- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
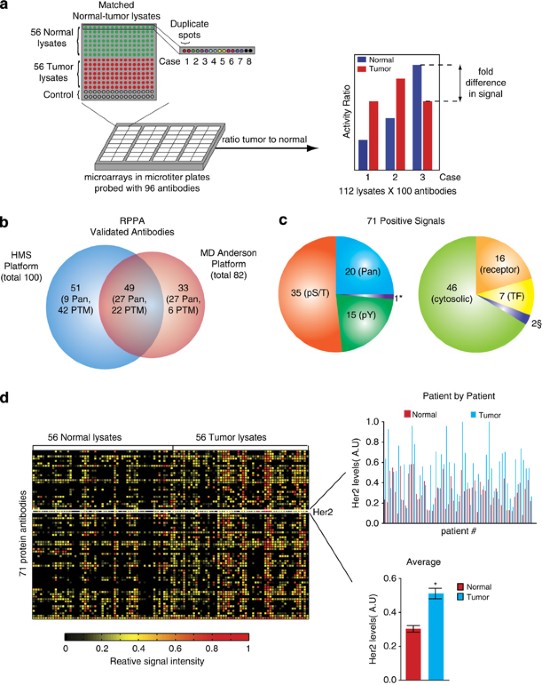
ABSTRACT Measuring the states of cell signaling pathways in tumor samples promises to advance the understanding of oncogenesis and identify response biomarkers. Here, we describe the use of
Reverse Phase Protein Arrays (RPPAs or RPLAs) to profile signaling proteins in 56 breast cancers and matched normal tissue. In RPPAs, hundreds to thousands of lysates are arrayed in dense
regular grids and each grid is probed with a different antibody (100 in the current work, of which 71 yielded strong signals with breast tissue). Although RPPA technology is quite widely
used, measuring changes in phosphorylation reflective of protein activation remains challenging. Using repeat deposition and well-validated antibodies, we show that diverse patterns of
phosphorylation can be monitored in tumor samples and changes mapped onto signaling networks in a coherent fashion. The patterns are consistent with biomarker-based classification of breast
cancers and known mechanisms of oncogenesis. We explore in detail one tumor-associated pattern that involves changes in the abundance of the Axl receptor tyrosine kinase (RTK) and
phosphorylation of the cMet RTK. Both cMet and Axl have been implicated in breast cancer, or in resistance to anticancer drugs, but the two RTKs are not known to be linked functionally.
Protein depletion and overexpression studies in a ‘triple-negative’ breast cell line reveal cross talk between Axl and cMet involving Axl-mediated modification of cMet, a requirement for
cMet in efficient and timely signal transduction by the Axl ligand Gas6 and the potential for the two receptors to interact physically. These findings have potential therapeutic
implications, as they imply that bi-specific receptor inhibitors (for example, ATP-competitive small-kinase inhibitors such as GSK1363089, BMS-777607 or MP470) may be more efficacious than
the mono-specific therapeutic antibodies currently in development (for example, Onartuzumab). Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn
more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS
OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS INTEGRATIVE ANALYSIS OF MULTI-PLATFORM
REVERSE-PHASE PROTEIN ARRAY DATA FOR THE PHARMACODYNAMIC ASSESSMENT OF RESPONSE TO TARGETED THERAPIES Article Open access 15 December 2020 A REVERSE PHASE PROTEIN ARRAY BASED
PHOSPHO-ANTIBODY CHARACTERIZATION APPROACH AND ITS APPLICABILITY FOR CLINICAL DERIVED TISSUE SPECIMENS Article Open access 26 December 2022 PAN-CANCER PROTEOGENOMIC INVESTIGATIONS IDENTIFY
POST-TRANSCRIPTIONAL KINASE TARGETS Article Open access 22 September 2021 REFERENCES * Vogelstein B, Kinzler KW . Cancer genes and the pathways they control. _Nat Med_ 2004; 10: 789–799.
Article CAS Google Scholar * Kolch W, Pitt A . Functional proteomics to dissect tyrosine kinase signalling pathways in cancer. _Nat Rev Cancer_ 2010; 10: 618–629. Article CAS Google
Scholar * Sevecka M, MacBeath G . State-based discovery: a multidimensional screen for small-molecule modulators of EGF signaling. _Nat Methods_ 2006; 3: 825–831. Article CAS Google
Scholar * Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW _et al_. Reverse phase protein microarrays which capture disease progression show activation of pro-survival
pathways at the cancer invasion front. _Gene_ 2001; 20: 1981–1989. CAS Google Scholar * Tibes R, Qiu YH, Lu Y, Hennessy B, Andreeff M, Mills GB _et al_. Reverse phase protein array:
validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. _Mol Cancer Ther_ 2006; 5: 2512. Article CAS Google Scholar
* Grubb RL, Calvert VS, Wulkuhle JD, Paweletz CP, Linehan WM, Phillips JL _et al_. Signal pathway profiling of prostate cancer using reverse phase protein arrays. _Proteomics_ 2003; 3:
2142–2146. Article CAS Google Scholar * VanMeter A, Signore M, Pierobon M, Espina V, Liotta LA, Petricoin I _et al_. Reverse-phase protein microarrays: application to biomarker discovery
and translational medicine. _Expert Rev Mol Diagn_ 2007; 7: 625–633. Article CAS Google Scholar * Hennessy BT, Lu Y, Gonzalez-Angulo AM, Carey MS, Myhre S, Ju Z _et al_. A technical
assessment of the utility of reverse phase protein arrays for the study of the functional proteome in non-microdissected human breast cancers. _Clin Proteomics_ 2010; 6: 129–151. Article
CAS Google Scholar * Sevecka M, Wolf-Yadlin A, Macbeath G . Lysate microarrays enable high-throughput, quantitative investigations of cellular signaling. _Mol Cell Proteomics_ 2011; 10:
M110 005363. Article Google Scholar * Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK _et al_. Transcript and protein expression profiles of the NCI-60 cancer cell
panel: an integromic microarray study. _Mol Cancer Ther_ 2007; 6: 820–832. Article CAS Google Scholar * Jiang R, Mircean C, Shmulevich I, Cogdell D, Jia Y, Tabus I _et al_. Pathway
alterations during glioma progression revealed by reverse phase protein lysate arrays. _Proteomics_ 2006; 6: 2964–2971. Article CAS Google Scholar * Nishizuka S, Charboneau L, Young L,
Major S, Reinhold WC, Waltham M _et al_. Proteomic profiling of the NCI-60 cancer cell lines using new high-density reverse-phase lysate microarrays. _Proc Natl Acad Sci USA_ 2003; 100:
14229–14234. Article CAS Google Scholar * Lin Y, Huang R, Cao X, Wang SM, Shi Q, Huang RP . Detection of multiple cytokines by protein arrays from cell lysate and tissue lysate. _Clin
Chem Lab Med_ 2003; 41: 139–145. Article CAS Google Scholar * Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK _et al_. Tissue ischemia time affects gene and protein expression
patterns within minutes following surgical tumor excision. _Biotechniques_ 2004; 36: 1030–1037. Article CAS Google Scholar * Sevecka M, Wolf-Yadlin A, MacBeath G . Lysate microarrays
enable high-throughput, quantitative investigations of cellular signaling. _Mol Cell Proteomics_ 2011; 10: M110 005363. Article Google Scholar * Luckert K, Gujral TS, Chan M, Joos TO,
Sorger PK, Macbeath G _et al_. A dual array-based approach to assess the abundance and posttranslational modification state of signaling proteins. _Sci Signal_ 2012; 5: pl1. Article Google
Scholar * Gonzalez-Angulo AM, Hennessy BT, Meric-Bernstam F, Sahin A, Liu W, Ju Z _et al_. Functional proteomics can define prognosis and predict pathologic complete response in patients
with breast cancer. _Clin Proteomics_ 2011; 8: 11. Article Google Scholar * Carey MS, Agarwal R, Gilks B, Swenerton K, Kalloger S, Santos J _et al_. Functional proteomic analysis of
advanced serous ovarian cancer using reverse phase protein array: TGF-beta pathway signaling indicates response to primary chemotherapy. _Clin Cancer Res_ 2010; 16: 2852–2860. Article CAS
Google Scholar * Nanjundan M, Byers LA, Carey MS, Siwak DR, Raso MG, Diao L _et al_. Proteomic profiling identifies pathways dysregulated in non-small cell lung cancer and an inverse
association of AMPK and adhesion pathways with recurrence. _J Thorac Oncol_ 2010; 5: 1894–1904. Article Google Scholar * Janes KA, Reinhardt HC, Yaffe MB . Cytokine-induced signaling
networks prioritize dynamic range over signal strength. _Cell_ 2008; 135: 343–354. Article CAS Google Scholar * Goentoro L, Kirschner MW . Evidence that fold-change, and not absolute
level, of beta-catenin dictates Wnt signaling. _Mol Cell_ 2009; 36: 872–884. Article CAS Google Scholar * Burstein HJ, Harris LN, Marcom PK, Lambert-Falls R, Havlin K, Overmoyer B _et
al_. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as
predictive factors, and cardiac surveillance algorithm. _J Clin Oncol_ 2003; 21: 2889–2895. Article CAS Google Scholar * Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL .
Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. _Science_ 1987; 235: 177–182. Article CAS Google Scholar * Isola JJ .
Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. _J Pathol_ 1993; 170: 31–35. Article CAS Google Scholar * Herrero
J, Valencia A, Dopazo J . A hierarchical unsupervised growing neural network for clustering gene expression patterns. _Bioinformatics_ 2001; 17: 126–136. Article CAS Google Scholar *
Vaske CJ, Benz SC, Sanborn JZ, Earl D, Szeto C, Zhu J _et al_. Inference of patient-specific pathway activities from multi-dimensional cancer genomics data using PARADIGM. _Bioinformatics_
2010; 26: i237–i245. Article CAS Google Scholar * Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA _et al_. Gene set enrichment analysis: a knowledge-based approach
for interpreting genome-wide expression profiles. _Proc Natl Acad Sci USA_ 2005; 102: 15545–15550. Article CAS Google Scholar * Storey JD, Tibshirani R . Statistical methods for
identifying differentially expressed genes in DNA microarrays. _Methods Mol Biol_ 2003; 224: 149–157. CAS PubMed Google Scholar * Pe’er D, Hacohen N . Principles and strategies for
developing network models in cancer. _Cell_ 2011; 144: 864–873. Article Google Scholar * Makretsov NA, Huntsman DG, Nielsen TO, Yorida E, Peacock M, Cheang MCU _et al_. Hierarchical
clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. _Clin Cancer Res_ 2004; 10: 6143. Article CAS Google Scholar
* Liu G, Fong E, Zeng X . GNCPro: navigate human genes and relationships through net-walking. _Adv Exp Med Biol_ 2010. 253–259. * Menashe I, Maeder D, Garcia-Closas M, Figueroa JD,
Bhattacharjee S, Rotunno M _et al_. Pathway analysis of breast cancer genome-wide association study highlights three pathways and one canonical signaling cascade. _Cancer Res_ 2010; 70:
4453. Article CAS Google Scholar * Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K _et al_. C-Met overexpression in node-positive breast cancer identifies patients with
poor clinical outcome independent of Her2/neu. _Int J Cancer_ 2005; 113: 678–682. Article CAS Google Scholar * Underiner TL, Herbertz T, Miknyoczki SJ . Discovery of small molecule c-Met
inhibitors: evolution and profiles of clinical candidates. _Anticancer Agents Med Chem_ 2010; 10: 7–27. Article CAS Google Scholar * Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev
YP, Orfi L _et al_. AXL is a potential target for therapeutic intervention in breast cancer progression. _Cancer Res_ 2008; 68: 1905. Article CAS Google Scholar * Gjerdrum C, Tiron C,
Høiby T, Stefansson I, Haugen H, Sandal T _et al_. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. _Proc Natl
Acad Sci USA_ 2010; 107: 1124. Article CAS Google Scholar * Bowers PM, Pellegrini M, Thompson MJ, Fierro J, Yeates TO, Eisenberg D . Prolinks: a database of protein functional linkages
derived from coevolution. _Genome Biol_ 2004; 5: R35. Article Google Scholar * Marcotte EM, Pellegrini M, Thompson MJ, Yeates TO, Eisenberg D . A combined algorithm for genome-wide
prediction of protein function. _Nature_ 1999; 402: 83–86. Article CAS Google Scholar * Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH, Ambs S, Caplen NJ . Identification of the receptor
tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. _Breast Cancer Res Treat_ 2011; 130: 663–679. Article CAS Google Scholar * Hafizi S, Dahlback B .
Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. _Cytokine Growth Factor Rev_ 2006; 17: 295–304. Article CAS Google Scholar * Bean J, Brennan C,
Shih JY, Riely G, Viale A, Wang L _et al_. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. _Proc Natl
Acad Sci USA_ 2007; 104: 20932–20937. Article CAS Google Scholar * Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY . Heterodimerization of insulin-like growth factor receptor/epidermal growth
factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. _Cancer Res_ 2006; 66: 10100–10111. Article CAS Google Scholar * Lim CT, Zhang Y .
Bead-based microfluidic immunoassays: the next generation. _Biosens Bioelectron_ 2007; 22: 1197–1204. Article CAS Google Scholar * Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider
C . Gas6 anti-apoptotic signaling requires NF-kappa B activation. _J Biol Chem_ 2001; 276: 31738–31744. Article CAS Google Scholar * Previdi S, Abbadessa G, Dalo F, France DS, Broggini M
. Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. _Mol Cancer Ther_ 2012; 11: 214–223.
Article CAS Google Scholar * Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C _et al_. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by
governing Axl expression in breast cancer. _Oncogene_ 2011; 30: 1436–1448. Article CAS Google Scholar * Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W _et al_. R428, a selective small
molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. _Cancer Res_ 2010; 70: 1544–1554. Article CAS Google Scholar * Zhang YX,
Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L _et al_. AXL is a potential target for therapeutic intervention in breast cancer progression. _Cancer Res_ 2008; 68: 1905–1915.
Article CAS Google Scholar * Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ . Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization
contributes to trastuzumab resistance of breast cancer cells. _Cancer Res_ 2005; 65: 11118–11128. Article CAS Google Scholar * Kataoka Y, Mukohara T, Tomioka H, Funakoshi Y, Kiyota N,
Fujiwara Y _et al_. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks.
_Invest New Drugs_ 2012; 30: 1352–1360. Article CAS Google Scholar * Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K _et al_. A novel tyrosine kinase switch is a mechanism
of imatinib resistance in gastrointestinal stromal tumors. _Oncogene_ 2007; 26: 3909–3919. Article CAS Google Scholar * Birchmeier C, Birchmeier W, Gherardi E, Woude GFV . Met,
metastasis, motility and more. _Nat Rev Mol Cell Biol_ 2003; 4: 915–925. Article CAS Google Scholar * Mood K, Saucier C, Bong YS, Lee HS, Park M, Daar IO . Gab1 is required for cell cycle
transition, cell proliferation, and transformation induced by an oncogenic met receptor. _Mol Biol Cell_ 2006; 17: 3717. Article CAS Google Scholar * Sam MR, Elliott BE, Mueller CR . A
novel activating role of SRC and STAT3 on HGF transcription in human breast cancer cells. _Mol Cancer_ 2007; 6: 69. Article Google Scholar * Wojcik E, Sharifpoor S, Miller N, Wright T,
Watering R, Tremblay E _et al_. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. _Oncogene_ 2006; 25: 2773–2784. Article CAS Google Scholar
* Yeh CY, Shin SM, Yeh HH, Wu TJ, Shin JW, Chang TY _et al_. Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder
cancer. _BMC Cancer_ 2011; 11: 139. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants from the National Institutes of Health (R33
CA128726, R21 CA126720, and 5RC1-HG005354) and from the Stand Up to Cancer Project (AACR-SU2C-DT0409). TSG is a Human Frontier Science Program Fellow. RLK is partially supported by NSF
0856285. Supplementary Information accompanies the paper on the Oncogene website. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Systems Biology, Harvard Medical School, Boston,
MA, USA T S Gujral, R L Karp, A Finski, M Chan, G MacBeath & P Sorger * Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA, USA T S Gujral & G MacBeath
* Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, USA A Finski * Protein Biotechnologies Inc., Ramona, CA, USA P E Schwartz Authors * T S Gujral View author
publications You can also search for this author inPubMed Google Scholar * R L Karp View author publications You can also search for this author inPubMed Google Scholar * A Finski View
author publications You can also search for this author inPubMed Google Scholar * M Chan View author publications You can also search for this author inPubMed Google Scholar * P E Schwartz
View author publications You can also search for this author inPubMed Google Scholar * G MacBeath View author publications You can also search for this author inPubMed Google Scholar * P
Sorger View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to G MacBeath or P Sorger. ETHICS DECLARATIONS COMPETING
INTERESTS GM is a Vice President and co-founder, and PKS a co-founder of Merrimack Pharmaceuticals, a biotechnology company that develops anti-cancer drugs. PES is a President and CEO of
Protein Biotechnologies, Inc. The remaining authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Oncogene website
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 3493 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gujral, T., Karp, R., Finski, A. _et
al._ Profiling phospho-signaling networks in breast cancer using reverse-phase protein arrays. _Oncogene_ 32, 3470–3476 (2013). https://doi.org/10.1038/onc.2012.378 Download citation *
Received: 23 March 2012 * Revised: 26 June 2012 * Accepted: 13 July 2012 * Published: 03 September 2012 * Issue Date: 18 July 2013 * DOI: https://doi.org/10.1038/onc.2012.378 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * reverse-phase protein arrays * breast cancer * tumor lysate * cell signaling * MET * AXL




:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)


