- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
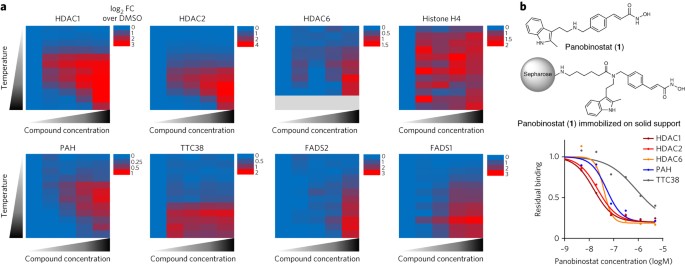
We describe a two-dimensional thermal proteome profiling strategy that can be combined with an orthogonal chemoproteomics approach to enable comprehensive target profiling of the marketed
histone deacetylase inhibitor panobinostat. The N-hydroxycinnamide moiety is identified as critical for potent and tetrahydrobiopterin-competitive inhibition of phenylalanine hydroxylase
leading to increases in phenylalanine and decreases in tyrosine levels. These findings provide a rationale for adverse clinical observations and suggest repurposing of the drug for treatment
of tyrosinemia.
We thank J. Stuhlfauth for cell culture; M. Jundt, K. Kammerer, M. Klös-Hudak, M. Paulmann and T. Rudi for expert technical assistance; and G. Drewes for helpful suggestions. We also thank
W.F. Mueller for providing cells for metabolomics experiments. P.F.F. acknowledges support from the Welch Foundation (grant AQ-1245). C.R.B. was supported by a VENI grant (project
722.013.009) from the Netherlands Organization for Scientific Research.
Isabelle Becher, Thilo Werner, Carola Doce, Ina Tögel, Anne Rueger, Marcel Muelbaier, Elsa Salzer, Marcus Bantscheff & Mikhail M Savitski
Genome Biology Unit, European Molecular Biology Laboratory, Heidelberg, Germany
Biomolecular Mass Spectrometry and Proteomics, Utrecht University, Utrecht, the Netherlands
Department of Biochemistry, University of Texas Health Science Center, San Antonio, Texas, USA
I.B., M.B. and M.M.S. conceived the project; I.B., T.W., M.B. and M.M.S. designed the biochemical, cell biological and MS experiments; I.B. and T.W. performed MS experiments; E.A.Z. and
C.R.B. designed and performed metabolomics experiments; A.R. and M.M. synthesized the panobinostat-amide and gave advice; P.F.F. and C.A.K. designed and performed enzyme activity
experiments; I.B., I.T. and E.S. performed biochemical and cell biological experiments; I.B., T.W., C.D., M.B. and M.M.S. contributed to data analysis; E.A.Z., C.R.B. and P.F.F. contributed
to the manuscript; I.B., M.B. and M.M.S. wrote the manuscript.
I.B., T.W., C.D., I.T., A.R., M.M., E.S., M.B. and M.M.S. are employees and/or shareholders of Cellzome GmbH and GlaxoSmithKline, which funded this work.
Supplementary Results, Supplementary Tables 1–6 and Supplementary Figures 1–17. (PDF 4463 kb)
HepG2 TPP TR (reference for melting temperature). (XLSX 3614 kb)
Affinity-based pulldowns using panobinostat beads, with and without detergent. (XLSX 3584 kb)
Affinity-based pulldowns using panobinostat beads, competition with panobinostat in SH-SY5Y cell extract. (XLSX 1919 kb)
Metabolomics data for amino acids intracellular and in cell medium after HepG2 treatment with panobinostat. (XLSX 43 kb)
Metabolomics data for intracellular and in cell medium amino acid levels after treatment with panobinostat (SH-SY5Y cells). (XLSX 40 kb)
Affinity-based pulldowns using panobinostat beads, competition with tetrahydrobiopterin. (XLSX 1921 kb)
Affinity-based pulldowns using panobinostat beads, competition with panobinostat-amide. (XLSX 1331 kb)
Affinity-based pulldowns using panobinostat beads, competition with belinostat. (XLSX 1932 kb)
Anyone you share the following link with will be able to read this content:







