- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
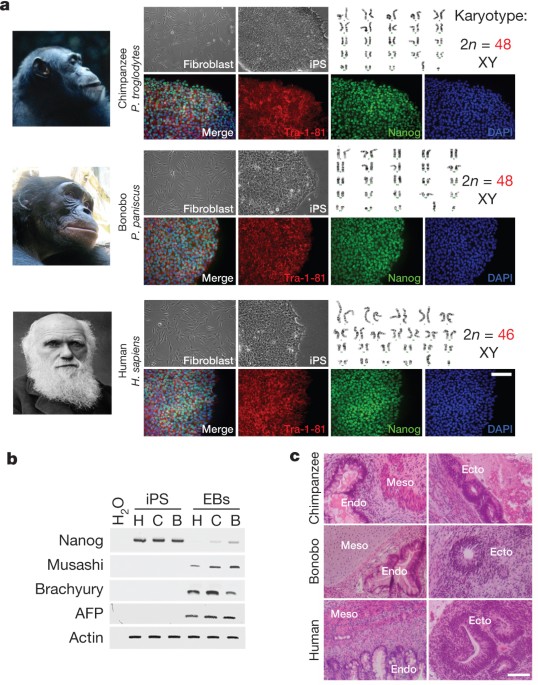
ABSTRACT Identifying cellular and molecular differences between human and non-human primates (NHPs) is essential to the basic understanding of the evolution and diversity of our own species.
Until now, preserved tissues have been the main source for most comparative studies between humans, chimpanzees (_Pan troglodytes_) and bonobos (_Pan paniscus_)1,2. However, these tissue
samples do not fairly represent the distinctive traits of live cell behaviour and are not amenable to genetic manipulation. We propose that induced pluripotent stem (iPS) cells could be a
unique biological resource to determine relevant phenotypical differences between human and NHPs, and that those differences could have potential adaptation and speciation value. Here we
describe the generation and initial characterization of iPS cells from chimpanzees and bonobos as new tools to explore factors that may have contributed to great ape evolution. Comparative
gene expression analysis of human and NHP iPS cells revealed differences in the regulation of long interspersed element-1 (L1, also known as LINE-1) transposons. A force of change in
mammalian evolution, L1 elements are retrotransposons that have remained active during primate evolution3,4,5. Decreased levels of L1-restricting factors APOBEC3B (also known as A3B)6 and
PIWIL2 (ref. 7) in NHP iPS cells correlated with increased L1 mobility and endogenous L1 messenger RNA levels. Moreover, results from the manipulation of A3B and PIWIL2 levels in iPS cells
supported a causal inverse relationship between levels of these proteins and L1 retrotransposition. Finally, we found increased copy numbers of species-specific L1 elements in the genome of
chimpanzees compared to humans, supporting the idea that increased L1 mobility in NHPs is not limited to iPS cells in culture and may have also occurred in the germ line or embryonic cells
developmentally upstream to germline specification during primate evolution. We propose that differences in L1 mobility may have differentially shaped the genomes of humans and NHPs and
could have continuing adaptive significance. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ZBTB12 IS A MOLECULAR BARRIER TO DEDIFFERENTIATION IN HUMAN PLURIPOTENT STEM CELLS Article
Open access 09 February 2023 PRIMATE CELL FUSION DISENTANGLES GENE REGULATORY DIVERGENCE IN NEURODEVELOPMENT Article 17 March 2021 THE EMERGENCE OF SOX AND POU TRANSCRIPTION FACTORS PREDATES
THE ORIGINS OF ANIMAL STEM CELLS Article Open access 14 November 2024 ACCESSION CODES ACCESSIONS GENE EXPRESSION OMNIBUS * GSE47626 DATA DEPOSITS RNA-seq and small RNA-seq data have been
deposited in the Gene Expression Omnibus under accession number GSE47626. CHANGE HISTORY * _ 27 NOVEMBER 2013 An image credit was added to the Figure 1 legend. _ REFERENCES * The Chimpanzee
Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. _Nature_ 437, 69–87 (2005) * Prüfer, K. et al. The bonobo genome compared
with the chimpanzee and human genomes. _Nature_ 486, 527–531 (2012) Article ADS Google Scholar * Lander, E. S. et al. Initial sequencing and analysis of the human genome. _Nature_ 409,
860–921 (2001) Article ADS CAS Google Scholar * Waterston, R. H. et al. Initial sequencing and comparative analysis of the mouse genome. _Nature_ 420, 520–562 (2002) Article ADS CAS
Google Scholar * Kazazian, H. H., Jr Mobile elements: drivers of genome evolution. _Science_ 303, 1626–1632 (2004) Article ADS CAS Google Scholar * Bogerd, H. P. et al. Cellular
inhibitors of long interspersed element 1 and Alu retrotransposition. _Proc. Natl Acad. Sci. USA_ 103, 8780–8785 (2006) Article ADS CAS Google Scholar * Aravin, A. A., Sachidanandam, R.,
Girard, A., Fejes-Toth, K. & Hannon, G. J. Developmentally regulated piRNA clusters implicate MILI in transposon control. _Science_ 316, 744–747 (2007) Article ADS CAS Google Scholar
* Varki, A. & Altheide, T. K. Comparing the human and chimpanzee genomes: searching for needles in a haystack. _Genome Res._ 15, 1746–1758 (2005) Article CAS Google Scholar *
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. _Cell_ 131, 861–872 (2007) Article CAS Google Scholar * Yu, J. et al. Induced
pluripotent stem cell lines derived from human somatic cells. _Science_ 318, 1917–1920 (2007) Article ADS CAS Google Scholar * Burns, K. H. & Boeke, J. D. Human transposon tectonics.
_Cell_ 149, 740–752 (2012) Article CAS Google Scholar * Kazazian, H. H., Jr Mobile elements and disease. _Curr. Opin. Genet. Dev._ 8, 343–350 (1998) Article CAS Google Scholar *
Muotri, A. R., Marchetto, M. C., Coufal, N. G. & Gage, F. H. The necessary junk: new functions for transposable elements. _Hum. Mol. Genet._ 16, R159–R167 (2007) Article CAS Google
Scholar * Beck, C. R., Garcia-Perez, J. L., Badge, R. M. & Moran, J. V. LINE-1 elements in structural variation and disease. _Annu. Rev. Genomics Hum. Genet._ 12, 187–215 (2011) Article
CAS Google Scholar * Wissing, S., Montano, M., Garcia-Perez, J. L., Moran, J. V. & Greene, W. C. Endogenous APOBEC3B restricts LINE-1 retrotransposition in transformed cells and
human embryonic stem cells. _J. Biol. Chem._ 286, 36427–36437 (2011) Article CAS Google Scholar * Chiu, Y. L. & Greene, W. C. The APOBEC3 cytidine deaminases: an innate defensive
network opposing exogenous retroviruses and endogenous retroelements. _Annu. Rev. Immunol._ 26, 317–353 (2008) Article CAS Google Scholar * Chen, H. et al. APOBEC3A is a potent inhibitor
of adeno-associated virus and retrotransposons. _Curr. Biol._ 16, 480–485 (2006) Article CAS Google Scholar * Ostertag, E. M., Prak, E. T., DeBerardinis, R. J., Moran, J. V. &
Kazazian, H. H., Jr Determination of L1 retrotransposition kinetics in cultured cells. _Nucleic Acids Res._ 28, 1418–1423 (2000) Article CAS Google Scholar * Xie, Y., Rosser, J. M.,
Thompson, T. L., Boeke, J. D. & An, W. Characterization of L1 retrotransposition with high-throughput dual-luciferase assays. _Nucleic Acids Res._ 39, e16 (2011) Article Google Scholar
* Garcia-Perez, J. L. et al. LINE-1 retrotransposition in human embryonic stem cells. _Hum. Mol. Genet._ 16, 1569–1577 (2007) Article CAS Google Scholar * Wissing, S. et al.
Reprogramming somatic cells into iPS cells activates LINE-1 retroelement mobility. _Hum. Mol. Genet._ 21, 208–218 (2012) Article Google Scholar * De Fazio, S. et al. The endonuclease
activity of Mili fuels piRNA amplification that silences LINE1 elements. _Nature_ 480, 259–263 (2011) Article ADS CAS Google Scholar * Mathews, L. M., Chi, S. Y., Greenberg, N.,
Ovchinnikov, I. & Swergold, G. D. Large differences between LINE-1 amplification rates in the human and chimpanzee lineages. _Am. J. Hum. Genet._ 72, 739–748 (2003) Article CAS Google
Scholar * Khan, H., Smit, A. & Boissinot, S. Molecular evolution and tempo of amplification of human LINE-1 retrotransposons since the origin of primates. _Genome Res._ 16, 78–87 (2006)
Article CAS Google Scholar * Lee, J. et al. Different evolutionary fates of recently integrated human and chimpanzee LINE-1 retrotransposons. _Gene_ 390, 18–27 (2007) Article CAS
Google Scholar * Mills, R. E. et al. Recently mobilized transposons in the human and chimpanzee genomes. _Am. J. Hum. Genet._ 78, 671–679 (2006) Article CAS Google Scholar * Campbell, M.
C. & Tishkoff, S. A. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. _Annu. Rev. Genomics Hum. Genet._ 9,
403–433 (2008) Article CAS Google Scholar * Gagneux, P. et al. Mitochondrial sequences show diverse evolutionary histories of African hominoids. _Proc. Natl Acad. Sci. USA_ 96, 5077–5082
(1999) Article ADS CAS Google Scholar * Bowden, R. et al. Genomic tools for evolution and conservation in the chimpanzee: _Pan troglodytes ellioti_ is a genetically distinct population.
_PLoS Genet._ 8, e1002504 (2012) Article CAS Google Scholar * Beck, C. R. et al. LINE-1 retrotransposition activity in human genomes. _Cell_ 141, 1159–1170 (2010) Article CAS Google
Scholar * Marchetto, M. C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. _Cell_ 143, 527–539 (2010) Article CAS Google
Scholar * Muotri, A. R., Nakashima, K., Toni, N., Sandler, V. M. & Gage, F. H. Development of functional human embryonic stem cell-derived neurons in mouse brain. _Proc. Natl Acad. Sci.
USA_ 102, 18644–18648 (2005) Article ADS CAS Google Scholar * Landry, S., Narvaiza, I., Linfesty, D. C. & Weitzman, M. D. APOBEC3A can activate the DNA damage response and cause
cell-cycle arrest. _EMBO Rep._ 12, 444–450 (2011) Article CAS Google Scholar * Kidd, J. M., Newman, T. L., Tuzun, E., Kaul, R. & Eichler, E. E. Population stratification of a common
_APOBEC_ gene deletion polymorphism. _PLoS Genet._ 3, e6 (2007) Article Google Scholar * Narvaiza, I. et al. Deaminase-independent inhibition of parvoviruses by the APOBEC3A cytidine
deaminase. _PLoS Pathog._ 5, e1000439 (2009) Article Google Scholar * Mizushima, S. & Nagata, S. pEF-BOS, a powerful mammalian expression vector. _Nucleic Acids Res._ 18, 5322 (1990)
Article CAS Google Scholar * Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013) Article CAS Google Scholar * Heinz, S. et al. Simple
combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010) Article CAS Google
Scholar * de Hoon, M. J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. _Bioinformatics_ 20, 1453–1454 (2004) Article CAS Google Scholar * Saldanha, A. J. Java
Treeview–extensible visualization of microarray data. _Bioinformatics_ 20, 3246–3248 (2004) Article CAS Google Scholar * Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a
Bioconductor package for differential expression analysis of digital gene expression data. _Bioinformatics_ 26, 139–140 (2010) Article CAS Google Scholar * Dennis, G., Jr et al. DAVID:
Database for Annotation, Visualization, and Integrated Discovery. _Genome Biol._ 4, P3 (2003) Article Google Scholar * Jurka, J. et al. Repbase Update, a database of eukaryotic repetitive
elements. _Cytogenet. Genome Res._ 110, 462–467 (2005) Article CAS Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nature Methods_ 9,
357–359 (2012) Article CAS Google Scholar * Sai Lakshmi, S. & Agrawal, S. piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. _Nucleic Acids Res._ 36,
D173–D177 (2008) Article CAS Google Scholar * Moran, J. V. et al. High frequency retrotransposition in cultured mammalian cells. _Cell_ 87, 917–927 (1996) Article CAS Google Scholar *
Bulliard, Y. et al. Structure–function analyses point to a polynucleotide-accommodating groove essential for APOBEC3A restriction activities. _J. Virol._ 85, 1765–1776 (2011) Article CAS
Google Scholar * Brouha, B. et al. Hot L1s account for the bulk of retrotransposition in the human population. _Proc. Natl Acad. Sci. USA_ 100, 5280–5285 (2003) Article ADS CAS Google
Scholar * Penzkofer, T., Dandekar, T. & Zemojtel, T. L1Base: from functional annotation to prediction of active LINE-1 elements. _Nucleic Acids Res._ 33, D498–D500 (2005) Article CAS
Google Scholar * Fujita, P. A. et al. The UCSC Genome Browser database: update 2011. _Nucleic Acids Res._ 39, D876–D882 (2011) Article CAS Google Scholar * Ladewig, J. et al. Small
molecules enable highly efficient neuronal conversion of human fibroblasts. _Nature Methods_ 9, 575–578 (2012) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The work was
supported by funds from the National Institutes of Health (NIH) (TR01: MH095741 and Eureka: MH08848 to F.H.G.), the Mathers Foundation and the Helmsley Foundation. This work was also
partially supported by funds from the NIH to A.R.M. (MH094753), M.D.W. (AI074967) and G.W.Y. (NS075449, HG004659 and GM084317). G.W.Y. is a recipient of the Alfred P. Sloan Research
Fellowship. We thank J. V. Moran and W. An for reagents. We would like to thank A. Varki, P. Gagneux, L. Fourgeaud and I. Guimont for discussions, N. Varki for help with teratoma analysis,
R. Keithley, I. Gallina and Y. Nunez for technical assistance, and M. L. Gage for editorial comments. AUTHOR INFORMATION Author notes * Maria C. N. Marchetto and Iñigo Narvaiza: These
authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Laboratory of Genetics, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, California
92037, USA, Maria C. N. Marchetto, Iñigo Narvaiza, Ahmet M. Denli, Christopher Benner, Thomas A. Lazzarini, Apuã C. M. Paquola & Fred H. Gage * Department of Cellular and Molecular
Medicine, University of California San Diego, Stem Cell Program, Institute for Genomic Medicine, Sanford Consortium for Regenerative Medicine, 2880 Torrey Pines Scenic Drive, La Jolla,
California 92037, USA, Jason L. Nathanson & Gene W. Yeo * Division of Biological Sciences, University of California San Diego, 9500 Gilman Drive, La Jolla, California 92093, USA, Keval
N. Desai * Department of Pediatrics/Rady Children’s Hospital San Diego, Department of Cellular & Molecular Medicine, University of California San Diego, School of Medicine, Stem Cell
Program, Sanford Consortium, MC 0695, 2880 Torrey Pines Scenic Drive, La Jolla, California 92093, USA, Roberto H. Herai & Alysson R. Muotri * Department of Pathology and Laboratory
Medicine, University of Pennsylvania Perelman School of Medicine and Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia, Philadelphia, 19104-4318,
Pennsylvania, USA Matthew D. Weitzman * Center for Academic Research and Training in Anthropogeny (CARTA), 9500 Gilman Drive, La Jolla, 92093, California, USA Alysson R. Muotri & Fred H.
Gage Authors * Maria C. N. Marchetto View author publications You can also search for this author inPubMed Google Scholar * Iñigo Narvaiza View author publications You can also search for
this author inPubMed Google Scholar * Ahmet M. Denli View author publications You can also search for this author inPubMed Google Scholar * Christopher Benner View author publications You
can also search for this author inPubMed Google Scholar * Thomas A. Lazzarini View author publications You can also search for this author inPubMed Google Scholar * Jason L. Nathanson View
author publications You can also search for this author inPubMed Google Scholar * Apuã C. M. Paquola View author publications You can also search for this author inPubMed Google Scholar *
Keval N. Desai View author publications You can also search for this author inPubMed Google Scholar * Roberto H. Herai View author publications You can also search for this author inPubMed
Google Scholar * Matthew D. Weitzman View author publications You can also search for this author inPubMed Google Scholar * Gene W. Yeo View author publications You can also search for this
author inPubMed Google Scholar * Alysson R. Muotri View author publications You can also search for this author inPubMed Google Scholar * Fred H. Gage View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS M.C.N.M. and I.N. are the leading authors. M.C.N.M., I.N. and A.M.D. contributed to the concept, designed and performed the
experiments, and analysed the data. M.C.N.M. reprogrammed NHP fibroblasts and performed iPS cell cultures and transduction assays. I.N. and M.C.N.M. performed L1 assays. I.N. designed and
performed biochemical experiments. A.M.D., C.B. and I.N. designed and performed comparative analysis of L1 insertions in the human and NHP genomes. T.A.L. produced lentiviruses and provided
tissue culture assistance. I.N. and K.N.D. generated the chimp-L1 reporter plasmid. C.B., A.C.M.P. and R.H.H performed bioinformatics analysis. I.N., C.B. and J.L.N. contributed to the
generation of libraries and analysis of RNA-seq data. M.D.W., G.W.Y. and A.R.M. contributed to concept and financial support. F.H.G. is the senior author. He contributed to the concept,
analysed the data, revised the manuscript and provided financial support. I.N., M.C.N.M., A.M.D. and F.H.G wrote the manuscript. All the authors read and approved the final manuscript.
CORRESPONDING AUTHOR Correspondence to Fred H. Gage. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED
DATA FIGURE 1 CELL LINES USED, NUMBER OF MAPPED READS PER SAMPLE IN RNA-SEQ AND GENE ONTOLOGY ENRICHMENT ANALYSIS FOR DIFFERENTIALLY EXPRESSED GENES. A, Origin of iPS cells used or generated
in this study. B, Total number of mapped reads per sample in RNA-seq. C, D, Gene ontology (GO) enrichment analysis of differentially expressed genes. C, Top 10 enriched GO terms for genes
with higher expression in human versus NHP iPS cells. D, Top 10 enriched GO terms for genes highly expressed in NHP versus human iPS cells. GO analysis was restricted to differentially
expressed protein-coding genes (FDR < 0.05 and fold change > 2). GO enrichment for biological processes (level 2) was performed using DAVID. Figure shows GO term, number of genes
(count), and _P_ values for EASE score and Benjamini adjustment. EXTENDED DATA FIGURE 2 AMINO ACID ALIGNMENT OF A3B AND PIWIL2. A, B, Protein sequences of human, chimp and bonobo A3B (A) or
PIWIL2 (B) were aligned using ClustalW. A, Alignment of A3B showing >93% identity between human and NHP proteins. B, Alignment of PIWIL2 showing >98% identity between human and NHP
proteins. EXTENDED DATA FIGURE 3 MRNA LEVELS OF _APOBEC3_ AND PIWI-LIKE PROTEIN FAMILY MEMBERS IN IPS CELLS. A, Comparative analysis of _PIWIL2_ mRNA levels. qRT–PCR analysis of _PIWIL2_
mRNA levels in human testis, human iPS cell lines, and available fibroblasts from which the iPS cell lines were derived. mRNA levels were normalized to _GAPDH_ and shown relative to human
testis (mean ± s.e.m.; _n_ = 3 biological replicates). Compared to testis, _PIWIL2_ levels are 20–40 fold lower in iPS cells and ∼1,100-fold lower in fibroblasts. B, C, Quantification of
mRNA levels of _APOBEC3_ and PIWI-like family members in human and NHP iPS cells by RNA-seq. Increased mRNA levels in human iPS cells are restricted for _APOBEC3B_ and _PIWIL2_. _y_ axes in
B and C denote the reads per kilobase per million mapped reads (RPKM). EXTENDED DATA FIGURE 4 L1 REPORTER ACTIVITY IN IPS CELLS. A, L1 retrotransposition reporter system. The L1-reporter
plasmid contains a retrotransposition-competent human L1 element and carries either an eGFP or a luciferase reporter construct in its 3′ UTR region. The reporter gene is interrupted by an
intron in the same transcriptional orientation as the L1 transcript. This arrangement ensures that eGFP/luciferase-positive cells will arise only when a transcript initiated from the
promoter driving L1 expression is spliced, reverse transcribed, and integrated into chromosomal DNA, thereby allowing expression of the reporter gene from a heterologous promoter. B–F,
Efficient A3B knockdown in human ES and iPS cells. B, Stable shRNA-mediated knockdown of A3B in human ES cells (HUES6) using lentivirus expressing different shRNAs against _A3B_ (shA3B-1 and
shA3B-2) or scrambled control (shScr). Levels of _A3B_ expression were normalized to _GAPDH_ and shown relative to shScr (mean ± s.e.m.; _n_ = 3 biological replicates). C, Western blot
confirming stable A3B knockdown in human ES cells. D–F, shRNA-mediated knockdown in human ES cells (HUES6) and iPS cell lines 1 and 2 (WT-33 and ADRC-40, respectively) was specific for
_A3B_. G–H, qRT–PCR analysis of plasmid expression in iPS cell lines transfected with L1–eGFP plasmid. Total RNA samples were obtained 60–72 h after transfection. L1 plasmid expression was
normalized to _GAPDH_ (G) or puromycin (H). L1–eGFP contains a puromycin expression cassette under _PGK_ promoter control. Thus, puromycin expression was used as normalizer for transfection.
iPS cells from two different individuals per species were transfected, and eGFP levels are shown as relative to human iPS cells. No significant differences were observed for L1 plasmid
expression between human and NHP iPS cell lines (mean ± s.e.m.; _n_ = 3 biological replicates). I, Relative L1 5′ UTR promoter activity. Human and chimp L1 promoters (L1 5′ UTR) controlling
firefly luciferase were transfected into human and NHP iPS cell lines. _Renilla_ luciferase was co-transfected as control. Luciferase activity was quantified as firefly luciferase units
relative to _Renilla_ luciferase units. Results are shown as normalized to human L1 5′ UTR activity in human iPS cell. iPS cells from two different individuals per species were transfected.
No significant differences were observed for L1 promoter activities between human and NHP iPS cell lines (mean ± s.e.m.; _n_ = 4 biological replicates). EXTENDED DATA FIGURE 5 NUCLEIC ACID
ALIGNMENT OF HUMAN AND CHIMPANZEE L1 ELEMENTS. Sequence of the chimpanzee L1Pt element cloned and used to generate the chimpanzee L1–eGFP tagged reporter plasmid (L1IN71) (top sequence).
_LRE3_: human L1 (bottom sequence). EXTENDED DATA FIGURE 6 IMMUNOPRECIPITATION OF PIRNAS ASSOCIATED WITH PIWIL2 IN HUMAN IPS CELLS AND ANNOTATED PIRNAS MAPPING TO CONSENSUS L1HS IN IPS
CELLS. A, Immunoprecipitation of PIWIL2 RNPs using Flag-tag antibodies from Tet-inducible Flag-tagged PIWIL2 human iPS cells after addition of doxyclycine to the culture media. HA-tag
antibody was used as control. B, [γ-32P]ATP end-labelling of RNAs associated with Flag–PIWIL2 RNPs. Signal in the piRNAs size range is detected only in anti-Flag but not in control antibody
anti-HA immunoprecipitates. C, Size distribution of RNA reads detected by small RNA-seq from small RNAs samples extracted from human iPS cell lines. D, Number of mapped reads per sample in
small RNA-seq. E, Number of annotated piRNAs (piRNAbank) detected by RNA-seq in human iPS cells 1 and 2. F, Characterization of 5′ end of piRNAs detected in human iPS cells relative to
annotated piRNAs. Read count distribution relative to piRNA 5′ ends (piRNAbank). G, Sequences of annotated piRNAs (piRNAbank) mapping to consensus L1Hs detected in human iPS cells 1 and 2.
The 26–33-nucleotide RNA reads from human iPS cell lines 1 and 2 characterized by RNA-seq are aligned to annotated piRNAs mapping to the consensus L1Hs sequence. Analysis of mapping
sequences was performed allowing two mismatches. EXTENDED DATA FIGURE 7 MAPPING OF 26–33-NUCLEOTIDE RNAS IN HUMAN IPS CELLS TO CONSENSUS L1HS. A, Mapping of annotated piRNAs (piRNAbank)
detected by RNA-seq from human iPS cell lines to the consensus sequence for L1Hs (from Repbase). All annotated piRNAs (piRNAbank) complementary to L1Hs are indicated (black bars). B, Total
26–33-nucleotide RNA reads characterized by small RNA-seq mapped to L1Hs. C, Similar analysis as in B of ENCODE data for small RNAs from H1 cells. Positive and negative values indicate sense
(+) and antisense (−) piRNAs, respectively. Schematic representation of the L1Hs element is shown (top). _y_ axes represent read counts normalized to 107 reads per experiment. EXTENDED DATA
FIGURE 8 HIGHER LEVELS OF ENDOGENOUS L1 RNA AND RECENT SPECIES-SPECIFIC L1 ELEMENTS IN CHIMPANZEE. A, Scheme of amplicons mapped to the L1Hs consensus sequence. Six primer pairs (two per
region) were used for quantification of 5′ UTR, ORF1 and ORF2. The primers were designed to recognize both species-specific and common families. B, Positions of the amplicons in L1Hs
consensus sequence and the number of _in silico_ PCR hits on the human and chimp genomes. C, qRT–PCR analysis using primers for different regions of L1 element show higher levels of L1 RNA
in NHP iPS cells regardless of the L1 region tested: 5′ UTR, ORF1 and ORF2 (mean ± s.e.m.; _n_ = 3 biological replicates; *_P_ < 0.01 between indicated groups, _t_-test). D–G,
Quantification of L1 elements in human and chimpanzee genomes using a population divergence model. Number of L1 elements found in the human and chimpanzee genomes for families: L1PA4 (D),
L1PA3 (E), L1PA2 (F) and L1Pt and L1Hs (G) plotted as a histogram relative to their divergence (number of mutations relative to the canonical element). The standard deviation describes the
differences in L1 density based on the sampling of different genomic regions and represents the variability of L1 coverage across the genomes (see Methods). EXTENDED DATA FIGURE 9 RELATIVE
_A3B_ AND _PIWIL2_ MRNA LEVELS IN IPS CELLS AND FIBROBLASTS. Relative expression of _A3B_ (A) and _PIWIL2_ (B) in human and NHP iPS cell lines, and the available source fibroblasts from
which iPS cells were derived. mRNA levels were normalized to _GAPDH_ and shown relative to human iPS cell line 1. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1 This file shows microRNAs
detected in human iPSCs by small RNA-seq. (XLSX 176 kb) SUPPLEMENTARY TABLE 2 This file shows annotated piRNAs detected in human iPSCs by small RNAseq. (XLSX 1622 kb) POWERPOINT SLIDES
POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Marchetto, M., Narvaiza, I., Denli, A. _et al._ Differential L1 regulation in pluripotent stem cells of humans and apes. _Nature_ 503, 525–529 (2013).
https://doi.org/10.1038/nature12686 Download citation * Received: 03 October 2012 * Accepted: 17 September 2013 * Published: 23 October 2013 * Issue Date: 28 November 2013 * DOI:
https://doi.org/10.1038/nature12686 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative