- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
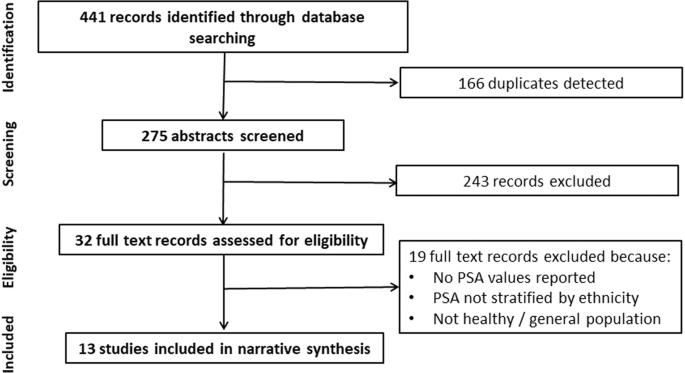
KEY POINTS * The phosphoinositide 3-kinase (PI3K) pathway is a crucial signal transduction system linking the activation of receptor tyrosine kinases (RTKs), G protein-coupled receptors
(GPCRs) and oncogenes such as _RAS_ to multiple essential cellular functions. * The PI3K pathway is tightly controlled by a class of PI3Ks that generate the lipid second messenger
phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3), and the tumor suppressor PTEN (phosphatase and tensin homologue), a lipid phosphatase that dephosphorylates PtdIns(3,4,5)P3,
thereby counteracting the actions of PI3Ks. * The PI3K pathway is one of the most frequently activated signalling pathways in human cancer. Oncogenic activation of this pathway commonly
occurs through activating mutations in the p110α isoform of PI3K or through loss of the PTEN tumour suppressor. * Inhibitors that target key components of this pathway, including PI3K, AKT
and mammalian target of rapamycin (mTOR), are being actively developed. Some of them have reached clinical trials in patients with various solid and haematological malignancies. Most PI3K
inhibitors developed to date are pan-PI3K inhibitors. * It may be desirable to generate isoform-specific PI3K inhibitors as PI3Ks have essential roles in a wide range of normal physiological
functions, including glucose homeostasis and immune responses. For example, by targeting p110α or p110β isoforms in solid tumours, potential drugs might avoid toxicity to the immune system,
which is largely dependent on p110δ and p110γ for function. * The presence of multiple nodes with feedback loops and crosstalk between pathways may affect therapeutic outcomes. Emerging
strategies, such as simultaneously targeting two kinases in the pathway or the combination of PI3K pathway inhibitors with drugs that target other pathways, may achieve optimal clinical
benefits. ABSTRACT The phosphoinositide 3-kinase (PI3K) pathway is a key signal transduction system that links oncogenes and multiple receptor classes to many essential cellular functions,
and is perhaps the most commonly activated signalling pathway in human cancer. This pathway therefore presents both an opportunity and a challenge for cancer therapy. Even as inhibitors that
target PI3K isoforms and other major nodes in the pathway, including AKT and mammalian target of rapamycin (mTOR), reach clinical trials, major issues remain. Here, we highlight recent
progress that has been made in our understanding of the PI3K pathway and discuss the potential of and challenges for the development of therapeutic agents that target this pathway in cancer.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this
journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS PHOSPHOINOSITIDE KINASES IN CANCER: FROM MOLECULAR MECHANISMS TO THERAPEUTIC OPPORTUNITIES Article 03 April 2025 THE PRESENT AND FUTURE OF PI3K
INHIBITORS FOR CANCER THERAPY Article 17 June 2021 TARGETING PI3K/AKT SIGNAL TRANSDUCTION FOR CANCER THERAPY Article Open access 16 December 2021 REFERENCES * Vivanco, I. & Sawyers, C.
L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. _Nature Rev. Cancer_ 2, 489–501 (2002). CAS Google Scholar * Bader, A. G., Kang, S., Zhao, L. & Vogt, P. K. Oncogenic
PI3K deregulates transcription and translation. _Nature Rev. Cancer_ 5, 921–929 (2005). CAS Google Scholar * Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of
phosphatidylinositol 3-kinases as regulators of growth and metabolism. _Nature Rev. Genet._ 7, 606–619 (2006). A RECENT COMPREHENSIVE REVIEW ON PI3K. CAS PubMed Google Scholar * Cully,
M., You, H., Levine, A. J. & Mak, T. W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. _Nature Rev. Cancer_ 6, 184–192 (2006). CAS
Google Scholar * Cantley, L. C. & Neel, B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. _Proc. Natl
Acad. Sci. USA_ 96, 4240–4245 (1999). CAS PubMed PubMed Central Google Scholar * Hennessy, B. T., Smith, D. L., Ram, P. T., Lu, Y. & Mills, G. B. Exploiting the PI3K/AKT pathway for
cancer drug discovery. _Nature Rev. Drug Discov._ 4, 988–1004 (2005). CAS Google Scholar * Fruman, D. A., Meyers, R. E. & Cantley, L. C. Phosphoinositide kinases. _Annu. Rev. Biochem._
67, 481–507 (1998). CAS PubMed Google Scholar * Katso, R. et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. _Annu. Rev. Cell
Dev. Biol._ 17, 615–675 (2001). CAS PubMed Google Scholar * Voigt, P., Dorner, M. B. & Schaefer, M. Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide
3-kinase gamma that is highly expressed in heart and interacts with PDE3B. _J. Biol. Chem._ 281, 9977–9986 (2006). CAS PubMed Google Scholar * Suire, S. et al. p84, a new Gβγ-activated
regulatory subunit of the type IB phosphoinositide 3-kinase p110γ. _Curr. Biol._ 15, 566–570 (2005). CAS PubMed Google Scholar * Chang, J. D. et al. Deletion of the phosphoinositide
3-kinase p110γ gene attenuates murine atherosclerosis. _Proc. Natl Acad. Sci. USA_ 104, 8077–8082 (2007). CAS PubMed PubMed Central Google Scholar * Patrucco, E. et al. PI3Kγ modulates
the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. _Cell_ 118, 375–387 (2004). CAS PubMed Google Scholar * Sasaki, T. et al. Function
of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. _Science_ 287, 1040–1046 (2000). CAS PubMed Google Scholar * Backer, J. M. The regulation and function of
Class III PI3Ks: novel roles for Vps34. _Biochem. J._ 410, 1–17 (2008). CAS PubMed Google Scholar * Scheid, M. P. & Woodgett, J. R. PKB/AKT: functional insights from genetic models.
_Nature Rev. Mol. Cell Biol._ 2, 760–768 (2001). CAS Google Scholar * Alessi, D. R. et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the
Drosophila DSTPK61 kinase. _Curr. Biol._ 7, 776–789 (1997). CAS PubMed Google Scholar * Stephens, L. et al. Protein kinase B kinases that mediate phosphatidylinositol
3,4,5-trisphosphate-dependent activation of protein kinase B. _Science_ 279, 710–714 (1998). CAS PubMed Google Scholar * Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M.
Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. _Science_ 307, 1098–1101 (2005). CAS PubMed Google Scholar * Manning, B. D. & Cantley, L. C. AKT/PKB signaling:
navigating downstream. _Cell_ 129, 1261–1274 (2007). CAS PubMed PubMed Central Google Scholar * Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism.
_Cell_ 124, 471–484 (2006). CAS PubMed Google Scholar * Sabatini, D. M. mTOR and cancer: insights into a complex relationship. _Nature Rev. Cancer_ 6, 729–734 (2006). CAS Google Scholar
* Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K. L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. _Nature Cell Biol._ 4, 648–657 (2002). CAS PubMed
Google Scholar * Manning, B. D., Tee, A. R., Logsdon, M. N., Blenis, J. & Cantley, L. C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a
target of the phosphoinositide 3-kinase/akt pathway. _Mol. Cell_ 10, 151–162 (2002). CAS PubMed Google Scholar * Vander Haar, E., Lee, S. I., Bandhakavi, S., Griffin, T. J. & Kim, D.
H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. _Nature Cell Biol._ 9, 316–323 (2007). CAS PubMed Google Scholar * Crino, P. B., Nathanson, K. L. & Henske, E.
P. The tuberous sclerosis complex. _N. Engl. J. Med._ 355, 1345–1356 (2006). CAS PubMed Google Scholar * Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. _Genes Dev._ 18,
1926–1945 (2004). CAS PubMed Google Scholar * Zhao, J. J. & Roberts, T. M. PI3 kinases in cancer: from oncogene artifact to leading cancer target. _Sci. STKE_ 2006, pe52 (2006).
PubMed Google Scholar * Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. _Science_ 318, 1108–1113 (2007). CAS PubMed Google Scholar * Thomas, R. K. et
al. High-throughput oncogene mutation profiling in human cancer. _Nature Genet._ 39, 347–351 (2007). CAS PubMed Google Scholar * Comprehensive genomic characterization defines human
glioblastoma genes and core pathways. _Nature_ 455, 1061–1068 (2008). * Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. _Science_ 321, 1807–1812
(2008). Article CAS PubMed PubMed Central Google Scholar * Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. _Science_ 304, 554 (2004). THIS STUDY
SHOWED THAT _PIK3CA_ IS FREQUENTLY MUTATED IN HUMAN CANCER. CAS PubMed Google Scholar * Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. _Nature_ 455,
1069–1075 (2008). CAS PubMed PubMed Central Google Scholar * Sansal, I. & Sellers, W. R. The biology and clinical relevance of the PTEN tumor suppressor pathway. _J. Clin. Oncol._
22, 2954–2963 (2004). CAS PubMed Google Scholar * Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple
advanced cancers. _Nature Genet._ 15, 356–362 (1997). CAS PubMed Google Scholar * Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. _Cell_ 133, 403–414
(2008). CAS PubMed Google Scholar * Parsons, R. Human cancer, PTEN and the PI-3 kinase pathway. _Semin. Cell Dev. Biol._ 15, 171–176 (2004). CAS PubMed Google Scholar * Knobbe, C. B.,
Lapin, V., Suzuki, A. & Mak, T. W. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. _Oncogene_ 27, 5398–5415 (2008). CAS PubMed
Google Scholar * Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. _Science_ 275, 1943–1947 (1997). CAS PubMed Google
Scholar * Di Cristofano, A., De Acetis, M., Koff, A., Cordon-Cardo, C. & Pandolfi, P. P. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. _Nature Genet._
27, 222–224 (2001). CAS PubMed Google Scholar * Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. Pten is essential for embryonic development and tumour suppression.
_Nature Genet._ 19, 348–355 (1998). CAS PubMed Google Scholar * Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer.
_Cancer Cell_ 4, 209–221 (2003). CAS PubMed Google Scholar * Stambolic, V. et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice.
_Cancer Res._ 60, 3605–3611 (2000). CAS PubMed Google Scholar * Stemke-Hale, K. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer.
_Cancer Res._ 68, 6084–6091 (2008). CAS PubMed PubMed Central Google Scholar * Zhao, J. J. et al. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in
human mammary epithelial cells. _Proc. Natl Acad. Sci. USA_ 102, 18443–18448 (2005). CAS PubMed PubMed Central Google Scholar * Isakoff, S. J. et al. Breast cancer-associated PIK3CA
mutations are oncogenic in mammary epithelial cells. _Cancer Res._ 65, 10992–11000 (2005). CAS PubMed Google Scholar * Samuels, Y. et al. Mutant PIK3CA promotes cell growth and invasion
of human cancer cells. _Cancer Cell_ 7, 561–573 (2005). CAS PubMed Google Scholar * Bader, A. G., Kang, S. & Vogt, P. K. Cancer-specific mutations in PIK3CA are oncogenic _in vivo_.
_Proc. Natl Acad. Sci. USA_ 103, 1475–1479 (2006). CAS PubMed PubMed Central Google Scholar * Lee, J. Y., Engelman, J. A. & Cantley, L. C. Biochemistry. PI3K charges ahead. _Science_
317, 206–207 (2007). CAS PubMed Google Scholar * Huang, C. H., Mandelker, D., Gabelli, S. B. & Amzel, L. M. Insights into the oncogenic effects of PIK3CA mutations from the structure
of p110α/p85α. _Cell Cycle_ 7, 1151–1156 (2008). CAS PubMed Google Scholar * Miled, N. et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic
subunit. _Science_ 317, 239–242 (2007). CAS PubMed Google Scholar * Huang, C. H. et al. The structure of a human p110α/p85α complex elucidates the effects of oncogenic PI3Kα mutations.
_Science_ 318, 1744–1748 (2007). REFERENCES 51 AND 52 REPORT CRYSTAL STRUCTURAL ANALYSES OF P110Α AND P85 ISH2 DOMAINS TO HELP ELUCIDATE THE EFFECTS OF THE CANCER-ASSOCIATED MUTATIONS IN
P110Α. CAS PubMed Google Scholar * Mizoguchi, M., Nutt, C. L., Mohapatra, G. & Louis, D. N. Genetic alterations of phosphoinositide 3-kinase subunit genes in human glioblastomas.
_Brain Pathol._ 14, 372–377 (2004). CAS PubMed Google Scholar * Philp, A. J. et al. The phosphatidylinositol 3-kinase p85α gene is an oncogene in human ovarian and colon tumors. _Cancer
Res._ 61, 7426–7429 (2001). CAS PubMed Google Scholar * Beeton, C. A., Chance, E. M., Foukas, L. C. & Shepherd, P. R. Comparison of the kinetic properties of the lipid- and
protein-kinase activities of the p110α and p110β catalytic subunits of class-Ia phosphoinositide 3-kinases. _Biochem. J._ 350, 353–359 (2000). CAS PubMed PubMed Central Google Scholar *
Benistant, C., Chapuis, H. & Roche, S. A specific function for phosphatidylinositol 3-kinase α (p85α-p110α) in cell survival and for phosphatidylinositol 3-kinase β (p85α-p110b) in _de
novo_ DNA synthesis of human colon carcinoma cells. _Oncogene_ 19, 5083–5090 (2000). CAS PubMed Google Scholar * Brugge, J., Hung., M. C. & Mills, G. B. A new mutational AKTivation in
the PI3K pathway. _Cancer Cell_ 12, 104–107 (2007). CAS PubMed Google Scholar * Carpten, J. D. et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer.
_Nature_ 448, 439–444 (2007). THIS PAPER DESCRIBES THE IDENTIFICATION OF AN ACTIVATING AKT1 MUTATION IN HUMAN CANCER. CAS PubMed Google Scholar * Davies, M. A. et al. A novel AKT3
mutation in melanoma tumours and cell lines. _Br. J. Cancer_ 99, 1265–1268 (2008). CAS PubMed PubMed Central Google Scholar * Vanhaesebroeck, B. & Alessi, D. R. The PI3K-PDK1
connection: more than just a road to PKB. _Biochem. J._ 346, 561–576 (2000). CAS PubMed PubMed Central Google Scholar * Hunter, C. et al. A hypermutation phenotype and somatic MSH6
mutations in recurrent human malignant gliomas after alkylator chemotherapy. _Cancer Res._ 66, 3987–3991 (2006). CAS PubMed PubMed Central Google Scholar * Knight, Z. A. & Shokat, K.
M. Chemically targeting the PI3K family. _Biochem. Soc. Trans._ 35, 245–249 (2007). CAS PubMed Google Scholar * Marone, R., Cmiljanovic, V., Giese, B. & Wymann, M. P. Targeting
phosphoinositide 3-kinase: moving towards therapy. _Biochim. Biophys. Acta_ 1784, 159–185 (2008). CAS PubMed Google Scholar * Knight, Z. A. et al. A pharmacological map of the PI3-K
family defines a role for p110α in insulin signaling. _Cell_ 125, 733–747 (2006). A COMPARATIVE ANALYSIS OF THE ROLES OF PI3K ISOFORMS USING SMALL-MOLECULE INHIBITORS. CAS PubMed PubMed
Central Google Scholar * Fan, Q. W. et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. _Cancer Cell_ 9, 341–349 (2006). CAS PubMed PubMed Central Google
Scholar * Maira, S. M. et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with
potent _in vivo_ antitumor activity. _Mol. Cancer Ther._ 7, 1851–1863 (2008). CAS PubMed Google Scholar * Serra, V. et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling
and inhibits the growth of cancer cells with activating PI3K mutations. _Cancer Res._ 68, 8022–8030 (2008). CAS PubMed Google Scholar * Garcia-Echeverria, C. & Sellers, W. R. Drug
discovery approaches targeting the PI3K/Akt pathway in cancer. _Oncogene_ 27, 5511–5526 (2008). CAS PubMed Google Scholar * Garlich, J. R. et al. A vascular targeted pan phosphoinositide
3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. _Cancer Res._ 68, 206–215 (2008). CAS PubMed Google Scholar * Ihle, N. T. et al. The
phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. _Mol.
Cancer Ther._ 4, 1349–1357 (2005). CAS PubMed PubMed Central Google Scholar * Hilgard, P. et al. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. _Eur. J. Cancer_
33, 442–446 (1997). CAS PubMed Google Scholar * Meuillet, E. J. et al. _In vivo_ molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. _Oncol. Res._ 14,
513–527 (2004). CAS PubMed Google Scholar * Gills, J. J. & Dennis, P. A. The development of phosphatidylinositol ether lipid analogues as inhibitors of the serine/threonine kinase,
Akt. _Expert Opin. Investig. Drugs_ 13, 787–797 (2004). CAS PubMed Google Scholar * Gills, J. J. et al. Spectrum of activity and molecular correlates of response to phosphatidylinositol
ether lipid analogues, novel lipid-based inhibitors of Akt. _Mol. Cancer Ther._ 5, 713–722 (2006). CAS PubMed Google Scholar * Rhodes, N. et al. Characterization of an Akt kinase
inhibitor with potent pharmacodynamic and antitumor activity. _Cancer Res._ 68, 2366–2374 (2008). CAS PubMed Google Scholar * Lindsley, C. W., Barnett, S. F., Yaroschak, M., Bilodeau, M.
T. & Layton, M. E. Recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. _Curr. Top. Med. Chem._ 7, 1349–1363 (2007). CAS PubMed Google Scholar *
Yang, H. et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. _Cancer Res._ 68, 425–433 (2008). CAS
PubMed Google Scholar * Vezina, C., Kudelski, A. & Sehgal, S. N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the
active principle. _J. Antibiot. (Tokyo)_ 28, 721–726 (1975). CAS Google Scholar * Yatscoff, R. W., LeGatt, D. F. & Kneteman, N. M. Therapeutic monitoring of rapamycin: a new
immunosuppressive drug. _Ther. Drug Monit._ 15, 478–482 (1993). CAS PubMed Google Scholar * Faivre, S., Kroemer, G. & Raymond, E. Current development of mTOR inhibitors as anticancer
agents. _Nature Rev. Drug Discov._ 5, 671–688 (2006). Article CAS Google Scholar * Guertin, D. A. & Sabatini, D. M. Defining the role of mTOR in cancer. _Cancer Cell_ 12, 9–22 (2007).
CAS PubMed Google Scholar * Hay, N. The Akt-mTOR tango and its relevance to cancer. _Cancer Cell_ 8, 179–183 (2005). CAS PubMed Google Scholar * Atkins, M. B. et al. Randomized phase
II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. _J. Clin. Oncol._ 22, 909–918
(2004). CAS PubMed Google Scholar * Guertin, D. A. et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. _Cancer Cell_ 15, 148–159 (2009).
CAS PubMed PubMed Central Google Scholar * Feldman, M. E. et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. _PLoS Biol._ 7, e38 (2009).
PubMed Google Scholar * Thoreen, C. C. et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. _J. Biol. Chem._ 284, 8023–8032
(2009). CAS PubMed PubMed Central Google Scholar * Fujita, N., Sato, S., Ishida, A. & Tsuruo, T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent
kinase-1. _J. Biol. Chem._ 277, 10346–10353 (2002). CAS PubMed Google Scholar * Solit, D. B., Basso, A. D., Olshen, A. B., Scher, H. I. & Rosen, N. Inhibition of heat shock protein 90
function down-regulates Akt kinase and sensitizes tumors to Taxol. _Cancer Res._ 63, 2139–2144 (2003). CAS PubMed Google Scholar * Solit, D. B. & Rosen, N. Hsp90: a novel target for
cancer therapy. _Curr. Top. Med. Chem._ 6, 1205–1214 (2006). CAS PubMed Google Scholar * Workman, P., Burrows, F., Neckers, L. & Rosen, N. Drugging the cancer chaperone HSP90:
combinatorial therapeutic exploitation of oncogene addiction and tumor stress. _Ann. NY Acad. Sci._ 1113, 202–216 (2007). CAS PubMed Google Scholar * Bi, L., Okabe, I., Bernard, D. J.
& Nussbaum, R. L. Early embryonic lethality in mice deficient in the p110β catalytic subunit of PI 3-kinase. _Mamm. Genome_ 13, 169–172 (2002). CAS PubMed Google Scholar * Bi, L.,
Okabe, I., Bernard, D. J., Wynshaw-Boris, A. & Nussbaum, R. L. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide
3-kinase. _J. Biol. Chem._ 274, 10963–10968 (1999). CAS PubMed Google Scholar * Jia, S. et al. Essential roles of PI3K-p110β in cell growth, metabolism and tumorigenesis. _Nature_ (2008).
THIS STUDY SHOWED THAT P110Β, NOT P110Α, CONTRIBUTED TO PTEN-DEFICIENCY-INDUCED PROSTATE CANCER IN MOUSE GENETIC MODELS. * Zhao, J. J. et al. The p110α isoform of PI3K is essential for
proper growth factor signaling and oncogenic transformation. _Proc. Natl Acad. Sci. USA_ 103, 16296–16300 (2006). CAS PubMed PubMed Central Google Scholar * Ciraolo, E. et al.
Phosphoinositide 3-kinase p110β activity: key role in metabolism and mammary gland cancer but not development. _Sci. Signal_ 1, ra3 (2008). A STUDY ON THE ROLE OF P110Β IN METABOLISM AND
HER2-INDUCED BREAST CANCER USING A KNOCK-IN MOUSE MODEL. PubMed PubMed Central Google Scholar * Guillermet-Guibert, J. et al. The p110β isoform of phosphoinositide 3-kinase signals
downstream of G protein-coupled receptors and is functionally redundant with p110γ. _Proc. Natl Acad. Sci. USA_ 105, 8292–8297 (2008). CAS PubMed PubMed Central Google Scholar *
Graupera, M. et al. Angiogenesis selectively requires the p110α isoform of PI3K to control endothelial cell migration. _Nature_ 453, 662–666 (2008). THIS WORK DEMONSTRATED A SPECIFIC ROLE OF
P110Α IN ENDOTHELIAL CELL MIGRATION. CAS PubMed Google Scholar * Foukas, L. C. et al. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation.
_Nature_ 441, 366–370 (2006). THIS STUDY SHOWED THE ROLE OF P110Α IN INSULIN SIGNALING AND METABOLISM USING AN INACTIVE-KINASE KNOCK-IN MOUSE MODEL. CAS PubMed Google Scholar * Utermark,
T., Schaffhausen, B. S., Roberts, T. M. & Zhao, J. J. The p110α isoform of phosphatidylinositol 3-kinase is essential for polyomavirus middle T antigen-mediated transformation. _J.
Virol._ 81, 7069–7076 (2007). CAS PubMed PubMed Central Google Scholar * Jackson, S. P. et al. PI3-kinase p110β: a new target for antithrombotic therapy. _Nature Med._ 11, 507–514
(2005). CAS PubMed Google Scholar * Yap, T. A. et al. Targeting the PI3K–AKT–mTOR pathway: progress, pitfalls, and promises. _Curr. Opin. Pharmacol._ 8, 393–412 (2008). CAS PubMed
Google Scholar * Torbett, N. E. et al. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition.
_Biochem. J._ 415, 97–110 (2008). CAS PubMed Google Scholar * Wee, S. et al. PTEN-deficient cancers depend on PIK3CB. _Proc. Natl Acad. Sci. USA_ 105, 13057–13062 (2008). CAS PubMed
PubMed Central Google Scholar * Oda, K. et al. PIK3CA cooperates with other phosphatidylinositol 3-kinase pathway mutations to effect oncogenic transformation. _Cancer Res._ 68, 8127–8136
(2008). CAS PubMed Google Scholar * Aguirre, V., Uchida, T., Yenush, L., Davis, R. & White, M. F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with
insulin receptor substrate-1 and phosphorylation of Ser307. _J. Biol. Chem._ 275, 9047–9054 (2000). CAS PubMed Google Scholar * Harrington, L. S., Findlay, G. M. & Lamb, R. F.
Restraining PI3K: mTOR signalling goes back to the membrane. _Trends Biochem. Sci._ 30, 35–42 (2005). CAS PubMed Google Scholar * Lee, Y. H., Giraud, J., Davis, R. J. & White, M. F.
c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. _J. Biol. Chem._ 278, 2896–2902 (2003). CAS PubMed Google Scholar * Hirosumi, J. et al. A
central role for JNK in obesity and insulin resistance. _Nature_ 420, 333–336 (2002). CAS PubMed Google Scholar * Um, S. H. et al. Absence of S6K1 protects against age- and diet-induced
obesity while enhancing insulin sensitivity. _Nature_ 431, 200–205 (2004). CAS PubMed Google Scholar * OReilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase
signaling and activates Akt. _Cancer Res._ 66, 1500–1508 (2006). CAS Google Scholar * Jun, T., Gjoerup, O. & Roberts, T. M. Tangled webs: evidence of cross-talk between c-Raf-1 and
Akt. _Sci. STKE_ 1999, PE1 (1999). CAS PubMed Google Scholar * Carracedo, A. et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human
cancer. _J. Clin. Invest._ 118, 3065–3074 (2008). CAS PubMed PubMed Central Google Scholar * Williams, R. et al. The skin and hair as surrogate tissues for measuring the target effect of
inhibitors of phosphoinositide-3-kinase signaling. _Cancer Chemother. Pharmacol._ 58, 444–450 (2006). CAS PubMed PubMed Central Google Scholar * Cho, H. et al. Insulin resistance and a
diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). _Science_ 292, 1728–1731 (2001). CAS PubMed Google Scholar * Brachmann, S. M., Ueki, K., Engelman, J. A.,
Kahn, R. C. & Cantley, L. C. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. _Mol. Cell Biol._
25, 1596–1607 (2005). CAS PubMed PubMed Central Google Scholar * Schnell, C. R. et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor
NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. _Cancer Res._ 68, 6598–6607 (2008). CAS PubMed Google Scholar * Bomanji, J. B., Costa, D. C. & Ell, P. J.
Clinical role of positron emission tomography in oncology. _Lancet Oncol._ 2, 157–164 (2001). CAS PubMed Google Scholar * Eichhorn, P. J. et al. Phosphatidylinositol 3-kinase
hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. _Cancer Res._ 68, 9221–9230 (2008). CAS PubMed PubMed
Central Google Scholar * Berns, K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. _Cancer Cell_ 12,
395–402 (2007). CAS PubMed Google Scholar * Zhang, J., Yang, P. L. & Gray, N. S. Targeting cancer with small molecule kinase inhibitors. _Nature Rev. Cancer_ 9, 28–39 (2009). Google
Scholar * Zunder, E. R., Knight, Z. A., Houseman, B. T., Apsel, B. & Shokat, K. M. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110α.
_Cancer Cell_ 14, 180–192 (2008). CAS PubMed PubMed Central Google Scholar * Engelman, J. A. et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small
cell lung cancer cell lines. _Proc. Natl Acad. Sci. USA_ 102, 3788–3793 (2005). CAS PubMed PubMed Central Google Scholar * Engelman, J. A. et al. MET amplification leads to gefitinib
resistance in lung cancer by activating ERBB3 signaling. _Science_ 316, 1039–1043 (2007). CAS PubMed Google Scholar * Sergina, N. V. et al. Escape from HER-family tyrosine kinase
inhibitor therapy by the kinase-inactive HER3. _Nature_ 445, 437–441 (2007). CAS PubMed PubMed Central Google Scholar * Apsel, B. et al. Targeted polypharmacology: discovery of dual
inhibitors of tyrosine and phosphoinositide kinases. _Nature Chem. Biol._ 4, 691–699 (2008). CAS Google Scholar * Gupta, S. et al. Binding of ras to phosphoinositide 3-kinase p110a is
required for ras-driven tumorigenesis in mice. _Cell_ 129, 957–968 (2007). CAS PubMed Google Scholar * Engelman, J. A. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras
G12D and PIK3CA H1047R murine lung cancers. _Nature Med._ 14, 1351–1356 (2008). THIS STUDY SHOWNED THAT A COMBINED TREATMENT WITH PI3K AND MEK INHIBITORS IS NECESSARY TO BLOCK ONCOGENIC
_KRAS_ -INDUCED LUNG CANCER IN A MURINE MODEL. CAS PubMed Google Scholar * Yu, K., Toral-Barza, L., Shi, C., Zhang, W. G. & Zask, A. Response and determinants of cancer cell
susceptibility to PI3K inhibitors: combined targeting of PI3K and Mek1 as an effective anticancer strategy. _Cancer Biol. Ther._ 7, 307–315 (2008). PubMed Google Scholar * Yuan, T. L. et
al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. _Proc. Natl Acad. Sci. USA_ 105, 9739–9744 (2008). CAS PubMed PubMed Central Google Scholar * Guba, M.
et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. _Nature Med._ 8, 128–135 (2002). CAS PubMed Google
Scholar * Stallone, G. et al. Sirolimus for Kaposis sarcoma in renal-transplant recipients. _N. Engl. J. Med._ 352, 1317–1323 (2005). CAS PubMed Google Scholar * Thomas, G. V. et al.
Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. _Nature Med._ 12, 122–127 (2006). CAS PubMed Google Scholar * Hudson, C. C. et al. Regulation of
hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. _Mol. Cell Biol._ 22, 7004–7014 (2002). CAS PubMed PubMed Central Google Scholar * Bernardi, R.
et al. PML inhibits HIF-1α translation and neoangiogenesis through repression of mTOR. _Nature_ 442, 779–785 (2006). CAS PubMed Google Scholar * Phung, T. L. et al. Pathological
angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. _Cancer Cell_ 10, 159–170 (2006). CAS PubMed PubMed Central Google Scholar * Hamada, K. et al. The
PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. _Genes Dev._ 19, 2054–2065 (2005). CAS PubMed PubMed Central Google Scholar * Bayascas, J. R., Leslie, N.
R., Parsons, R., Fleming, S. & Alessi, D. R. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN+/− mice. _Curr. Biol._ 15, 1839–1846 (2005). CAS PubMed Google Scholar *
Chen, M. L. et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. _Genes Dev._ 20, 1569–1574 (2006). CAS PubMed PubMed Central Google Scholar *
Massion, P. P. et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway.
_Cancer Res._ 62, 3636–3640 (2002). CAS PubMed Google Scholar * Massion, P. P. et al. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. _Am.
J. Respir. Crit. Care Med._ 170, 1088–1094 (2004). PubMed Google Scholar * Okudela, K. et al. PIK3CA mutation and amplification in human lung cancer. _Pathol. Int._ 57, 664–671 (2007). CAS
PubMed Google Scholar * Kawano, O. et al. PIK3CA gene amplification in Japanese non-small cell lung cancer. _Lung Cancer_ 58, 159–160 (2007). PubMed Google Scholar * Ma, Y. Y. et al.
PIK3CA as an oncogene in cervical cancer. _Oncogene_ 19, 2739–2744 (2000). CAS PubMed Google Scholar * Wu, G. et al. Somatic mutation and gain of copy number of PIK3CA in human breast
cancer. _Breast Cancer Res._ 7, R609–R616 (2005). CAS PubMed PubMed Central Google Scholar * Woenckhaus, J. et al. Genomic gain of PIK3CA and increased expression of p110α are associated
with progression of dysplasia into invasive squamous cell carcinoma. _J. Pathol._ 198, 335–342 (2002). CAS PubMed Google Scholar * Pedrero, J. M. et al. Frequent genetic and biochemical
alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. _Int. J. Cancer_ 114, 242–248 (2005). CAS PubMed Google Scholar * Fenic, I., Steger, K., Gruber, C.,
Arens, C. & Woenckhaus, J. Analysis of PIK3CA and Akt/protein kinase B in head and neck squamous cell carcinoma. _Oncol. Rep._ 18, 253–259 (2007). CAS PubMed Google Scholar * Byun, D.
S. et al. Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIK3CA amplification in gastric carcinoma. _Int. J. Cancer_ 104, 318–327 (2003). CAS PubMed Google
Scholar * Wu, G. et al. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. _J. Clin. Endocrinol. Metab._ 90, 4688–4693 (2005). CAS PubMed Google Scholar
* Miller, C. T. et al. Gene amplification in esophageal adenocarcinomas and Barretts with high-grade dysplasia. _Clin. Cancer Res._ 9, 4819–4825 (2003). CAS PubMed Google Scholar *
Miyake, T. et al. PIK3CA gene mutations and amplifications in uterine cancers, identified by methods that avoid confounding by PIK3CA pseudogene sequences. _Cancer Lett._ 261, 120–126
(2008). CAS PubMed Google Scholar * Nakayama, K. et al. Amplicon profiles in ovarian serous carcinomas. _Int. J. Cancer_ 120, 2613–2617 (2007). CAS PubMed Google Scholar * Nakayama, K.
et al. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. _Cancer Biol. Ther._ 5, 779–785 (2006). CAS PubMed Google Scholar * Kita, D.,
Yonekawa, Y., Weller, M. & Ohgaki, H. PIK3CA alterations in primary (_de novo_) and secondary glioblastomas. _Acta Neuropathol._ 113, 295–302 (2007). CAS PubMed Google Scholar *
Bleeker, F. E. et al. AKT1E17K in human solid tumours. _Oncogene_ 27, 5648–5650 (2008). CAS PubMed Google Scholar * Kim, M. S., Jeong, E. G., Yoo, N. J. & Lee, S. H. Mutational
analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. _Br. J. Cancer_ 98, 1533–1535 (2008). CAS PubMed PubMed Central Google Scholar * Malanga, D. et al.
Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. _Cell Cycle_ 7, 665–669 (2008). CAS PubMed Google Scholar *
Staal, S. P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. _Proc. Natl Acad. Sci. USA_ 84,
5034–5037 (1987). CAS PubMed PubMed Central Google Scholar * Bellacosa, A. et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. _Int. J. Cancer_ 64,
280–285 (1995). CAS PubMed Google Scholar * Cheng, J. Q. et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human
ovarian carcinomas. _Proc. Natl Acad. Sci. USA_ 89, 9267–9271 (1992). CAS PubMed PubMed Central Google Scholar * Ruggeri, B. A., Huang, L., Wood, M., Cheng, J. Q. & Testa, J. R.
Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. _Mol. Carcinog._ 21, 81–86 (1998). CAS PubMed Google Scholar * Oki, E. et al.
Impact of loss of heterozygosity of encoding phosphate and tensin homolog on the prognosis of gastric cancer. _J. Gastroenterol. Hepatol._ 21, 814–818 (2006). CAS PubMed Google Scholar *
Li, Y. L., Tian, Z., Wu, D. Y., Fu, B. Y. & Xin, Y. Loss of heterozygosity on 10q23.3 and mutation of tumor suppressor gene PTEN in gastric cancer and precancerous lesions. _World J.
Gastroenterol._ 11, 285–288 (2005). CAS PubMed PubMed Central Google Scholar * Feilotter, H. E. et al. Analysis of the 10q23 chromosomal region and the PTEN gene in human sporadic breast
carcinoma. _Br. J. Cancer_ 79, 718–723 (1999). CAS PubMed PubMed Central Google Scholar * Freihoff, D. et al. Exclusion of a major role for the PTEN tumour-suppressor gene in breast
carcinomas. _Br. J. Cancer_ 79, 754–758 (1999). CAS PubMed PubMed Central Google Scholar * Garcia, J. M. et al. Allelic loss of the PTEN region (10q23) in breast carcinomas of poor
pathophenotype. _Breast Cancer Res. Treat._ 57, 237–243 (1999). CAS PubMed Google Scholar * Tokunaga, E. et al. Coexistence of the loss of heterozygosity at the PTEN locus and HER2
overexpression enhances the Akt activity thus leading to a negative progesterone receptor expression in breast carcinoma. _Breast Cancer Res. Treat._ 101, 249–257 (2007). CAS PubMed Google
Scholar * Pollock, P. M. et al. PTEN inactivation is rare in melanoma tumours but occurs frequently in melanoma cell lines. _Melanoma Res._ 12, 565–575 (2002). CAS PubMed Google Scholar
* Celebi, J. T., Shendrik, I., Silvers, D. N. & Peacocke, M. Identification of PTEN mutations in metastatic melanoma specimens. _J. Med. Genet._ 37, 653–657 (2000). CAS PubMed PubMed
Central Google Scholar * Birck, A., Ahrenkiel, V., Zeuthen, J., Hou-Jensen, K. & Guldberg, P. Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma
biopsies. _J. Invest. Dermatol._ 114, 277–280 (2000). CAS PubMed Google Scholar * Reifenberger, J. et al. Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour
suppressor gene in primary and metastatic malignant melanomas. _Virchows Arch._ 436, 487–493 (2000). CAS PubMed Google Scholar * Cairns, P. et al. Frequent inactivation of PTEN/MMAC1 in
primary prostate cancer. _Cancer Res._ 57, 4997–5000 (1997). CAS PubMed Google Scholar * Feilotter, H. E., Nagai, M. A., Boag, A. H., Eng, C. & Mulligan, L. M. Analysis of PTEN and
the 10q23 region in primary prostate carcinomas. _Oncogene_ 16, 1743–1748 (1998). CAS PubMed Google Scholar * Pesche, S. et al. PTEN/MMAC1/TEP1 involvement in primary prostate cancers.
_Oncogene_ 16, 2879–2883 (1998). CAS PubMed Google Scholar * Gray, I. C. et al. Mutation and expression analysis of the putative prostate tumour-suppressor gene PTEN. _Br. J. Cancer_ 78,
1296–1300 (1998). CAS PubMed PubMed Central Google Scholar * Wang, S. I., Parsons, R. & Ittmann, M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate
adenocarcinomas. _Clin. Cancer Res._ 4, 811–815 (1998). CAS PubMed Google Scholar * Bostrom, J. et al. Mutation of the PTEN (MMAC1) tumor suppressor gene in a subset of glioblastomas but
not in meningiomas with loss of chromosome arm 10q. _Cancer Res._ 58, 29–33 (1998). CAS PubMed Google Scholar * Wang, S. I. et al. Somatic mutations of PTEN in glioblastoma multiforme.
_Cancer Res._ 57, 4183–4186 (1997). CAS PubMed Google Scholar * Smith, J. S. et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma
multiforme. _J. Natl Cancer Inst._ 93, 1246–1256 (2001). CAS PubMed Google Scholar * Kim, N. et al. The p110δ catalytic isoform of PI3K is a key player in NK-cell development and
cytokine secretion. _Blood_ 110, 3202–3208 (2007). CAS PubMed Google Scholar * Clayton, E. et al. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell
development and activation. _J. Exp. Med._ 196, 753–763 (2002). CAS PubMed PubMed Central Google Scholar * Jou, S. T. et al. Essential, nonredundant role for the phosphoinositide
3-kinase p110δ in signaling by the B-cell receptor complex. _Mol. Cell Biol._ 22, 8580–8591 (2002). CAS PubMed PubMed Central Google Scholar * Puri, K. D. et al. Mechanisms and
implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. _Blood_ 103, 3448–3456 (2004). CAS PubMed Google Scholar * Okkenhaug, K. et al.
Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. _Science_ 297, 1031–1034 (2002). CAS PubMed Google Scholar * Ali, K. et al. Essential role for the p110δ
phosphoinositide 3-kinase in the allergic response. _Nature_ 431, 1007–1011 (2004). CAS PubMed Google Scholar * Guo, H., Samarakoon, A., Vanhaesebroeck, B. & Malarkannan, S. The
p110δ of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. _J. Exp. Med._ 205, 2419–2435 (2008). CAS PubMed PubMed Central Google Scholar *
Hirsch, E. et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. _Science_ 287, 1049–1053 (2000). CAS PubMed Google Scholar * MacDonald, P. E. et al.
Impaired glucose-stimulated insulin secretion, enhanced intraperitoneal insulin tolerance, and increased β-cell mass in mice lacking the p110γ isoform of phosphoinositide 3-kinase.
_Endocrinology_ 145, 4078–4083 (2004). CAS PubMed Google Scholar * Crackower, M. A. et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways.
_Cell_ 110, 737–749 (2002). CAS PubMed Google Scholar * Li, Z. et al. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. _Science_ 287, 1046–1049 (2000).
CAS PubMed Google Scholar * Laffargue, M. et al. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. _Immunity_ 16, 441–451 (2002). CAS PubMed Google Scholar *
Rodriguez-Borlado, L. et al. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. _J. Immunol._ 170, 4475–4482 (2003). CAS PubMed Google Scholar * Webb, L.
M., Vigorito, E., Wymann, M. P., Hirsch, E. & Turner, M. Cutting edge: T cell development requires the combined activities of the p110γ and p110δ catalytic isoforms of
phosphatidylinositol 3-kinase. _J. Immunol._ 175, 2783–2787 (2005). CAS PubMed Google Scholar * Swat, W. et al. Essential role of PI3Kδ and PI3Kγ in thymocyte survival. _Blood_ 107,
2415–2422 (2006). CAS PubMed PubMed Central Google Scholar * Tassi, I. et al. p110γ and p110δ phosphoinositide 3-kinase signaling pathways synergize to control development and functions
of murine NK cells. _Immunity_ 27, 214–227 (2007). CAS PubMed Google Scholar * Terauchi, Y. et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of
phosphoinositide 3-kinase. _Nature Genet._ 21, 230–235 (1999). CAS PubMed Google Scholar * Suzuki, H. et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of
phosphoinositide 3-kinase. _Science_ 283, 390–392 (1999). CAS PubMed Google Scholar * Chen, D. et al. p50α/p55α phosphoinositide 3-kinase knockout mice exhibit enhanced insulin
sensitivity. _Mol. Cell Biol._ 24, 320–329 (2004). CAS PubMed PubMed Central Google Scholar * Fruman, D. A. et al. Impaired B cell development and proliferation in absence of
phosphoinositide 3-kinase p85α. _Science_ 283, 393–397 (1999). CAS PubMed Google Scholar * Fruman, D. A. et al. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all
isoforms of phosphoinositide 3-kinase p85α. _Nature Genet._ 26, 379–382 (2000). CAS PubMed Google Scholar * Ueki, K. et al. Increased insulin sensitivity in mice lacking p85β subunit of
phosphoinositide 3-kinase. _Proc. Natl Acad. Sci. USA_ 99, 419–424 (2002). CAS PubMed Google Scholar * Taniguchi, C. M. et al. Divergent regulation of hepatic glucose and lipid metabolism
by phosphoinositide 3-kinase via Akt and PKCλ/ζ. _Cell. Metab._ 3, 343–353 (2006). CAS PubMed Google Scholar * Luo, J. et al. Loss of class IA PI3K signaling in muscle leads to impaired
muscle growth, insulin response, and hyperlipidemia. _Cell Metab._ 3, 355–366 (2006). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank N. Gray and Q. Liu for
providing compound structures and helpful discussions. We thank the reviewers for their helpful suggestions. We apologize to colleagues whose primary papers were not cited owing to space
constraints. This work was supported in part by the National Institutes of Health (CA030002, CA089021 and CA050661 to T.M.R. and CA134502-01 to J.J.Z.), the Department of Defense for Cancer
Research (BC051565 to J.J.Z.), the V Foundation (J.J.Z.) and the Claudia Barr Program (J.J.Z.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Cancer Biology, Dana–Farber
Cancer Institute, Pathology, Harvard Medical School, Boston, 02115, Massachusetts, USA Pixu Liu, Hailing Cheng, Thomas M. Roberts & Jean J. Zhao Authors * Pixu Liu View author
publications You can also search for this author inPubMed Google Scholar * Hailing Cheng View author publications You can also search for this author inPubMed Google Scholar * Thomas M.
Roberts View author publications You can also search for this author inPubMed Google Scholar * Jean J. Zhao View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Jean J. Zhao. ETHICS DECLARATIONS COMPETING INTERESTS T.M.R. and J.J.Z. hold consulting positions at Novartis. RELATED LINKS RELATED LINKS
DATABASES OMIM Bannayan–Riley–Ruvalcabas syndrome Cowden's syndrome FURTHER INFORMATION Jean J. Zhao's homepage GLOSSARY * Germline mutation A heritable change in the DNA that
occurred in a germ cell or the zygote at the single-cell stage. When transmitted to the next generation, a germline mutation is incorporated in every cell of the body. * Somatic mutation
Also referred to as an 'acquired mutation', this is an alteration in DNA that occurs in a somatic cell, in contrast to a mutation in a germ cell. * Allosteric inhibitor A molecule
that inhibits an enzyme by binding to a site other than the active site, causing a conformational change in the active site of the enzyme and thereby inhibiting its catalytic function. *
Thrombosis The formation or presence of a blood clot in a blood vessel. * Biomarker A characteristic that can be objectively measured and evaluated as an indicator of normal biological
processes, pathogenic processes, or pharmacological responses to a therapeutic intervention. * Tumour angiogenesis The formation of new blood vessels that grow into the tumour, supplying
nutrients and oxygen to assist tumour growth. * Cancer chemoprevention The use of chemical compounds to intervene in the early precancerous stages of carcinogenesis, thereby preventing
tumour formation. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, P., Cheng, H., Roberts, T. _et al._ Targeting the phosphoinositide 3-kinase
pathway in cancer. _Nat Rev Drug Discov_ 8, 627–644 (2009). https://doi.org/10.1038/nrd2926 Download citation * Issue Date: August 2009 * DOI: https://doi.org/10.1038/nrd2926 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative