- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Mitochondrial DNA (mtDNA) is normally present at thousands of copies per cell and is packaged into several hundred higher-order structures termed nucleoids1. The abundant
mtDNA-binding protein TFAM (transcription factor A, mitochondrial) regulates nucleoid architecture, abundance and segregation2. Complete mtDNA depletion profoundly impairs oxidative
phosphorylation, triggering calcium-dependent stress signalling and adaptive metabolic responses3. However, the cellular responses to mtDNA instability, a physiologically relevant stress
observed in many human diseases and ageing, remain poorly defined4. Here we show that moderate mtDNA stress elicited by TFAM deficiency engages cytosolic antiviral signalling to enhance the
expression of a subset of interferon-stimulated genes. Mechanistically, we find that aberrant mtDNA packaging promotes escape of mtDNA into the cytosol, where it engages the DNA sensor cGAS
(also known as MB21D1) and promotes STING (also known as TMEM173)–IRF3-dependent signalling to elevate interferon-stimulated gene expression, potentiate type I interferon responses and
confer broad viral resistance. Furthermore, we demonstrate that herpesviruses induce mtDNA stress, which enhances antiviral signalling and type I interferon responses during infection. Our
results further demonstrate that mitochondria are central participants in innate immunity, identify mtDNA stress as a cell-intrinsic trigger of antiviral signalling and suggest that cellular
monitoring of mtDNA homeostasis cooperates with canonical virus sensing mechanisms to fully engage antiviral innate immunity. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per
year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MITOCHONDRIAL DNA
REPLICATION STRESS TRIGGERS A PRO-INFLAMMATORY ENDOSOMAL PATHWAY OF NUCLEOID DISPOSAL Article 08 February 2024 VRK2 IS INVOLVED IN THE INNATE ANTIVIRAL RESPONSE BY PROMOTING
MITOSTRESS-INDUCED MTDNA RELEASE Article 30 March 2021 THE POXVIRUS F17 PROTEIN COUNTERACTS MITOCHONDRIALLY ORCHESTRATED ANTIVIRAL RESPONSES Article Open access 30 November 2023 ACCESSION
CODES PRIMARY ACCESSIONS GENE EXPRESSION OMNIBUS * GSE63767 DATA DEPOSITS Microarray data have been submitted to the NCBI Gene Expression Omnibus under accession number GSE63767. CHANGE
HISTORY * _ 22 APRIL 2015 The bar graph in Fig. 3d was corrected. _ REFERENCES * Spelbrink, J. N. Functional organization of mammalian mitochondrial DNA in nucleoids: history, recent
developments, and future challenges. _IUBMB Life_ 62, 19–32 (2010) CAS PubMed Google Scholar * Kasashima, K., Sumitani, M. & Endo, H. Human mitochondrial transcription factor A is
required for the segregation of mitochondrial DNA in cultured cells. _Exp. Cell Res._ 317, 210–220 (2011) CAS PubMed Google Scholar * Ryan, M. T. & Hoogenraad, N. J.
Mitochondrial–nuclear communications. _Annu. Rev. Biochem._ 76, 701–722 (2007) CAS PubMed Google Scholar * Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases,
aging, and cancer: a dawn for evolutionary medicine. _Annu. Rev. Genet._ 39, 359–407 (2005) CAS PubMed PubMed Central Google Scholar * Larsson, N. G. et al. Mitochondrial transcription
factor A is necessary for mtDNA maintenance and embryogenesis in mice. _Nature Genet._ 18, 231–236 (1998) CAS PubMed Google Scholar * Woo, D. K. et al. Mitochondrial genome instability
and ROS enhance intestinal tumorigenesis in _APC_Min/+ mice. _Am. J. Pathol._ 180, 24–31 (2012) CAS PubMed PubMed Central Google Scholar * Rusinova, I. et al. Interferome v2.0: an
updated database of annotated interferon-regulated genes. _Nucleic Acids Res._ 41, D1040–D1046 (2013) CAS PubMed Google Scholar * Schoggins, J. W. & Rice, C. M. Interferon-stimulated
genes and their antiviral effector functions. _Curr. Opin. Virol._ 1, 519–525 (2011) CAS PubMed PubMed Central Google Scholar * West, A. P., Shadel, G. S. & Ghosh, S. Mitochondria in
innate immune responses. _Nature Rev. Immunol._ 11, 389–402 (2011) CAS Google Scholar * Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis.
_Immunity_ 36, 401–414 (2012) CAS PubMed PubMed Central Google Scholar * Nicholls, T. J. & Minczuk, M. In D-loop: 40 years of mitochondrial 7S DNA. _Exp. Gerontol._ 56, 175–181
(2014) CAS PubMed Google Scholar * Ban-Ishihara, R., Ishihara, T., Sasaki, N., Mihara, K. & Ishihara, N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes
to cristae reformation and release of cytochrome c. _Proc. Natl Acad. Sci. USA_ 110, 11863–11868 (2013) ADS CAS PubMed PubMed Central Google Scholar * Malena, A., Loro, E., Di Re, M.,
Holt, I. J. & Vergani, L. Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. _Hum. Mol. Genet._ 18, 3407–3416 (2009) CAS PubMed Google Scholar *
Cymerman, I. A., Chung, I., Beckmann, B. M., Bujnicki, J. M. & Meiss, G. EXOG, a novel paralog of Endonuclease G in higher eukaryotes. _Nucleic Acids Res._ 36, 1369–1379 (2008) CAS
PubMed PubMed Central Google Scholar * Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway.
_Science_ 339, 786–791 (2013) ADS CAS PubMed Google Scholar * Ablasser, A. et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. _J. Immunol._ 192,
5993–5997 (2014) CAS PubMed Google Scholar * Cai, X., Chiu, Y.-H. & Chen, Z. J. The cGAS–cGAMP–STING pathway of cytosolic DNA sensing and signaling. _Mol. Cell_ 54, 289–296 (2014) CAS
PubMed Google Scholar * Atianand, M. K. & Fitzgerald, K. A. Molecular basis of DNA recognition in the immune system. _J. Immunol._ 190, 1911–1918 (2013) CAS PubMed Google Scholar
* Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. _Immunity_ 38, 855–869 (2013) CAS PubMed PubMed Central Google Scholar * Pohjoismäki, J. L. O. et al.
Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. _Nucleic Acids Res._ 34,
5815–5828 (2006) PubMed PubMed Central Google Scholar * Wiedmer, A. et al. Epstein–Barr virus immediate-early protein Zta co-opts mitochondrial single-stranded DNA binding protein to
promote viral and inhibit mitochondrial DNA replication. _J. Virol._ 82, 4647–4655 (2008) CAS PubMed PubMed Central Google Scholar * Saffran, H. A., Pare, J. M., Corcoran, J. A., Weller,
S. K. & Smiley, J. R. Herpes simplex virus eliminates host mitochondrial DNA. _EMBO Rep._ 8, 188–193 (2007) CAS PubMed Google Scholar * Corcoran, J. A., Saffran, H. A., Duguay, B. A.
& Smiley, J. R. Herpes simplex virus UL12.5 targets mitochondria through a mitochondrial localization sequence proximal to the N terminus. _J. Virol._ 83, 2601–2610 (2009) CAS PubMed
PubMed Central Google Scholar * Duguay, B. A. & Smiley, J. R. Mitochondrial nucleases ENDOG and EXOG participate in mitochondrial DNA depletion initiated by herpes simplex virus 1
UL12.5. _J. Virol._ 87, 11787–11797 (2013) CAS PubMed PubMed Central Google Scholar * Duguay, B. A. et al. Elimination of mitochondrial DNA is not required for herpes simplex virus 1
replication. _J. Virol._ 88, 2967–2976 (2014) PubMed PubMed Central Google Scholar * Crow, M. K. & Kirou, K. A. Interferon-α in systemic lupus erythematosus. _Curr. Opin. Rheumatol._
16, 541–547 (2004) CAS PubMed Google Scholar * Khodarev, N. N. et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells.
_Cancer Res._ 67, 9214–9220 (2007) CAS PubMed Google Scholar * Lee, H.-T. et al. Leukocyte mitochondrial DNA alteration in systemic lupus erythematosus and its relevance to the
susceptibility to lupus nephritis. _Int. J. Mol. Sci._ 13, 8853–8868 (2012) CAS PubMed PubMed Central Google Scholar * Lee, H.-M., Sugino, H., Aoki, C. & Nishimoto, N.
Underexpression of mitochondrial-DNA encoded ATP synthesis-related genes and DNA repair genes in systemic lupus erythematosus. _Arthritis Res. Ther._ 13, R63 (2011) CAS PubMed PubMed
Central Google Scholar * Wallace, D. C. Mitochondria and cancer. _Nature Rev. Cancer_ 12, 685–698 (2012) CAS Google Scholar * Weinberg, F. et al. Mitochondrial metabolism and ROS
generation are essential for Kras-mediated tumorigenicity. _Proc. Natl Acad. Sci. USA_ 107, 8788–8793 (2010) ADS CAS PubMed PubMed Central Google Scholar * Ishikawa, H. & Barber, G.
N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. _Nature_ 455, 674–678 (2008) ADS CAS PubMed PubMed Central Google Scholar * Stetson, D. B. &
Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. _Immunity_ 24, 93–103 (2006) CAS PubMed Google Scholar * Tal, M. C. et al. Absence of
autophagy results in reactive oxygen species-dependent amplification of RLR signaling. _Proc. Natl Acad. Sci. USA_ 106, 2770–2775 (2009) ADS CAS PubMed PubMed Central Google Scholar *
Dalton, K. P. & Rose, J. K. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus
particles. _Virology_ 279, 414–421 (2001) CAS PubMed Google Scholar * Desai, P. & Person, S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1
capsid. _J. Virol._ 72, 7563–7568 (1998) CAS PubMed PubMed Central Google Scholar * Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing
local memory T cells. _Nature_ 491, 463–467 (2012) ADS CAS PubMed PubMed Central Google Scholar * Fuerst, T. R., Niles, E. G., Studier, F. W. & Moss, B. Eukaryotic
transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. _Proc. Natl Acad. Sci. USA_ 83, 8122–8126 (1986) ADS CAS PubMed PubMed
Central Google Scholar * Pang, I. K., Pillai, P. S. & Iwasaki, A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. _Proc. Natl
Acad. Sci. USA_ 110, 13910–13915 (2013) ADS CAS PubMed PubMed Central Google Scholar * Yordy, B., Iijima, N., Huttner, A., Leib, D. & Iwasaki, A. A neuron-specific role for
autophagy in antiviral defense against herpes simplex virus. _Cell Host Microbe_ 12, 334–345 (2012) CAS PubMed PubMed Central Google Scholar * Cardenas, I. et al. Placental viral
infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. _Am. J. Reprod. Immunol._ 65, 110–117 (2011) CAS PubMed Google Scholar * Marshall, H. D. et al.
Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4+ cell properties during viral infection. _Immunity_ 35, 633–646 (2011) CAS PubMed PubMed Central Google
Scholar * Welsh, R. M. & Seedhom, M. O. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. _Curr. Protoc. Microbiol._ UNIT 15A. 1,
http://dx.doi.org/10.1002/9780471729259.mc15a01s8 (2008) * McCausland, M. M. & Crotty, S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. _J. Virol.
Methods_ 147, 167–176 (2008) CAS PubMed Google Scholar * Parr, M. B. et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. _Lab.
Invest._ 70, 369–380 (1994) CAS PubMed Google Scholar * Malin, S. A., Davis, B. M. & Molliver, D. C. Production of dissociated sensory neuron cultures and considerations for their use
in studying neuronal function and plasticity. _Nature Protocols_ 2, 152–160 (2007) CAS PubMed Google Scholar * Holden, P. & Horton, W. A. Crude subcellular fractionation of cultured
mammalian cell lines. _BMC Res. Notes_ 2, 243 (2009) PubMed PubMed Central Google Scholar * Raimundo, N. et al. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness.
_Cell_ 148, 716–726 (2012) CAS PubMed PubMed Central Google Scholar * Saeed, A. I. et al. TM4: a free, open-source system for microarray data management and analysis. _Biotechniques_ 34,
374–378 (2003) CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank N. Chandel for _Tfam__fl/fl_ mice, D. Martin for _Tfam__+/−_ MEFs, J. Schoggins and S. Virgin for
_cGas__−/−_ MEFs, G. Barber for _Sting__−/−_ MEFs, G. Sen for IFIT3 antibodies, J. Rose for VSV antibodies and recombinant vaccinia virus, K. Bahl and J. Schell for advice with VSV
infections, and S. Ding for advice with HSV-1 gene expression analysis. This work was supported by a joint grant from the United Mitochondrial Disease Foundation and Mitocon, NIH R01
AG047632 and P01 ES011163 (G.S.S.), NIH R01 AI054359 and R01 AI081884 (A.I.), Canadian Institutes for Health Research grant MOP37995 and a Canada Research Chair in Molecular Virology
(J.R.S.), American Cancer Society Postdoctoral Fellowship PF-13-035-01-DMC (A.P.W.), NIH T32 AI055403 (W.K.-H.), NIH F31 AG039163 (M.C.T.), NIH NRSA F32 DK091042 (M.B.), Alberta
Innovates-Health Solutions and a Queen Elizabeth II Graduate Scholarship (B.A.D.), and a United Mitochondrial Disease Foundation Postdoctoral Fellowship (N.R.). AUTHOR INFORMATION Author
notes * Michal C. Tal, Megan Bestwick & Nuno Raimundo Present address: Present addresses: Institute for Stem Cell Biology and Regenerative Medicine, Stanford University School of
Medicine, Stanford, California 94305, USA (M.C.T.); Department of Chemistry, Linfield College, McMinnville, Oregon 97128, USA (M.B.); Institute for Cellular Biochemistry, Universitätsmedizin
Göttingen, 37073 Göttingen, Germany (N.R.)., AUTHORS AND AFFILIATIONS * Department of Pathology, Yale School of Medicine, New Haven, 06520, Connecticut, USA A. Phillip West, Cristiana M.
Pineda, Sabine M. Lang, Megan Bestwick, Nuno Raimundo, Robert E. Means & Gerald S. Shadel * Department of Immunobiology, Yale School of Medicine, New Haven, 06520, Connecticut, USA
William Khoury-Hanold, Matthew Staron, Michal C. Tal, Susan M. Kaech & Akiko Iwasaki * Department of Medical Microbiology and Immunology, Li Ka Shing Institute of Virology, University of
Alberta, Edmonton, Alberta T6G 2S2, Canada, Brett A. Duguay & James R. Smiley * Department of Pathology and Immunology, Washington University School of Medicine, St Louis, 63110,
Missouri, USA Donna A. MacDuff * Howard Hughes Medical Institute, Chevy Chase, 20815-6789, Maryland, USA Susan M. Kaech & Akiko Iwasaki * Department of Genetics, Yale School of Medicine,
New Haven, 06520, Connecticut, USA Gerald S. Shadel Authors * A. Phillip West View author publications You can also search for this author inPubMed Google Scholar * William Khoury-Hanold
View author publications You can also search for this author inPubMed Google Scholar * Matthew Staron View author publications You can also search for this author inPubMed Google Scholar *
Michal C. Tal View author publications You can also search for this author inPubMed Google Scholar * Cristiana M. Pineda View author publications You can also search for this author inPubMed
Google Scholar * Sabine M. Lang View author publications You can also search for this author inPubMed Google Scholar * Megan Bestwick View author publications You can also search for this
author inPubMed Google Scholar * Brett A. Duguay View author publications You can also search for this author inPubMed Google Scholar * Nuno Raimundo View author publications You can also
search for this author inPubMed Google Scholar * Donna A. MacDuff View author publications You can also search for this author inPubMed Google Scholar * Susan M. Kaech View author
publications You can also search for this author inPubMed Google Scholar * James R. Smiley View author publications You can also search for this author inPubMed Google Scholar * Robert E.
Means View author publications You can also search for this author inPubMed Google Scholar * Akiko Iwasaki View author publications You can also search for this author inPubMed Google
Scholar * Gerald S. Shadel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.P.W. designed and performed experiments, analysed data,
interpreted results and wrote the paper; W.K.H. provided viral stocks, advice on viral infection protocols, and performed _in vivo_ HSV-1 infections; M.S. performed LCMV and influenza
infections; M.C.T. aided in experimental design and assisted with viral infections; C.M.P. performed experiments and analysed data; M.B. performed steady-state mitochondrial transcript
analysis; N.R. assisted with gene expression array analysis; D.A.M. generated _cGas__−/−_ MEFs; B.A.D. and J.R.S. generated and provided HSV-1 UL12 constructs and HSV-1 ΔUL12 viruses; S.M.K.
provided reagents and facilities for LCMV infections and interpreted results; S.M.L. and R.E.M. provided reagents and advice and performed viral infections; A.I. supplied reagents, designed
experiments, and interpreted results; G.S.S. designed experiments, interpreted results and wrote the paper. CORRESPONDING AUTHOR Correspondence to Gerald S. Shadel. ETHICS DECLARATIONS
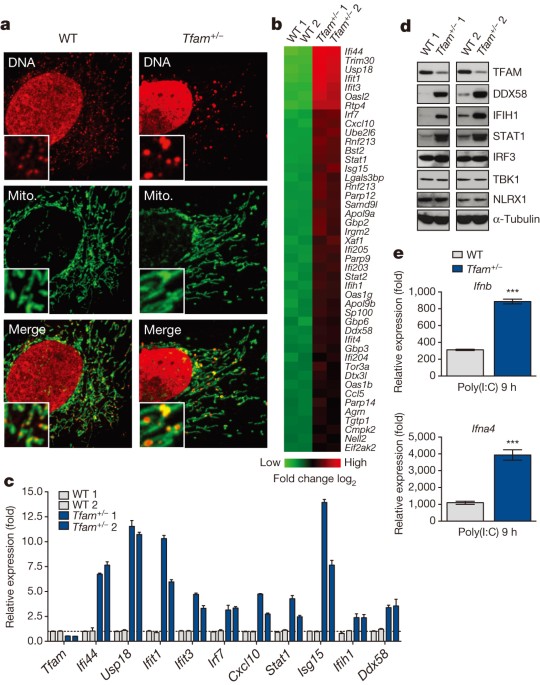
COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 TFAM DEFICIENCY INDUCES MTDNA DEPLETION, NUCLEOID STRESS,
ELEVATED ISG EXPRESSION AND AUGMENTED TYPE I INTERFERON RESPONSES, BUT DOES NOT DRASTICALLY ALTER OXYGEN CONSUMPTION AND MITOCHONDRIAL TRANSCRIPTION RATES. A, Quantitative PCR analysis of
relative mtDNA copy number from wild-type (WT) and _Tfam__+/−_ MEFs. B, Basal oxygen consumption rate of wild-type and _Tfam__+/−_ MEFs as determined by Seahorse Bioscience XF96
Extracellular Flux assay. C, qRT–PCR of mtDNA-encoded rRNA (16s) and mRNA (ND6, Cytb, Cox1) transcripts in wild-type and _Tfam__+/−_ MEFs. D–F, Untransfected _Tfam__+/−_ (D) or wild-type
MEFs transfected with control (siCtrl) or _Tfam_ (_siTfam_) siRNAs (D–F) were stained with anti-HSP60 (Mito.) and anti-DNA (DNA) antibodies. Nucleoid area from multiple independent images
was calculated, stratified into groups, and graphed as percentage of the total number of nucleoids counted for each sample (D). Inset panels are 3× magnification to enhance viewing of
mitochondrial network and nucleoid architecture (E). TFAM and ISG mRNA expression were measured by qRT–PCR (F). G–I, _Tfam__flox/flox__ ER-cre__−_ or _Tfam__flox/flox__ ER-cre_+ BMDMs were
incubated in 4OHT for 96 h to induce TFAM depletion. Immunofluorescence staining was performed as described above (G). ISG mRNA and protein expression was monitored by qRT–PCR and western
blotting (H). qRT–PCR analysis of type I interferon and _Il6_ expression in 4OHT-treated _Tfam__fl/fl__ ER-cre_−/+ BMDMs 2 h post-cytosolic delivery of interferon-stimulatory DNA (ISD) or
poly(I:C) (PIC) (I). Error bars indicate ±s.e.m. of triplicates and data are representative of three independent experiments. _P_ < 0.05; _P_ < 0.01; _P_ < 0.001; NS, not
significant. EXTENDED DATA FIGURE 2 TFAM DEFICIENCY PROMOTES ACCUMULATION OF CYTOSOLIC MTDNA. A, Wild-type (WT) or _Tfam__+/−_ MEFs were subjected to digitonin fractionation as described in
the Methods and whole-cell extracts (WCE), pellets (Pel) or cytosolic extracts (Cyt) were blotted using the indicated antibodies. B, DNA was extracted from digitonin extracts of wild-type
and _Tfam__+/−_ MEFs or _Tfam__fl/fl__ ER-cre__−_ or _Tfam__fl/fl__ ER-cre_+ BMDMs incubated in 4OHT for 72 h. Cytosolic mtDNA was quantitated via qPCR using a mitochondrial _Dloop_ primer
set (mt-Dloop3). Normalization was performed as described in the Methods. C, Samples were prepared as described in B, and cytosolic mtDNA was quantitated via qPCR using the indicated primer
sets. Error bars indicate ±s.e.m. of triplicates and data are representative of three independent experiments. _P_ < 0.01, _P_ < 0.001. EXTENDED DATA FIGURE 3 MITOCHONDRIAL HYPERFUSION
REGULATES THE ACCUMULATION OF MTDNA NUCLEOID STRESS IN TFD MEFS. A, B, Wild-type (WT) MEFs were transfected with control or _Tfam_ siRNAs for 96 h. Cells were fixed and processed for
electron microscopy analysis (A). Mitochondrial perimeter measurements were obtained from multiple independent images, stratified into groups, and graphed as a percentage of the total number
of mitochondria counted for each sample (B). C–E, Wild-type MEFs were transfected with control, _Mfn1_ and/or _Tfam_ siRNAs for 96 h. Cells were fixed and stained with an anti-HSP60
antibody (Mito.) and an anti-DNA antibody (DNA) for confocal microscopy (C). Nucleoid area from multiple independent images was calculated as previously described (D). RNA was extracted for
ISG expression analysis by qRT–PCR (E). F, Wild-type and _Tfam__+/−_ MEFs were transfected with the indicated siRNAs for 96 h and ISG expression analysed by qRT–PCR. Error bars indicate
±s.e.m. of triplicates and data are representative of two independent experiments. _P_ < 0.01; _P_ < 0.001. EXTENDED DATA FIGURE 4 MTDNA STRESS IN TFD MEFS AND BMDMS POTENTIATES TYPE I
INTERFERON RESPONSES TO VIRAL INFECTION AND ENHANCES VIRAL CLEARANCE. A, B, Wild-type (WT) and _Tfam__+/−_ MEFs were infected with VSV-GFP (A) or MHV68-GFP (B) and, after the indicated
times, cytokine and ISG mRNA expression was determined by qRT–PCR, or cytokine secretion was determined by ELISA. C–F, _Tfam__fl/fl__ ER-cre__−_ or _Tfam__fl/fl__ ER-cre_+ BMDMs were
incubated in 4OHT for 96 h to induce TFAM depletion. Cells were infected with HSV-1-GFP (C, E, F) or VSV-GFP (D, E), incubated for the indicated times, and viral gene expression was
determined by qRT–PCR (C, D) and western blotting (E), or cytokine and ISG mRNA expression was determined by qRT–PCR (F). Error bars indicate ±s.e.m. of triplicates and data are
representative of two independent experiments. _P_ < 0.01; _P_ < 0.001; A.U., arbitrary units; ND, not detected; NS, not significant. EXTENDED DATA FIGURE 5 TISSUES FROM _TFAM__+/−_
MICE DISPLAY ELEVATED ISG EXPRESSION, AND DDC ABROGATES MTDNA STRESS, ISG EXPRESSION AND VIRAL RESISTANCE PHENOTYPES OF TFD CELLS. A, RNA was extracted from the liver and kidneys of
8-week-old wild-type (WT) and _Tfam__+/−_ mice (_n_ = 2 each) and subjected to qRT–PCR analysis for basal ISG expression. B-D, Relative mtDNA copy number (B), mtDNA nucleoid area (C) and ISG
expression (D) of wild-type and _Tfam__+/−_ MEFs exposed to ddC for 96 h. E, F, mtDNA nucleoid area (E) and ISG expression (F) of wild-type MEFs transfected with control or _Tfam_ siRNAs
for 96 h in the presence or absence of ddC. G–I, _Tfam__fl/fl__ ER-cre__−_ or _Tfam__fl/fl__ ER-cre_+ BMDMs were incubated in 4OHT for 96 h to induce TFAM depletion in the presence of ddC.
ddC was washed out and cells allowed to recover overnight before infection. Cells were infected with VSV-GFP (G) or HSV-1-GFP (H) at MOI 1, or wild-type BMDMs were transfected with poly(I:C)
or interferon-stimulatory DNA (ISD) (I), and incubated for the indicated times. _Ifnb_ expression or viral gene expression was determined by qRT–PCR. Error bars indicate ±s.e.m. of
triplicates and data are representative of two independent experiments. _P_ < 0.05; _P_ < 0.01; _P_ < 0.001; NS, not significant. EXTENDED DATA FIGURE 6 ALPHA- AND
GAMMAHERPESVIRUSES INDUCE MTDNA STRESS, BUT INFLUENZA, LCMV, AND VACCINIA DO NOT. A, Relative mtDNA copy number of wild-type (WT) MEFs 24 h post-infection with VSV-GFP, HSV-1-GFP or mock
infection at the indicated MOIs. B, Wild-type MEFs were infected with MHV68-GFP at MOI 0.5. After the indicated times cells were stained and subjected to confocal microscopy or the relative
mtDNA copy number was determined. C, Wild-type MEFs were infected with HSV-2, influenza-GFP or LCMV-GFP at MOI 10. After 6 h, cells were stained and subjected to confocal microscopy. D,
Wild-type MEFs were infected with vaccinia virus at MOI 10 (for microscopy) or 1. After the indicated times cells were stained and subjected to confocal microscopy or the relative mtDNA copy
number was determined. Error bars indicate ±s.e.m. of triplicates and data are representative of two independent experiments. _P_ < 0.05; _P_ < 0.01; _P_ < 0.001; A.U., arbitrary
units; ND, not detected; NS, not significant. EXTENDED DATA FIGURE 7 HSV-1 UL12 M185 EXPRESSION IS SUFFICIENT TO TRIGGER MTDNA STRESS, TFAM DEPLETION AND ANTIVIRAL PRIMING IN BMDMS;
INFECTION WITH UL12-DEFICIENT HSV-1 FAILS TO INDUCE MTDNA STRESS, ELICITS LOWER VAGINAL TYPE I INTERFERON RESPONSES AND SPREADS MORE READILY TO DORSAL ROOT GANGLIA. A, Wild-type (WT) BMDMs
were transduced with HSV-1-UL12-M185-expressing- or empty retroviruses (RV) and relative mtDNA abundance, protein expression, and ISG mRNA expression determined. B, Wild-type MEFs were
infected with HSV-1 (UL12–FLAG) or UL12-deficient HSV-1 (ΔUL12 + UL98–FLAG) at MOI 10 for 3 h and analysed by confocal microscopy. C, Wild-type MEFs were infected with HSV-1 (UL12–FLAG) or
UL12-deficient HSV-1 (ΔUL12 + UL98–FLAG) at MOI 2 for 24 h and mtDNA abundance was determined by qPCR. D, The vaginas of wild-type mice (_n_ = 3 per condition) were inoculated with 106
plaque-forming units of HSV-1 (UL12–FLAG) or UL12-deficient HSV-1 (ΔUL12 + UL98–FLAG) and 24 h post-infection, vaginal RNA was extracted and gene expression analysed by qRT–PCR. E, Mice (_n_
= 3 per condition) were infected as previously described and 6 days post-infection, DNA from dorsal root ganglia was isolated for mtDNA and HSV-1 genome abundance measurements by qPCR.
Error bars indicate ±s.e.m. of triplicates and data are representative of two independent experiments. _P_ < 0.05; _P_ < 0.01; _P_ < 0.001; NS, not significant. EXTENDED DATA FIGURE
8 MODEL ILLUSTRATING MTDNA STRESS-DEPENDENT ANTIVIRAL PRIMING. TFAM depletion, induced genetically or during herpesvirus infection, triggers mtDNA stress, characterized by nucleoid loss and
enlargement. This results in the release of fragmented mtDNA that recruits and activates peri-mitochondrial cGAS to generate the second messenger cyclic GMP-AMP (cGAMP) and activate
endoplasmic-reticulum-resident STING. STING then activates TBK1, which phosphorylates IRF3 to induce dimerization and nuclear translocation. Active IRF3 elevates basal gene expression of
ISGs with antiviral signalling and effector functions. Signalling molecules encoded by ISGs, such as IRF7, ISG15, STAT1 and STAT2, cooperate with IRF3 to potentiate the RIG-I-like receptor
(RLR), interferon-stimulatory DNA (ISD) and type I interferon (IFN-I) responses, while effector molecules encoded by ISGs, such as IFI44, IFIT1, IFIT3 and OASL2, augment viral resistance.
Both outcomes collectively and robustly boost innate antiviral defences to dampen viral replication. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT
SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE West, A., Khoury-Hanold, W., Staron, M. _et al._
Mitochondrial DNA stress primes the antiviral innate immune response. _Nature_ 520, 553–557 (2015). https://doi.org/10.1038/nature14156 Download citation * Received: 04 July 2014 * Accepted:
15 December 2014 * Published: 02 February 2015 * Issue Date: 23 April 2015 * DOI: https://doi.org/10.1038/nature14156 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative