the Drosha complex are required for processing of rRNA and a subset of microRNAs. _Nat Cell Biol_ 2007; 9: 604–611. Article CAS Google Scholar * Wallace M, Coates PJ, Wright EG, Ball
KL . Differential post-translational modification of the tumour suppressor proteins Rb and p53 modulate the rates of radiation-induced apoptosis _in vivo_. _Oncogene_ 2001; 20: 3597–3608.
Article CAS Google Scholar * Coates PJ, Lorimore SA, Lindsay KJ, Wright EG . Tissue-specific p53 responses to ionizing radiation and their genetic modification: the key to tissue-specific
tumour susceptibility? _J Pathol_ 2003; 201: 377–388. Article CAS Google Scholar * Midgley CA, Owens B, Briscoe CV, Thomas DB, Lane DP, Hall PA . Coupling between gamma irradiation, p53
induction and the apoptotic response depends upon cell type _in vivo_. _J Cell Sci_ 1995; 108: 1843–1848. CAS Google Scholar * MacCallum DE, Hupp TR, Midgley CA, Stuart D, Campbell SJ,
Harper A _et al_. The p53 response to ionising radiation in adult and developing murine tissues. _Oncogene_ 1996; 13: 2575–2587. CAS PubMed Google Scholar * Fei P, Bernhard EJ, El-Deiry
WS . Tissue-specific induction of p53 targets _in vivo_. _Cancer Res_ 2002; 62: 7316–7327. CAS Google Scholar * Lorimore SA, Pragnell IB, Eckmann L, Wright EG . Synergistic interactions
allow colony formation _in vitro_ by murine haemopoietic stem cells. _Leuk Res_ 1990; 14: 481–489. Article CAS Google Scholar * Lotem J, Sachs L . Hematopoietic cells from mice deficient
in wild-type p53 are more resistant to induction of apoptosis by some agents. _Blood_ 1993; 82: 1092–1096. CAS PubMed Google Scholar * Lorimore SA, Goodhead DT, Wright EG . The effect of
p53 status on the radiosensitivity of haemopoietic stem cells. _Cell Death Differ_ 1995; 2: 233–234. CAS PubMed Google Scholar * Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G,
Crook T, Hsieh JK _et al_. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. _Nat Genet_ 2003; 33: 162–167. Article CAS Google Scholar * Budram-Mahadeo V, Morris
PJ, Latchman DS . The Brn-3a transcription factor inhibits the pro-apoptotic effect of p53 and enhances cell cycle arrest by differentially regulating the activity of the p53 target genes
encoding Bax and p21(CIP1/Waf1). _Oncogene_ 2002; 21: 6123–6131. Article CAS Google Scholar * Hudson CD, Morris PJ, Latchman DS, Budhram-Mahadeo VS . Brn-3a transcription factor blocks
p53-mediated activation of proapoptotic target genes Noxa and Bax _in vitro_ and _in vivo_ to determine cell fate. _J Biol Chem_ 2005; 280: 11851–11858. Article CAS Google Scholar * Das
S, Raj L, Zhao B, Kimura Y, Bernstein A, Aaronson SA _et al_. Hzf Determines cell survival upon genotoxic stress by modulating p53 transactivation. _Cell_ 2007; 130: 624–637. CAS PubMed
PubMed Central Google Scholar * Aylon Y, Oren M . Living with p53, dying of p53. _Cell_ 2007; 130: 597–600. Article CAS Google Scholar * Espinosa JM, Verdun RE, Emerson BM . p53
functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. _Mol Cell_ 2003; 12: 1015–1027. Article CAS Google Scholar
* Morachis JM, Murawsky CM, Emerson BM . Regulation of the p53 transcriptional response by structurally diverse core promoters. _Genes Dev_ 2010; 24: 135–147. Article CAS Google Scholar *
Lu X, Burbidge SA, Griffin S, Smith HM . Discordance between accumulated p53 protein level and its transcriptional activity in response to u.v. radiation. _Oncogene_ 1996; 13: 413–418. CAS
PubMed Google Scholar * Chene P, Fuchs J, Bohn J, Garcia-Echeverria C, Furet P, Fabbro D . A small synthetic peptide, which inhibits the p53-hdm2 interaction, stimulates the p53 pathway
in tumour cell lines. _J Mol Biol_ 2000; 299: 245–253. Article CAS Google Scholar * Bouvard V, Zaitchouk T, Vacher M, Duthu A, Canivet M, Choisy-Rossi C _et al_. Tissue and cell-specific
expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionising irradiation in mice. _Oncogene_ 2000; 19: 649–660. Article CAS Google Scholar * Causevic M,
Hislop RG, Kernohan NM, Carey FA, Kay RA, Steele RJ _et al_. Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. _Oncogene_ 2001; 20: 7734–7743.
Article CAS Google Scholar * Yang L, Lin C, Liu ZR . Phosphorylations of DEAD box p68 RNA helicase are associated with cancer development and cell proliferation. _Mol Cancer Res_ 2005; 3:
355–363. Article CAS Google Scholar * Wortham NC, Ahamed E, Nicol SM, Thomas RS, Periyasamy M, Jiang J _et al_. The DEAD-box protein p72 regulates ERalpha-/oestrogen-dependent
transcription and cell growth, and is associated with improved survival in ERalpha-positive breast cancer. _Oncogene_ 2009; 28: 4053–4064. Article CAS Google Scholar * Hoy CA, Carswell C,
Schimke RT . Bromodeoxyuridine/DNA analysis of replication in CHO cells after exposure to UV light. _Mutat Res_ 1993; 290: 217–230. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank Colin Henderson for helpful discussions. This work was supported by grants from Cancer Research UK (C8745/A11216) and the Association for International Cancer
Research (06–613). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Cancer Research, University of Dundee, Ninewells Hospital & Medical School, Dundee, UK S M Nicol, S A
Lorimore, E G Wright, D W Meek & F V Fuller-Pace * Tayside Tissue Bank, University of Dundee, Ninewells Hospital & Medical School, Dundee, UK S E Bray & P J Coates * Medical
School Resource Unit, University of Dundee, Ninewells Hospital & Medical School, Dundee, UK H Derek Black * p53 Lab, Immunos, A*Star, Singapore, Singapore D P Lane Authors * S M Nicol
View author publications You can also search for this author inPubMed Google Scholar * S E Bray View author publications You can also search for this author inPubMed Google Scholar * H Derek
Black View author publications You can also search for this author inPubMed Google Scholar * S A Lorimore View author publications You can also search for this author inPubMed Google
Scholar * E G Wright View author publications You can also search for this author inPubMed Google Scholar * D P Lane View author publications You can also search for this author inPubMed
Google Scholar * D W Meek View author publications You can also search for this author inPubMed Google Scholar * P J Coates View author publications You can also search for this author
inPubMed Google Scholar * F V Fuller-Pace View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to F V Fuller-Pace. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Oncogene website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY TABLE S1 (DOC 78 KB) SUPPLEMENTARY TABLE S2 (DOC 142 KB) SUPPLEMENTARY FIGURE S1 (JPG 3340 KB) SUPPLEMENTARY FIGURE S2 (JPG 570 KB) SUPPLEMENTARY FIGURE S3 (JPG
3034 KB) SUPPLEMENTARY FIGURE S4 (JPG 614 KB) SUPPLEMENTARY FIGURE S5 (JPG 1231 KB) SUPPLEMENTARY FIGURE S6 (JPG 1237 KB) SUPPLEMENTARY FIGURE S7 (JPG 1055 KB) SUPPLEMENTARY FIGURE S8 (JPG
1498 KB) SUPPLEMENTARY FIGURE S9 (JPG 4919 KB) SUPPLEMENTARY FIGURE S10 (JPG 4319 KB) SUPPLEMENTARY FIGURE S11 (JPG 1241 KB) SUPPLEMENTARY FIGURE S12 (PPT 583 KB) RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nicol, S., Bray, S., Derek Black, H. _et al._ The RNA helicase p68 (DDX5) is selectively required for the induction of
p53-dependent p21 expression and cell-cycle arrest after DNA damage. _Oncogene_ 32, 3461–3469 (2013). https://doi.org/10.1038/onc.2012.426 Download citation * Received: 16 April 2012 *
Revised: 06 July 2012 * Accepted: 31 July 2012 * Published: 17 September 2012 * Issue Date: 18 July 2013 * DOI: https://doi.org/10.1038/onc.2012.426 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * p68 (DDX5) RNA helicase * p53 * DNA damage * cell-cycle arrest * apoptosis