- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Microglia are a unique lineage of tissue macrophages that are distinct from any other type of myeloid cell inside and outside the brain. They originate exclusively from
erythromyeloid precursors in the yolk sac. Microglia are long-lived and self-renew to ensure cell expansion. * Microglia can be distinguished from other myeloid cells in the brain by
characteristic gene expression profiles. The molecules CSF1R (colony-stimulating factor 1 receptor), DAP12 (DNAX-activation protein 12), IRF8 (interferon regulatory factor 8) and
transcription factor PU.1 are essential for the development and activity of microglia. * Microglia are vital for normal brain function and sensitive to degeneration. Microglial dysfunction
can cause neuropsychiatric diseases that we have named microgliopathies. * Therapeutic benefit in neurological and psychiatric disorders can come from targeting bone marrow-derived myeloid
cells to the CNS. Preclinical evidence is provided in animal models of Alzheimer's disease and Rett syndrome. * Innate immune cells in the CNS (microglia, monocytes, macrophages and
dendritic cells) show complex interactions in response to pathogens, tissue damage and lymphocyte interactions, and reprogramme their function in an adaptive process termed polarization.
ABSTRACT Mononuclear phagocytic cells in the CNS used to be defined according to their anatomical location and surface marker expression. Recently, this concept has been challenged by the
results of developmental and gene expression profiling studies that have used novel molecular biological tools to unravel the origin of microglia and to define their role as specialized
tissue macrophages with long lifespans. Here, we describe how these results have redefined microglia and helped us to understand how different myeloid cell populations operate in the CNS
based on their cell-specific gene expression signatures, distinct ontogeny and differential functions. Moreover, we describe the vulnerability of microglia to dysfunction and propose that
myelomonocytic cells might be used in the treatment of neurological and psychiatric disorders that are characterized by primary or secondary 'microgliopathy'. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $189.00 per year only $15.75 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS BRAIN MACROPHAGE DEVELOPMENT, DIVERSITY AND DYSREGULATION IN HEALTH AND DISEASE Article Open access 26 June 2023 ARG1-EXPRESSING MICROGLIA SHOW A DISTINCT MOLECULAR
SIGNATURE AND MODULATE POSTNATAL DEVELOPMENT AND FUNCTION OF THE MOUSE BRAIN Article Open access 11 May 2023 MONOCYTE-DERIVED MACROPHAGES ACT AS REINFORCEMENTS WHEN MICROGLIA FALL SHORT IN
ALZHEIMER’S DISEASE Article 06 January 2025 REFERENCES * Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in
neurodegeneration. _Nature Neurosci._ 14, 1227–1235 (2011). Article CAS PubMed Google Scholar * Gomez, P. E., Schulz, C. & Geissmann, F. Development and homeostasis of “resident”
myeloid cells: the case of the microglia. _Glia_ 61, 112–120 (2013). Article Google Scholar * Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. _Science_
327, 656–661 (2010). Article CAS PubMed PubMed Central Google Scholar * Sieweke, M. H. & Allen, J. E. Beyond stem cells: self-renewal of differentiated macrophages. _Science_ 342,
1242974 (2013). Article CAS PubMed Google Scholar * Prinz, M. & Mildner, A. Microglia in the CNS: immigrants from another world. _Glia_ 59, 177–187 (2011). Article PubMed Google
Scholar * Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. _Nature Immunol._ 13,
1118–1128 (2012). Article CAS Google Scholar * Chow, A., Brown, B. D. & Merad, M. Studying the mononuclear phagocyte system in the molecular age. _Nature Rev. Immunol._ 11, 788–798
(2011). Article CAS Google Scholar * Davies, L. C., Jenkins, S. J., Allen, J. E. & Taylor, P. R. Tissue-resident macrophages. _Nature Immunol._ 14, 986–995 (2013). Article CAS
Google Scholar * Butovsky, O. et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. _J. Clin. Invest._ 122, 3063–3087 (2012). Article CAS
PubMed PubMed Central Google Scholar * Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. _Science_ 330, 841–845 (2010). Article CAS
PubMed PubMed Central Google Scholar * Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. _Nature Neurosci._ 16, 273–280 (2013).
Article CAS PubMed Google Scholar * Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. _Science_ 336, 86–90 (2012). Article CAS PubMed
Google Scholar * Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. _Nature Neurosci._ 16, 1618–1626 (2013). REFERENCES
10–13 USE FATE-MAPPING AND GENETIC TOOLS TO SHOW THAT MICROGLIA ARE DERIVED FROM THE YOLK SAC. Article CAS PubMed Google Scholar * Yona, S. et al. Fate mapping reveals origins and
dynamics of monocytes and tissue macrophages under homeostasis. _Immunity_ 38, 79–91 (2013). THIS STUDY ESTABLISHES A NEW LINE OF TRANSGENIC MICE IN WHICH CRE IS EXPRESSED SPECIFICALLY IN
MICROGLIA. Article CAS PubMed Google Scholar * Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. _Cell_ 155,
1596–1609 (2013). Article CAS PubMed PubMed Central Google Scholar * Prinz, M. et al. Distinct and nonredundant _in vivo_ functions of IFNAR on myeloid cells limit autoimmunity in the
central nervous system. _Immunity_ 28, 675–686 (2008). Article CAS PubMed Google Scholar * Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial
paralysis. _Nature Med._ 11, 146–152 (2005). Article CAS PubMed Google Scholar * Pfrieger, F. W. & Slezak, M. Genetic approaches to study glial cells in the rodent brain. _Glia_ 60,
681–701 (2012). Article PubMed Google Scholar * Ding, Z. et al. Antiviral drug ganciclovir is a potent inhibitor of microglial proliferation and neuroinflammation. _J. Exp. Med._ 211,
189–198 (2014). Article CAS PubMed PubMed Central Google Scholar * Blank, T. & Prinz, M. Microglia as modulators of cognition and neuropsychiatric disorders. _Glia_ 61, 62–70
(2013). Article PubMed Google Scholar * Priller, J. in _Neuroglia_ 3rd edn (eds Kettenmann, H. & Ransom, B. R.) 906–916 (Oxford Univ. Press, 2013). Google Scholar * Hickey, W. F.
& Kimura, H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen _in vivo_. _Science_ 239, 290–292 (1988). Article CAS PubMed Google Scholar *
Hickey, W. F., Vass, K. & Lassmann, H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. _J. Neuropathol.
Exp. Neurol._ 51, 246–256 (1992). Article CAS PubMed Google Scholar * Bertrand, J. Y. et al. Three pathways to mature macrophages in the early mouse yolk sac. _Blood_ 106, 3004–3011
(2005). Article CAS PubMed Google Scholar * Cumano, A. & Godin, I. Ontogeny of the hematopoietic system. _Annu. Rev. Immunol._ 25, 745–785 (2007). Article CAS PubMed Google
Scholar * Alliot, F., Lecain, E., Grima, B. & Pessac, B. Microglial progenitors with a high proliferative potential in the embryonic and adult mouse brain. _Proc. Natl Acad. Sci. USA_
88, 1541–1545 (1991). Article CAS PubMed Google Scholar * Alliot, F., Godin, I. & Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate
in the brain. _Brain Res. Dev. Brain Res._ 117, 145–152 (1999). Article CAS PubMed Google Scholar * Ashwell, K. The distribution of microglia and cell death in the fetal rat forebrain.
_Brain Res. Dev. Brain Res._ 58, 1–12 (1991). Article CAS PubMed Google Scholar * Lawson, L. J., Perry, V. H. & Gordon, S. Turnover of resident microglia in the normal adult mouse
brain. _Neuroscience_ 48, 405–415 (1992). Article CAS PubMed Google Scholar * Hutchins, K. D., Dickson, D. W., Rashbaum, W. K. & Lyman, W. D. Localization of morphologically distinct
microglial populations in the developing human fetal brain: implications for ontogeny. _Brain Res. Dev. Brain Res._ 55, 95–102 (1990). Article CAS PubMed Google Scholar * Rezaie, P.
& Male, D. Colonisation of the developing human brain and spinal cord by microglia: a review. _Microsc. Res. Tech._ 45, 359–382 (1999). Article CAS PubMed Google Scholar * Rezaie,
P., Dean, A., Male, D. & Ulfig, N. Microglia in the cerebral wall of the human telencephalon at second trimester. _Cereb. Cortex_ 15, 938–949 (2005). Article PubMed Google Scholar *
Esiri, M. M., al Izzi, M. S. & Reading, M. C. Macrophages, microglial cells, and HLA-DR antigens in fetal and infant brain. _J. Clin. Pathol._ 44, 102–106 (1991). Article CAS PubMed
PubMed Central Google Scholar * Verney, C., Monier, A., Fallet-Bianco, C. & Gressens, P. Early microglial colonization of the human forebrain and possible involvement in
periventricular white-matter injury of preterm infants. _J. Anat._ 217, 436–448 (2010). Article PubMed PubMed Central Google Scholar * Kierdorf, K. & Prinz, M. Factors regulating
microglia activation. _Front. Cell Neurosci._ 7, 44 (2013). Article CAS PubMed PubMed Central Google Scholar * Ashwell, K. Microglia and cell death in the developing mouse cerebellum.
_Brain Res. Dev. Brain Res._ 55, 219–230 (1990). Article CAS PubMed Google Scholar * Chan, W. Y., Kohsaka, S. & Rezaie, P. The origin and cell lineage of microglia: new concepts.
_Brain Res. Rev._ 53, 344–354 (2007). Article CAS PubMed Google Scholar * Lichanska, A. M. & Hume, D. A. Origins and functions of phagocytes in the embryo. _Exp. Hematol._ 28,
601–611 (2000). Article CAS PubMed Google Scholar * Dai, X. M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear
phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. _Blood_ 99, 111–120 (2002). Article CAS PubMed Google Scholar * Erblich, B., Zhu, L.,
Etgen, A. M., Dobrenis, K. & Pollard, J. W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. _PLoS ONE_
6, e26317 (2011). Article CAS PubMed PubMed Central Google Scholar * Wegiel, J. et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient
osteopetrotic op/op mice. _Brain Res._ 804, 135–139 (1998). Article CAS PubMed Google Scholar * Yoshida, H. et al. The murine mutation osteopetrosis is in the coding region of the
macrophage colony stimulating factor gene. _Nature_ 345, 442–444 (1990). Article CAS PubMed Google Scholar * Pixley, F. J. & Stanley, E. R. CSF-1 regulation of the wandering
macrophage: complexity in action. _Trends Cell Biol._ 14, 628–638 (2004). Article CAS PubMed Google Scholar * Metcalf, D. The granulocyte-macrophage colony stimulating factors. _Cell_
43, 5–6 (1985). Article CAS PubMed Google Scholar * Lagasse, E. & Weissman, I. L. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis
in op/op mice. _Cell_ 89, 1021–1031 (1997). Article CAS PubMed Google Scholar * Nakahata, T., Gross, A. J. & Ogawa, M. A stochastic model of self-renewal and commitment to
differentiation of the primitive hemopoietic stem cells in culture. _J. Cell. Physiol._ 113, 455–458 (1982). Article CAS PubMed Google Scholar * Lin, H. et al. Discovery of a cytokine
and its receptor by functional screening of the extracellular proteome. _Science_ 320, 807–811 (2008). Article CAS PubMed Google Scholar * Wei, S. et al. Functional overlap but
differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. _J. Leukoc. Biol._ 88, 495–505 (2010). Article CAS PubMed PubMed Central Google
Scholar * Greter, M. et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. _Immunity_ 37, 1050–1060 (2012).
Article CAS PubMed PubMed Central Google Scholar * Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. _Nature
Immunol._ 13, 753–760 (2012). Article CAS Google Scholar * Herbomel, P., Thisse, B. & Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina,
and epidermis through a M-CSF receptor-dependent invasive process. _Dev. Biol._ 238, 274–288 (2001). Article CAS PubMed Google Scholar * Otero, K. et al. Macrophage colony-stimulating
factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. _Nature Immunol._ 10, 734–743 (2009). Article CAS Google Scholar * Rosenbauer, F.
& Tenen, D. G. Transcription factors in myeloid development: balancing differentiation with transformation. _Nature Rev. Immunol._ 7, 105–117 (2007). Article CAS Google Scholar *
McKercher, S. R. et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. _EMBO J._ 15, 5647–5658 (1996). Article CAS PubMed PubMed Central Google
Scholar * Beers, D. R. et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. _Proc. Natl Acad. Sci. USA_ 103, 16021–16026 (2006).
Article CAS PubMed Google Scholar * Zusso, M. et al. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. _J.
Neurosci._ 32, 11285–11298 (2012). Article CAS PubMed PubMed Central Google Scholar * Jin, H. et al. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative
feedback loop inhibiting Pu.1 expression. _Blood_ 119, 5239–5249 (2012). Article CAS PubMed PubMed Central Google Scholar * Holtschke, T. et al. Immunodeficiency and chronic myelogenous
leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. _Cell_ 87, 307–317 (1996). Article CAS PubMed Google Scholar * Masuda, T. et al. IRF8 is a critical
transcription factor for transforming microglia into a reactive phenotype. _Cell Rep._ 1, 334–340 (2012). Article CAS PubMed PubMed Central Google Scholar * Horiuchi, M. et al.
Interferon regulatory factor 8/interferon consensus sequence binding protein is a critical transcription factor for the physiological phenotype of microglia. _J. Neuroinflammation_ 9, 227
(2012). Article CAS PubMed PubMed Central Google Scholar * Minten, C., Terry, R., Deffrasnes, C., King, N. J. & Campbell, I. L. IFN regulatory factor 8 is a key constitutive
determinant of the morphological and molecular properties of microglia in the CNS. _PLoS ONE_ 7, e49851 (2012). Article CAS PubMed PubMed Central Google Scholar * Hashimoto, D., Miller,
J. & Merad, M. Dendritic cell and macrophage heterogeneity _in vivo_. _Immunity_ 35, 323–335 (2011). Article CAS PubMed PubMed Central Google Scholar * Nimmerjahn, A., Kirchhoff,
F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma _in vivo_. _Science_ 308, 1314–1318 (2005). Article CAS PubMed Google Scholar *
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury _in vivo_. _Nature Neurosci._ 8, 752–758 (2005). Article CAS PubMed Google Scholar * Sieger, D., Moritz,
C., Ziegenhals, T., Prykhozhij, S. & Peri, F. Long-range Ca2+ waves transmit brain-damage signals to microglia. _Dev. Cell_ 22, 1138–1148 (2012). Article CAS PubMed Google Scholar *
Hanisch, U. K. & Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. _Nature Neurosci._ 10, 1387–1394 (2007). Article CAS PubMed
Google Scholar * Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. _Nature Immunol._ 11, 889–896 (2010). Article CAS
Google Scholar * Roca, H. et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. _J. Biol. Chem._ 284,
34342–34354 (2009). Article CAS PubMed PubMed Central Google Scholar * Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines _in
vivo_: granulomatous pathology is shaped by the pattern of L-arginine metabolism. _J. Immunol._ 167, 6533–6544 (2001). Article CAS PubMed Google Scholar * Wynn, T. A. Fibrotic disease
and the TH1/TH2 paradigm. _Nature Rev. Immunol._ 4, 583–594 (2004). Article CAS Google Scholar * David, S. & Kroner, A. Repertoire of microglial and macrophage responses after spinal
cord injury. _Nature Rev. Neurosci._ 12, 388–399 (2011). Article CAS Google Scholar * Kim, H. J. et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate
therapy in patients with multiple sclerosis. _J. Immunol._ 172, 7144–7153 (2004). Article CAS PubMed Google Scholar * Durafourt, B. A. et al. Comparison of polarization properties of
human adult microglia and blood-derived macrophages. _Glia_ 60, 717–727 (2012). Article PubMed Google Scholar * Kigerl, K. A. et al. Identification of two distinct macrophage subsets with
divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. _J. Neurosci._ 29, 13435–13444 (2009). Article CAS PubMed PubMed Central Google Scholar
* Hu, X. et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. _Stroke_ 43, 3063–3070 (2012). Article CAS PubMed
Google Scholar * Wang, G. et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. _J. Cereb. Blood Flow Metab._ 33, 1864–1874 (2013). Article CAS
PubMed PubMed Central Google Scholar * Choi, S. H., Aid, S., Kim, H. W., Jackson, S. H. & Bosetti, F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial
activation during neuroinflammation. _J. Neurochem._ 120, 292–301 (2012). Article CAS PubMed Google Scholar * Komohara, Y., Ohnishi, K., Kuratsu, J. & Takeya, M. Possible
involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. _J. Pathol._ 216, 15–24 (2008). Article CAS PubMed Google Scholar * Pyonteck, S. M. et al. CSF-1R
inhibition alters macrophage polarization and blocks glioma progression. _Nature Med._ 19, 1264–1272 (2013). Article CAS PubMed Google Scholar * Dal, B. A. et al. Multiple sclerosis and
Alzheimer's disease. _Ann. Neurol._ 63, 174–183 (2008). Article Google Scholar * Vogel, D. Y. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate
activation status. _J. Neuroinflammation_ 10, 35 (2013). Article CAS PubMed PubMed Central Google Scholar * Takahashi, K., Prinz, M., Stagi, M., Chechneva, O. & Neumann, H.
TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. _PLoS_ _Med._ 4, e124 (2007). Google Scholar *
Guerreiro, R. et al. _TREM2_ variants in Alzheimer's disease. _N. Engl. J. Med._ 368, 117–127 (2013). Article CAS PubMed Google Scholar * Heneka, M. T. et al. NLRP3 is activated in
Alzheimer's disease and contributes to pathology in APP/PS1 mice. _Nature_ 493, 674–678 (2012). Article CAS PubMed PubMed Central Google Scholar * Moore, C. S. et al. miR-155 as a
multiple sclerosis-relevant regulator of myeloid cell polarization. _Ann. Neurol._ 74, 709–720 (2013). Article CAS PubMed Google Scholar * Wang, Y. et al. Transforming growth factor
beta-activated kinase 1 (TAK1)-dependent checkpoint in the survival of dendritic cells promotes immune homeostasis and function. _Proc. Natl Acad. Sci. USA_ 109, E343–E352 (2012). Article
PubMed Google Scholar * Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. _Nature Neurosci._ 16, 1211–1218 (2013). Article
CAS PubMed Google Scholar * Mikita, J. et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical
status by M2 activated monocyte administration. _Mult. Scler._ 17, 2–15 (2011). Article CAS PubMed Google Scholar * Chiu, I. M. et al. A neurodegeneration-specific gene-expression
signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. _Cell Rep._ 4, 385–401 (2013). Article CAS PubMed PubMed Central Google Scholar * Ajami, B.,
Bennett, J. L., Krieger, C., Tetzlaff, W. & Rossi, F. M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. _Nature Neurosci._ 10, 1538–1543
(2007). Article CAS PubMed Google Scholar * Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not
contribute to the resident microglia pool. _Nature Neurosci._ 14, 1142–1149 (2011). Article CAS PubMed Google Scholar * Giovanoli, S. et al. Stress in puberty unmasks latent
neuropathological consequences of prenatal immune activation in mice. _Science_ 339, 1095–1099 (2013). Article CAS PubMed Google Scholar * Lazic, S. E. Comment on “Stress in puberty
unmasks latent neuropathological consequences of prenatal immune activation in mice”. _Science_ 340, 811 (2013). Article CAS PubMed Google Scholar * Roumier, A. et al. Impaired synaptic
function in the microglial KARAP/DAP12-deficient mouse. _J. Neurosci._ 24, 11421–11428 (2004). Article CAS PubMed PubMed Central Google Scholar * Wake, H., Moorhouse, A. J., Jinno, S.,
Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses _in vivo_ and determine the fate of ischemic terminals. _J. Neurosci._ 29, 3974–3980
(2009). Article CAS PubMed PubMed Central Google Scholar * Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. _Science_ 333, 1456–1458
(2011). Article CAS PubMed Google Scholar * Tremblay, M. E., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. _PLoS_ _Biol._
8, e1000527 (2010). Google Scholar * Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. _Neuron_ 74, 691–705 (2012). Article
CAS PubMed PubMed Central Google Scholar * Schafer, D. P. & Stevens, B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. _Curr. Opin. Neurobiol._
23, 1034–1040 (2013). Article CAS PubMed PubMed Central Google Scholar * Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. _Nature Neurosci._ 16,
1896–1905 (2013). Article CAS PubMed Google Scholar * Paloneva, J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical
disease phenotype. _Am. J. Hum. Genet._ 71, 656–662 (2002). Article CAS PubMed PubMed Central Google Scholar * Mosher, K. I. & Wyss-Coray, T. Microglial dysfunction in brain aging
and Alzheimer's disease. _Biochem. Pharmacol._ http://dx.doi.org/10.1016/j.bcp.2014.01.008 (2014). * Hollingworth, P. et al. Common variants at _ABCA7_, _MS4A6A/MS4A4E_, _EPHA1_, _CD33_
and _CD2AP_ are associated with Alzheimer's disease. _Nature Genet._ 43, 429–435 (2011). Article CAS PubMed Google Scholar * Naj, A. C. et al. Common variants at _MS4A4/MS4A6E_,
_CD2AP_, _CD33_ and _EPHA1_ are associated with late-onset Alzheimer's disease. _Nature Genet._ 43, 436–441 (2011). Article CAS PubMed Google Scholar * Griciuc, A. et al.
Alzheimer's disease risk gene _CD33_ inhibits microglial uptake of amyloid beta. _Neuron_ 78, 631–643 (2013). Article CAS PubMed PubMed Central Google Scholar * Guerreiro, R. J. et
al. Using exome sequencing to reveal mutations in _TREM2_ presenting as a frontotemporal dementia-like syndrome without bone involvement. _JAMA Neurol._ 70, 78–84 (2013). Article PubMed
PubMed Central Google Scholar * Rademakers, R. et al. Mutations in the colony stimulating factor 1 receptor (_CSF1R_) gene cause hereditary diffuse leukoencephalopathy with spheroids.
_Nature Genet._ 44, 200–205 (2012). THE FIRST DESCRIPTION OF A PRIMARY 'MICROGLIOPATHY' IN HUMANS. Article CAS Google Scholar * De Jager, P. L. et al. Meta-analysis of genome
scans and replication identify _CD6_, _IRF8_ and _TNFRSF1A_ as new multiple sclerosis susceptibility loci. _Nature Genet._ 41, 776–782 (2009). Article CAS PubMed Google Scholar *
International Multiple Sclerosis Genetics Consortium. The genetic association of variants in _CD6_, _TNFRSF1A_ and _IRF8_ to multiple sclerosis: a multicenter case-control study. _PLoS ONE_
6, e18813 (2011). * Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. _Immunity_ 19, 71–82 (2003). Article
CAS PubMed Google Scholar * Carlin, L. M. et al. _Nr4a1_-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. _Cell_ 153, 362–375 (2013). Article CAS
PubMed PubMed Central Google Scholar * Michaud, J. P., Bellavance, M. A., Prefontaine, P. & Rivest, S. Real-time _in vivo_ imaging reveals the ability of monocytes to clear vascular
amyloid β. _Cell Rep._ 5, 646–653 (2013). Article CAS PubMed Google Scholar * Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of
Alzheimer's disease. _J. Neurosci._ 31, 11159–11171 (2011). Article CAS PubMed PubMed Central Google Scholar * Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular
macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. _Proc. Natl Acad. Sci. USA_ 106, 1261–1266 (2009). Article PubMed Google Scholar * Fiala, M. et al. Ineffective
phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. _J. Alzheimers. Dis._ 7, 221–232 (2005). Article CAS PubMed Google Scholar * Fiala, M. et al. Innate
immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. _Proc. Natl Acad. Sci. USA_ 104, 12849–12854 (2007).
Article CAS PubMed Google Scholar * Saunders, A. M. et al. Association of apolipoprotein E allele ɛ 4 with late-onset familial and sporadic Alzheimer's disease. _Neurology_ 43,
1467–1472 (1993). Article CAS PubMed Google Scholar * Jiang, Q. et al. ApoE promotes the proteolytic degradation of Aβ. _Neuron_ 58, 681–693 (2008). Article CAS PubMed PubMed Central
Google Scholar * Lucin, K. M. et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease. _Neuron_ 79, 873–886 (2013). Article
CAS PubMed PubMed Central Google Scholar * Krabbe, G. et al. Functional impairment of microglia coincides with β-amyloid deposition in mice with Alzheimer-like pathology. _PLoS ONE_ 8,
e60921 (2013). Article CAS PubMed PubMed Central Google Scholar * Yan, S. D. et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. _Nature_ 382, 685–691 (1996).
Article CAS PubMed Google Scholar * Xie, Z. et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42- and lipopolysaccharide-activated microglia. _J. Neurosci._ 22, 3484–3492
(2002). Article CAS PubMed Google Scholar * Grathwohl, S. A. et al. Formation and maintenance of Alzheimer's disease β-amyloid plaques in the absence of microglia. _Nature
Neurosci._ 12, 1361–1363 (2009). Article CAS PubMed Google Scholar * Doody, R. S. et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. _N. Engl. J. Med._
370, 311–321 (2014). Article CAS PubMed Google Scholar * Salloway, S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. _N. Engl. J. Med._ 370,
322–333 (2014). Article CAS PubMed PubMed Central Google Scholar * Holmes, C. et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised,
placebo-controlled phase I trial. _Lancet_ 372, 216–223 (2008). Article CAS PubMed Google Scholar * Shechter, R. et al. Infiltrating blood-derived macrophages are vital cells playing an
anti-inflammatory role in recovery from spinal cord injury in mice. _PLoS_ _Med._ 6, e1000113 (2009). Google Scholar * Popovich, P. G. et al. Depletion of hematogenous macrophages promotes
partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. _Exp. Neurol._ 158, 351–365 (1999). Article CAS PubMed Google Scholar * Shechter, R. et al.
Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. _Immunity_ 38, 555–569 (2013). Article CAS PubMed PubMed Central Google
Scholar * King, I. L., Dickendesher, T. L. & Segal, B. M. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease.
_Blood_ 113, 3190–3197 (2009). Article CAS PubMed PubMed Central Google Scholar * Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the
central nervous system. _Brain_ 132, 2487–2500 (2009). Article PubMed Google Scholar * Boche, D., Denham, N., Holmes, C. & Nicoll, J. A. Neuropathology after active Aβ42
immunotherapy: implications for Alzheimer's disease pathogenesis. _Acta Neuropathol._ 120, 369–384 (2010). Article CAS PubMed Google Scholar * Priller, J. et al. Targeting
gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. _Nature Med._ 7, 1356–1361 (2001). Article CAS PubMed
Google Scholar * Djukic, M. et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. _Brain_ 129,
2394–2403 (2006). Article PubMed Google Scholar * Priller, J. et al. Early and rapid engraftment of bone marrow-derived microglia in scrapie. _J. Neurosci._ 26, 11753–11762 (2006).
Article CAS PubMed PubMed Central Google Scholar * Simard, A. R. & Rivest, S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully
differentiated parenchymal microglia. _FASEB J._ 18, 998–1000 (2004). Article CAS PubMed Google Scholar * Nakano, K., Migita, M., Mochizuki, H. & Shimada, T. Differentiation of
transplanted bone marrow cells in the adult mouse brain. _Transplantation_ 71, 1735–1740 (2001). Article CAS PubMed Google Scholar * Corti, S. et al. Neuroectodermal and microglial
differentiation of bone marrow cells in the mouse spinal cord and sensory ganglia. _J. Neurosci. Res._ 70, 721–733 (2002). Article CAS PubMed Google Scholar * Vallieres, L. &
Sawchenko, P. E. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. _J. Neurosci._ 23, 5197–5207 (2003). Article CAS PubMed PubMed
Central Google Scholar * Flugel, A., Bradl, M., Kreutzberg, G. W. & Graeber, M. B. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS
inflammation and during the retrograde response to axotomy. _J. Neurosci. Res._ 66, 74–82 (2001). Article CAS PubMed Google Scholar * Cogle, C. R. et al. Bone marrow transdifferentiation
in brain after transplantation: a retrospective study. _Lancet_ 363, 1432–1437 (2004). Article CAS PubMed Google Scholar * Unger, E. R. et al. Male donor-derived cells in the brains of
female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific _in situ_ hybridization study. _J. Neuropathol. Exp. Neurol._ 52, 460–470 (1993). Article CAS PubMed
Google Scholar * Kennedy, D. W. & Abkowitz, J. L. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation
model. _Blood_ 90, 986–993 (1997). CAS PubMed Google Scholar * Lassmann, H. & Hickey, W. F. Radiation bone marrow chimeras as a tool to study microglia turnover in normal brain and
inflammation. _Clin. Neuropathol._ 12, 284–285 (1993). CAS PubMed Google Scholar * Krall, W. J., Challita, P. M., Perlmutter, L. S., Skelton, D. C. & Kohn, D. B. Cells expressing
human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. _Blood_ 83, 2737–2748 (1994). CAS
PubMed Google Scholar * Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. _Nature Neurosci._ 10, 1544–1553 (2007).
TOGETHER WITH REFERENCE 90, THIS STUDY PROVIDES THE FIRST DESCRIPTION OF MICROGLIAL SELF-RENEWAL OCCURRING INDEPENDENTLY OF CIRCULATING MYELOID CELLS. Article CAS PubMed Google Scholar *
Capotondo, A. et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. _Proc. Natl Acad. Sci. USA_ 109,
15018–15023 (2012). Article PubMed Google Scholar * Bottcher, C., Fernandez-Klett, F., Gladow, N., Rolfes, S. & Priller, J. Targeting myeloid cells to the brain using
non-myeloablative conditioning. _PLoS ONE_ 8, e80260 (2013). Article CAS PubMed PubMed Central Google Scholar * Wilkinson, F. L. et al. Busulfan conditioning enhances engraftment of
hematopoietic donor-derived cells in the brain compared with irradiation. _Mol. Ther._ 21, 868–876 (2013). Article CAS PubMed PubMed Central Google Scholar * Kierdorf, K., Katzmarski,
N., Haas, C. A. & Prinz, M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. _PLoS ONE_ 8, e58544 (2013). Article CAS PubMed PubMed Central
Google Scholar * Cartier, N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. _Science_ 326, 818–823 (2009). Article CAS PubMed
Google Scholar * Biffi, A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. _Science_ 341, 1233158 (2013). Article CAS PubMed Google Scholar
* Biffi, A., Aubourg, P. & Cartier, N. Gene therapy for leukodystrophies. _Hum. Mol. Genet._ 20, R42–R53 (2011). Article CAS PubMed Google Scholar * Eichler, F. S. et al. Is
microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? _Ann. Neurol._ 63, 729–742 (2008). Article PubMed Google Scholar * Boillee, S. et al. Onset and
progression in inherited ALS determined by motor neurons and microglia. _Science_ 312, 1389–1392 (2006). Article CAS PubMed Google Scholar * Simard, A. R., Soulet, D., Gowing, G.,
Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. _Neuron_ 49, 489–502 (2006). Article
CAS PubMed Google Scholar * El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. _Nature Med._ 13, 432–438 (2007).
Article CAS PubMed Google Scholar * Chen, S. K. et al. Hematopoietic origin of pathological grooming in _Hoxb8_ mutant mice. _Cell_ 141, 775–785 (2010). Article CAS PubMed PubMed
Central Google Scholar * Castro, J., Mellios, N. & Sur, M. Mechanisms and therapeutic challenges in autism spectrum disorders: insights from Rett syndrome. _Curr. Opin. Neurol._ 26,
154–159 (2013). Article CAS PubMed Google Scholar * Derecki, N. C. et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. _Nature_ 484, 105–109 (2012). TOGETHER
WITH REFERENCE 158, THIS REPORT SUGGESTS THAT DYSFUNCTION OF MICROGLIA MAY INDUCE BEHAVIOURAL DISTURBANCES. Article CAS PubMed PubMed Central Google Scholar * Hoeffel, G. et al. Adult
Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. _J. Exp. Med._ 209, 1167–1181 (2012). Article CAS
PubMed PubMed Central Google Scholar * Chorro, L. et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the
epidermal LC network. _J. Exp. Med._ 206, 3089–3100 (2009). Article CAS PubMed PubMed Central Google Scholar * Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally
throughout adult life with minimal contribution from circulating monocytes. _Immunity_ 38, 792–804 (2013). Article CAS PubMed Google Scholar * Guilliams, M. et al. Alveolar macrophages
develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. _J. Exp. Med._ 210, 1977–1992 (2013). Article CAS PubMed PubMed Central Google
Scholar * Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. _Immunity_ 39, 925–938
(2013). Article CAS PubMed Google Scholar * Hsu, A. P. et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. _Blood_ 121,
3830–3837 (2013). Article CAS PubMed PubMed Central Google Scholar * Hambleton, S. et al. IRF8 mutations and human dendritic-cell immunodeficiency. _N. Engl. J. Med._ 365, 127–138
(2011). Article CAS PubMed PubMed Central Google Scholar * Bigley, V. & Collin, M. Dendritic cell, monocyte, B and NK lymphoid deficiency defines the lost lineages of a new GATA-2
dependent myelodysplastic syndrome. _Haematologica_ 96, 1081–1083 (2011). Article PubMed PubMed Central Google Scholar * Blevins, G. & Fedoroff, S. Microglia in colony-stimulating
factor 1-deficient op/op mice. _J. Neurosci. Res._ 40, 535–544 (1995). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors apologize to all of those
colleagues whose work was discussed without proper quotation owing space constraints. The authors thank S. M. Brendecke for critically reading the manuscript and M. Knust for help with the
artwork. M.P. and J.P. are joint coordinators of the DFG-funded research unit (FOR) 1336. In addition, M.P. is supported by the BMBF-funded competence network of multiple sclerosis (KKNMS),
the Gemeinnützige Hertie-Stiftung (GHST), the Fritz Thyssen Stiftung, the competence network of neurodegenerative disorders (KNDD) and the DFG (SFB 992). J.P. receives additional funding
from the DFG (SFB/TRR43 and the Cluster of Excellence NeuroCure), the BMBF (Forschungsnetz zu psychischen Erkrankungen) and the Berlin Institute of Health. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Institute of Neuropathology, University of Freiburg, Breisacherstraße 64, Freiburg, 79106, Germany Marco Prinz * BIOSS Centre for Biological Signalling Studies, University of
Freiburg, Freiburg, 79104, Germany Marco Prinz * Department of Neuropsychiatry and Laboratory of Molecular Psychiatry, Charité – Universitätsmedizin Berlin, Charitéplatz 1, Berlin, 10117,
Germany Josef Priller * Cluster of Excellence NeuroCure, Charitéplatz 1, Berlin, 10117, Germany Josef Priller Authors * Marco Prinz View author publications You can also search for this
author inPubMed Google Scholar * Josef Priller View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Marco Prinz or
Josef Priller. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2
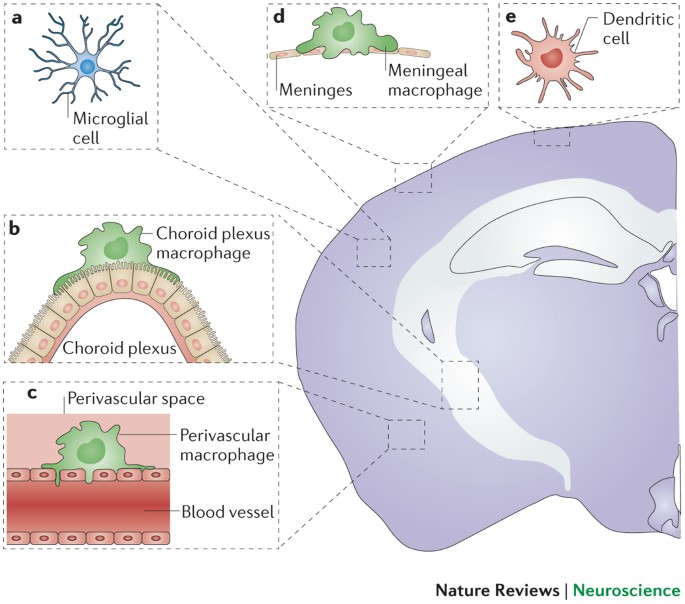
POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR TABLE 1 GLOSSARY * Macrophages Tissue-resident cells of the mononuclear phagocyte
system that are characterized by their ability to phagocytose foreign particulate material, debris and colloidal material. * Mononuclear phagocytes A mononuclear cell type of the myeloid
lineage (macrophages, monocytes or dendritic cells) that have the ability to phagocytose. * Dendritic cells Also known as an interdigitating reticular cells because of their branched
morphology. Dendritic cells are the most potent stimulators of T cell responses. * Monocyte A type of mononuclear leukocyte that is derived from the bone marrow and circulates in the
bloodstream. Monocytes typically migrate into tissues, where they can differentiate into various types of macrophages. * Haematopoietic stem cells (HSCs). Rare multipotent cells that give
rise to all blood cells, including myeloid and lymphoid lineages. * Leukocytes White blood cells derived from multipotent haematopoietic stem cells in the bone marrow. Leukocytes are of
myeloid or lymphoid lineage and are found in the blood and lymphatic system. * Yolk sac A membranous sac attached to the embryo that provides early nourishment in the form of yolk. It
functions as the developmental circulatory system of the embryo before internal circulation begins. * CD45 (Also known as leukocyte common antigen and PTPRC). A type I transmembrane protein
present on all haematopoietic cells that assists in cell activation and the levels of which are reduced in mature parenchymal microglia. * Neuroepithelium The ectodermal epithelium in the
embryo from which the CNS and its main cellular constituents (neurons, astrocytes, oligodendrocytes and ependymal cells) are derived. * Natural killer cells A type of cytotoxic lymphocyte
that are crucial for the innate immune system. * Myelopoiesis The regulated formation of myeloid cells, including macrophages, monocytes, dendritic cells and granulocytes. Myelopoiesis takes
place in the bone marrow or the yolk sac. * Deep RNA sequencing An approach enabled by next-generation sequencing technology that is particularly useful for identifying low-abundance RNAs
or low-frequency mutations. * Parabiotic mice Mice in which shared blood circulation is created via surgical intervention. This procedure enables the fate of labelled donor cells to be
followed in the parabiotic partner. * Graft-versus-host disease A complication following an allogeneic tissue transplant in which immune cells (white blood cells) in the tissue (the graft)
recognize the recipient (the host) as 'foreign'. * X-linked adrenoleukodystrophy A rare X chromosome-linked disorder resulting from mutations in _ABCD1_ (ATP-binding cassette
subfamily D member 1) that cause defects in peroxisomal β-oxidation and lead to the accumulation of very-long-chain fatty acids, particularly in the CNS and adrenal cortex. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Prinz, M., Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric
disease. _Nat Rev Neurosci_ 15, 300–312 (2014). https://doi.org/10.1038/nrn3722 Download citation * Published: 09 April 2014 * Issue Date: May 2014 * DOI: https://doi.org/10.1038/nrn3722
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative








