- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
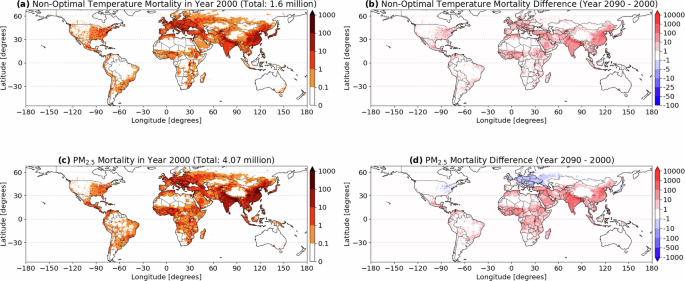
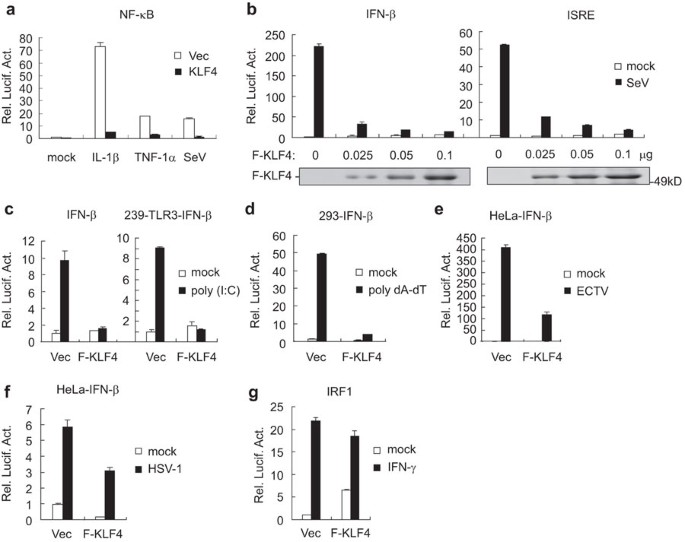
Access through your institution Buy or subscribe The IACUC Chairperson was correct, in that the drawing of blood for clinical cases does not require an IACUC-approved protocol. If the
veterinarian had drawn the blood before Burbank asked for a 1-ml sample, and there was extra blood already drawn, that would have been acceptable. Madela is also correct. One could consider
the request for extra blood to be drawn as two separate collections: the usual amount for the clinical studies and the additional 1 ml requested for the research project. Because the
additional 1 ml was drawn specifically to obtain data for a research proposal, an IACUC-approved protocol was necessary. A situation similar to the drawing of blood involves the use of
archived tissues that are collected at necropsy from animals on an approved project. Years later, another investigator may examine these archived materials to provide preliminary data for a
proposed project. Although the tissues were not collected for the preliminary data study, they are available for that study. If the investigator had requested access to these tissues before
they were collected, then that would require an approved IACUC amendment to the project involving the animals at the time of necropsy. If tissues and blood are routinely collected at
necropsy and are later available for preliminary data, then an IACUC-approved protocol should not be necessary. This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Animal Welfare
Act, 7 USC 2157, Sec. 28 (a)(2)(B). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Veterinary Medicine, Harrison is Research Scientist, Tulane National Primate
Research Center, Covington, LA., Richard M. Harrison PhD Authors * Richard M. Harrison PhD View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Harrison, R. Response to Protocol Review Scenario: Better Safe Than Sorry. _Lab Anim_ 33, 18–19 (2004).
https://doi.org/10.1038/laban0104-18b Download citation * Issue Date: January 2004 * DOI: https://doi.org/10.1038/laban0104-18b SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative