- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Perennially cold habitats are diminishing as a result of climate change; however, little is known of the diversity or biogeography of microbes that thrive in such environments. Here
we use targeted 16S rRNA gene surveys to evaluate the global affinities of cold-dwelling cyanobacteria from lake, stream and ice communities living at the northern limit of High Arctic
Canada. Pigment signature analysis by HPLC confirmed the dominance of cyanobacteria in the phototrophic communities of these High Arctic microbial mats, with associated populations of
chlorophytes and chromophytes. Microscopic analysis of the cyanobacteria revealed a diverse assemblage of morphospecies grouping into orders Oscillatoriales, Nostocales and Chroococcales.
The 16S rRNA gene sequences from six clone libraries grouped into a total of 24 ribotypes, with a diversity in each mat ranging from five ribotypes in ice-based communities to 14 in
land-based pond communities. However, no significant differences in composition were observed between these two microbial mat systems. Based on clone-library and phylogenetic analysis,
several of the High Arctic ribotypes were found to be >99% similar to Antarctic and alpine sequences, including to taxa previously considered endemic to Antarctica. Among the latter, one
High Arctic sequence was found 99.8% similar to _Leptolyngbya antarctica_ sequenced from the Larsemann Hills, Antarctica. More than 68% of all identified ribotypes at each site matched only
cyanobacterial sequences from perennially cold terrestrial ecosystems, and were <97.5% similar to sequences from warmer environments. These results imply the global distribution of
low-temperature cyanobacterial ecotypes throughout the cold terrestrial biosphere. SIMILAR CONTENT BEING VIEWED BY OTHERS A GENUS IN THE BACTERIAL PHYLUM AQUIFICOTA APPEARS TO BE ENDEMIC TO
AOTEAROA-NEW ZEALAND Article Open access 02 January 2024 BACTERIAL AND ARCHAEAL COMMUNITY DISTRIBUTIONS AND COSMOPOLITANISM ACROSS PHYSICOCHEMICALLY DIVERSE HOT SPRINGS Article Open access
18 August 2023 LINKING THE COMPOSITION OF CRYOCONITE PROKARYOTIC COMMUNITIES IN THE ARCTIC, ANTARCTIC, AND CENTRAL CAUCASUS WITH THEIR CHEMICAL CHARACTERISTICS Article Open access 09 July
2024 INTRODUCTION Recently attention has been focused on how the Earth's atmosphere has rapidly warmed over the last decade; however, vast regions of the planet remain at temperatures
near or below freezing. Extreme cold is a defining feature of High Arctic, Antarctic and high alpine sites, which are separated by large distances and climatic barriers. The ecology of these
cryoenvironments is mostly microbial, and existence of a perennially cold terrestrial biosphere has implications for microbial speciation, dispersal, biogeography and gene exchange at a
planetary scale. Globally dispersed microbial ecotypes have been described from hot springs and other geothermal environments (Papke et al., 2003; Bhaya et al., 2007; Ward et al., 2008), but
microbiota at the opposite thermal extreme, cold-dwelling taxa, have received little attention. Cyanobacteria are common throughout the terrestrial North and South Polar Regions, where they
form benthic mats and films at the bottom of lakes, ponds and streams (Zakhia et al., 2007). These communities often dominate total ecosystem biomass and productivity, and must contend with
persistent low temperatures, repeated freeze–thaw cycles and highly variable light, nutrient and osmotic regimes (Vincent, 2000). Filamentous, mucilage-producing Oscillatoriales are
responsible for much of the biomass and three-dimensional structure of these polar mat consortia. They have been shown to tolerate a wide range of conditions and to maintain slow net growth
despite the frigid ambient temperatures (Tang et al., 1997). Previous work on polar cyanobacteria using both morphological and molecular methods in the Polar Regions, has mostly been
performed in the Antarctic, where cosmopolitan and endemic taxa are reported (Komárek, 1999; Taton et al., 2003, 2006a, 2006b; Jungblut et al., 2005; Comte et al., 2007). By comparison,
little is known about Arctic cyanobacteria, which although inhabiting a similar environment, are potentially more connected to temperate latitudes than Antarctica cyanobacteria, which are
isolated by the Southern Ocean. In the present study we evaluated the global distribution of cyanobacteria by comparing communities from the most northern reaches of North America (High
Arctic Canada) with those from analogous sites in Antarctica. We determined the diversity and community structure of cyanobacterial mats collected from lakes, ponds and streams on land, and
from meltwater lakes on ice shelves, at the northern limit of the North American Arctic, specifically Ward Hunt Island (latitude 83.1°N) and its vicinity in Quttinirpaaq (‘top of the world’
in Inuktitut) National Park, Nunavut, Canada. Cyanobacterial diversity was determined in the microbial mats by way of morphological characters, 16S rRNA gene similarity and pigment
biomarkers. MATERIALS AND METHODS STUDY SITES The samples from Ellesmere Island in Quttinirpaaq National Park (Supplementary Figure S1), Canadian High Arctic, were taken between 8 and 15
July 2007 from the following sites: Ward Hunt Lake (WH-Lake) 83°N 05.289, 74°W 10.048; Quttinirpaaq Lagoon (Q-Lagoon) 83°N 05.843, 74°W 15.018; Markham Ice Shelf (MIS) 83°N 01.898, 71°W
30.812; Ward Hunt Ice Shelf (WIS) 83°N 04.949, 74°W 26.281; Antoniades Pond (Pond-A) 82°N 58.957, 75°W 24.161 and the inflow from Lake B into Lake A (Inflow-A) 82°N 58.801, 75°W 25.372. All
environmental measurements and samples were from 10- to 20-cm water depths. WH-Lake has a maximum depth of 5.5 m and a total area of 0.37 km2, and is the most northern lake of North America
(Villeneuve et al., 2001). The ice-free littoral zones were completely covered by cohesive microbial mats. Q-Lagoon is located between the northern coastline of Ward Hunt Island and an ice
rise (thick ice on land). It has an area of 3 km2, with meltwater inflows from the island and the ice rise. The shallow inshore region of the lagoon along Ward Hunt Island was covered with
thick accumulation of microbial mat flakes. WIS (400 km2) is a floating mass of landfast ice approximately 40 m thick, with a ridge and trough morphology. The troughs were filled with
meltwater ponds up to 15 km long, approximately 3 m deep and 10–20 m wide, with localized accumulations of sediments and loose aggregates of microbial mats (‘matlets’; Mueller and Vincent,
2006). In July 2007, MIS had an area of 50 km2 and a third of its surface was covered with sediment. Mat-containing sediments occurred on raised mounds of the ice and in meltwater ponds in
the form of microbial matlets (Mueller et al., 2006). Inflow A was approximately 10 m wide and 30 cm deep, and its submerged berm was coated with mucilaginous benthic microbial mats. Pond-A,
with an area of approximately 300 m2, contained thick mucilaginous orange pigmented microbial mats that covered the littoral zone. SAMPLING AND WATER ANALYSIS Two or more samples were
obtained from all the sites, except for Pond-A and Inflow-A where only single samples were taken. For the ice shelf sites, adjacent meltwater ponds were considered replicates and replicates
for the lakes were from sites up to several hundred meters apart along the shorelines. After collection the samples were divided into subsamples for pigment, DNA and morphological analysis.
Mat material for DNA and pigments was stored at −80 °C and the subsamples for morphological characterization were kept in the dark at 4 °C until examination by microscopy. Water temperature,
pH and conductivity were determined at each site using a portable instrument (pH/Con 10 Series; Oakton Instruments, Vernon Hills, IL, USA). Water samples for nutrient analysis were
collected from just above the microbial mats, in acid-washed bottles, and stored at 4 °C until analysis. Total nitrogen and total phosphorus were determined by standard methods (Strainton et
al., 1977; QuikChem 10-107-06-2-K) at Institut National de la Recherche Scientifique (Quebec City, QC, Canada). MICROSCOPIC CHARACTERIZATION Cyanobacteria in the mats were examined at ×
1000 magnification using an Olympus inverted light microscope (model IX71) equipped with DIC and phase contrast. Images were taken and measurements were taken using an ocular micrometer.
Separation of taxa was based on morphological descriptions (Geitler, 1932; Anagnostidis and Komárek, 1988, 1990; Komárek and Anagnostidis, 1989, 1998; Villeneuve et al., 2001; Taton et al.,
2008). PIGMENT ANALYSIS Total pigments from subsamples were extracted in the dark by grinding the frozen material for 2 min followed by sonication (3 × 20 s at 20 W) in 4 or 6 ml 90%
acetone:water (vol/vol) mixture, and left overnight at −20 °C under an argon gas atmosphere. The extracts were recovered following centrifugation at 4150 r.p.m. for 15 min at 4 °C. The
supernatant was then filtered through a 0.2 μm pore size PTFE Acrodisc filter (Pall Corporation, Ann Arbor, MI, USA) and stored in the dark at −70 °C under an argon atmosphere until
high-performance liquid chromatography (HPLC) analysis. This extraction procedure was repeated for the residual material until no further coloration was detected in the extract solution.
HPLC analysis was performed on 50 μl of injected sample using a ProStar HPLC system (Varian, Palo Alto, CA, USA) equipped with a Symmetry C8 column (3.5 μm pore size, 4.6 × 150 mm; Waters
Corporation, Milford, MA, USA) at 25 °C with a C8 guard column (5 μm pore size, 3.9 × 20 mm; Waters Corporation). The HPLC peaks were detected by diode-array spectroscopy (350–750 nm).
Absorbance chromatograms at 384 nm (for scytonemin), 440 nm (for chlorophylls) and 450 nm (for carotenoids) were recorded. Chlorophylls were also detected by fluorescence (excitation, 40 nm;
emission, 650 nm). The HPLC solvent protocol was based on gradient dilution with two solvent mixtures (Zapata et al., 2000). The flow rate was 1 ml min−1, with an equilibrium time of 5 min.
Standards (chlorophyll (chl.)-_a_, _b_ and _c_2; β,β-carotene, canthaxanthin, diadinoxanthin, echinenone, fucoxanthin, lutein, myxoxanthophyll and zeaxanthin) were obtained for
identification and quantification of detected pigments (Sigma Inc., St Louis, MO, USA; DHI Water & Environments, Hørsholm, Denmark; Bonilla et al., 2005). Other carotenoids were
quantified based on the published extinction coefficients of related pigments: HFU-like, 142 l g−1 cm−1; uriolide-like, 166 l g−1 cm−1; 19′-hexanoylofucoxanthin-like, 142 l g−1 cm−1;
peridinin-like, 136 l g−1 cm−1; astaxanthin, 210 l g−1 cm−1; antheraxanthin-like, 244 l g−1 cm−1; diatoxanthin, 210 l g−1 cm−1 and monadoxanthin-like, 250 l g−1 cm−1. The extinction
coefficient of β,β-carotene (250 l g−1 cm−1) was used for unknown carotenoids (Jeffrey et al., 1997) and scytonemin was quantified as described by Garcia-Pichel et al. (1992). DNA EXTRACTION
On return from the field, the mat material for DNA analysis was freeze-dried and suspended in 800 μl XS-buffer (1% potassium-methyl-xanthogenate; 800 mM ammonium acetate; 20 mM EDTA; 1%
sodium dodel sulfate; 100 mM Tris-HCl (pH 7.4); Tillett and Neilan, 2000). The mixture was vortex-mixed and incubated at 65 °C for 4 h. The extracts were cooled overnight at −20 °C and cell
debris were removed by centrifugation at 12 000 _g_ for 10 min. An equal volume of phenol–chloroform–isoamyl alcohol (25:24:1) was then added to the removed aqueous phase and centrifuged at
3000 _g_ for 3 min. The two steps were repeated twice. DNA was precipitated overnight by addition of 1 volume of isopropanol and 1/10 volume of 4 M ammonium acetate at −20 °C. The
precipitated DNA was pelleted by centrifugation at 12 000 _g_ for 10 min and washed with 70% ethanol. The extracted DNA was then resuspended in 100 μl of sterile water. PCR All PCR reactions
were performed using Advantage 2 PCR kits with proof-reading ability, in a 20 μl reaction mix using 10 × Advantage 2 SA PCR buffer and 0.2 mM dNTPs (Fermentas, Foster City, CA, USA),
according to the manufacturer (Clontech, Mountain View, CA, USA). PCR amplification of cyanobacterial 16S rDNA was performed using 0.5 μM of each cyanobacteria-specific primer 27F1
(5′-AGAGTTTGATCCTGGCTCAG-3′) and 809R (5′-GCTTCGGCACGGCTCGGGTCGATA-3′). As described by Jungblut et al. (2005), these primers provide broad coverage of cyanobacterial taxa. CLONING, RFLP
ANALYSIS AND SEQUENCING Prior to cloning, the amplified PCR products were verified by gel electrophoresis and amplicons of the target size were purified with the Qiaquick PCR Purification
kit (Qiagen, Mississauga, CA, USA). PCR products were cloned using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA, USA). Ligation and transformation were performed according to the
manufacturer's protocols. Positive clones were transferred to 96-well plates containing Luria Bertani medium with 7% glycerol. The inserted 16S rRNA sequences were amplified using
vector-specific primers M13f and M13r, and subjected to restriction-fragment length polymorphism (RFLP) screening. Amplicons (4 μl) were digested (overnight in separate incubations with 5 U
of restriction enzymes _Alu_I and _Hpa_II; Fermentas, Hanover, NH, USA) in a final reaction volume of 10 μl with the appropriate buffer at 37 °C. The resulting digests were run on 2.5%,
low-melting point agarose gel and the generated RFLP patterns were visualized using the Bio-Rad Laboratories Gel Doc imaging system and Quantity One software (Bio-Rad Laboratories, Hercules,
CA, USA, version 4.5.1). At least two clones for each unique RFLP pattern were sequenced using the vector-specific T7 universal primer (single read) at the Centre Hospitalier de
l'Université Laval (CHUL, QC, Canada), using an ABI 3730xl system (Applied Biosystems, Foster City, CA, USA), which included a purification step. TOTAL-MAT-COMMUNITY RFLP Communities
were compared using direct RFLP analysis of PCR products from total genomic DNA using the same restriction enzymes as above. Five units of _Alu_I and _Hpa_II (Fermentas) were added to 6 μl
of PCR product for a final volume of 10 μl. The generated RFLP patterns were run on 2.5%, low-melting point agarose gel and analyzed as described above. The RFLP community patterns were
compared using Restdist and Neighbor in PHYLIP version 3.67 (Felsenstein, 1989). PHYLOGENETIC ANALYSIS AND DIVERSITY CALCULATIONS All sequences were checked for chimeras using the Chimera
check program at Ribosomal Data Project II (Maidak et al., 2001) and they were excluded from further analysis. Sequences were edited and trimmed using 4Peaks (version 1.7). The approximately
750-nt sequences were aligned using ClustalX (version 1.8; Thompson et al., 1994) and were checked manually using BBEdit Lite (version 6.1). Reference sequences were from GenBank and for
each phylotype the closest match based on a BLAST search (Altschul et al., 1990) of GenBank was selected as a reference sequence. If the closest match was an uncultured clone, we also
included the closest isolated strain. For comparisons, we also searched for environmental 16S rDNA sequence data from other Arctic and Antarctic sites (Priscu et al., 1998; Nadeau _et al._,
2001; Taton et al., 2003, 2006a, 2006b; Jungblut et al., 2005). The genetic differences between the cyanobacterial communities from the clone-libraries were calculated using Unifrac
(Lozupone et al., 2006). Each pair of environments was compared with a weighted Unifrac matrix that takes abundances of different sequences into account using Unifrac Significance test.
Phylogenetic trees were constructed using neighbor-joining with the Kimura-2-Parameter distance matrix (DNAdist, Neighbor) and maximum-likelihood (DNAml) was computed with PHYLIP (version
3.67, _19_). Aligned partial 16S rRNA gene sequences corresponding to _Escherichia coli_ sequence positions 129–775 were used. Confidence levels were calculated for each method by
bootstrapping with 1000 and 100 reassembly events for neighbor-joining and maximum-likelihood, respectively (Seqboot, Consense). One representative for each ribotype was included in the
phylogenetic analysis, and individual ribotypes or Operational Taxonomic Units (OTUs) were defined as groups of sequences, which were at least 97.5% similar (Stackebrandt and Göbel, 1994;
Taton et al., 2003). Library coverage, the Shannon–Wiener diversity index (H'), Chao1 non-parametric richness estimates and rarefaction curves were calculated using DOTUR (Schloss and
Handelsman, 2005) on a Juke–Cantor distance matrix. 16S rRNA gene sequences are available under GenBank accession numbers FJ977098–FJ977164 (Supplementary Table S1). RESULTS ENVIRONMENTAL
PROPERTIES The six collection sites spanned a range of environmental conditions, with overlying water temperatures from 0.9 °C in the WIS meltwater ponds to 6 °C in Pond-A (Supplementary
Table S2). Pond-A had the highest pH among all the sites (8.28), and the lowest pH values were recorded in the meltwater ponds of WIS (6.45) and MIS (6.53). Highest conductivities were at
the ice-based sites, with 637, 384.8 and 269.0 μS cm−1 on MIS, WIS and Q-Lagoon, respectively. Land-based sites had conductivities of 137 μS cm−1 or less. Nutrient concentrations were
highest in Pond-A, with 0.961 mg l−1 total nitrogen and 0.016 mg l−1 total phosphorus. Total nitrogen concentrations were similar between the two ice-shelf sites (0.149 mg l−1 for MIS and
0.156 mg l−1 for WIS), whereas total phosphorus concentrations were 0.009 and 0.014 mg l−1, respectively. The lowest nutrient concentrations were in Q-Lagoon and WH-Lake, with 0.033 and
0.089 mg l−1 total nitrogen, and 0.004 and 0.003 mg l−1 of total phosphorus, respectively. PIGMENT DIVERSITY Each microbial community contained diverse pigments, including chlorophylls,
scytonemins, carotenoids and their degradation products (Supplementary Table S3). Chl.-_a_ concentrations ranged from 3.9 μg cm−2 (Pond-A) to 42.6 μg cm−2 (WIS). Chl.-_b_ was identified in
all the sites except WH-Lake, with concentrations of 3.5 μg cm−2 or less, whereas chl.-_c_ was only identified in Pond-A (0.5 μg cm−2). The cyanobacterial pigment scytonemin and its reduced
derivative, red-scytonemin, were the most abundant pigments in mats from WH-Lake, WIS and MIS, with concentrations of up to 474.8 μg cm−2 (WIS). Low concentrations of scytonemin were
detected in Pond-A, with 0.28 μg cm−2, and none in Q-Lagoon. High concentrations of the carotenoids zeaxanthin, echinenone, β-carotene and a lutein-like carotenoid were present in all mat
samples. The pigments canthaxanthin, fucoxanthin, 19′-hexanoylofucoxanthin and 4-ketomyxol-like carotenoid were only separated in some of the microbial mats, with diatoxanthin; astaxanthin
and diadinoxanthin-like, antheraxanthin-like, monadoxanthin-like, peridinin-like carotenoids identified only in Pond-A mats. MORPHOLOGICAL CLASSIFICATION Microscopic analyses confirmed that
cyanobacteria constituted the greatest proportion of biomass in all of the High Arctic communities. Based on morphological criteria, they were found to be composed of taxa within orders
Chroococcales, Nostocales and Oscillatoriales (Supplementary Table S4). Five known Chroococcales genera were identified (_Gloeocapsa_ cf. _alpina_, _Chroococcus_ cf. _prescotii_,
_Chlorogloea_, _Aphanocapsa_ cf. _hyalina_ and _Merismopedia_ cf. _angularis_), along with one unclassified coccoid morphotype. Genera within order Nostocales included _Nostoc_, _Dichothrix_
and _Tolypothrix_, and within the Oscillatoriales the identified genera were _Leptolyngbya_ (_Leptolyngbya_ cf. _frigida_), _Pseudanabaena_ (_Pseudanabaena_ cf. _amphigranulata_),
_Phormidium_ (_Phormidium autumnale_) and _Oscillatoria_ (_Oscillatoria sancta_). Overall we distinguished six Chroococcales, five Nostocales and 13 Oscillatoriales based on classical
morphological characters. CYANOBACTERIAL 16S RRNA GENE ANALYSIS We constructed targeted 16S rRNA gene clone libraries using genomic environmental DNA from all the sites, yielding a total of
426 clones with the correct insert. Initial RFLP analysis showed that there were much larger differences among sites than between duplicate samples from the same site (Supplementary Figure
S2). The highest diversities were from the three land-based sites, with 12–14 OTUs, defined as >97.5% similarity, per site. Similarly, Chao statistics of the land-based sites ranged from
21.7 to 34.7, while the WIS mats contained only five OTUs (Table 1). However, the six cyanobacterial communities did not differ in pairwise comparisons in a weighted Unifrac matrix (Lozupone
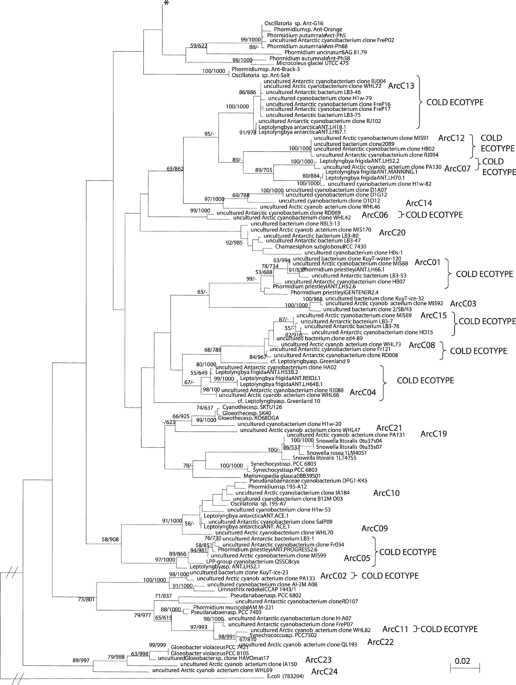
et al., 2006). Six OTUs were from order Chroococcales (Figure 1 and Table 2). Three of these were most similar to cultured representatives: clone ArC22 was up to 99.1% similar to
_Synechococcus_ sp. PCC 7502 (AF448080); ArC20 98.5% to _Chamaesiphon subglobosus_ PCC 7430 (AY170472) and ArC19 98.8% to _Snowella litoralis_ 1LT47S05, AJ781041). The novel ribotype,
ArcC21, had highest similarity of 93.5–93.8% to _Gloeothece_ sp. SK40 (AB067576). Two other ribotypes had 93.1–93.4% (ArC23) and 95.8% (ArC24) similarity to uncultured _Gloeobacter_ sp.
HAVOmat17 (EF032784). Three of the OTUs were within order Nostocales, and one within Stigonematales (Figure 2 and Table 2). Nostocales had the highest sequence similarity to cultured
_Nostoc_ spp., including _Nostoc commune_ KU002 (ArcC17, 98.2–98.4% similarity to AB088375) and _Nostoc_ sp. PCC 7906 (ArcC18, 97.7% similarity to AB325908), and therefore were
conservatively classified as cosmopolitan ribotypes. OTU ArcC16 had less than 94.9% sequence similarity to _Stigonema ocellatum_ SAG 48.90 (AJ544082) and appears to be a novel phylotype
within order Stigonematales. Fifteen of the OTUs grouped within order Oscillatoriales (Figure 2 and Table 2). Eleven of these had highest similarities (97.5% or usually >99) to sequences
from cold environments and seven OTUs (ArcC04–07 and ArcC11–13) were grouped with clones or strains previously identified solely from Antarctic microbial communities, including the Vestfold
and Larsemann Hills (East Antarctica), McMurdo Ice Shelf, Lake Fryxell and Lake Bonney (McMurdo Dry Valleys) (Priscu et al., 1998; Taton et al., 2003; Jungblut et al., 2005). At five of the
Arctic sites, these clones made up between 20.5 and 70% of the total diversity (Figure 3). The second set of OTUs included ArcC01, 08 and 15, and was grouped with sequences from Antarctic
and other cold environments (glaciers and glacial surface snow, Kuytun Glacier 51, Tian Shan Mountains, China; AY493581, AY151728, DQ181742). This set accounted for 12.0–94.4% of the total
diversity. The oscillatorian ribotype ArcC02 shared the highest similarity with an environmental sequence from Kuytun Glacier 51 (surface snow, China; EU263766) and had a relative abundance
of 1.3% in one of the High Arctic sites. All of these three categories of phylotypes were classified as cold ecotypes since they have only been reported from cold habitats to date.
DISCUSSION PHOTOTROPHIC COMMUNITY DIVERSITY Polar cyanobacteria withstand the extremes of their environment through production of photoprotective screening and quenching pigments, as well as
by their highly efficient light-capturing systems, nutrient storage ability and freeze–thaw tolerance (Hawes and Schwarz, 2001; Zakhia et al., 2007). The HPLC pigment signatures of the High
Arctic assemblages that we sampled in the present study provided semi-quantitative information on phototrophic community composition. Chl.-_a_ concentrations in WH-Lake and WIS were similar
to that in earlier reports (Bonilla et al., 2005; Mueller et al., 2005, 2006). Previous studies reported higher concentrations of chl.-_a_ in mats from WIS and MIS than from Antarctic
microbial ice-shelf mats (Howard-Williams et al., 1989, 1990; Mueller et al., 2005). Cyanobacteria-specific markers, such as scytonemin, echinenone, zeaxanthin and canthaxanthin, dominated
the microbial mats in WIS, MIS, WH-Lake and Q-Lagoon, consistent with cyanobacterial dominance of the total phototrophic biomass (Vincent et al., 2004; Bonilla et al., 2005; Mueller et al.,
2005), and similar markers have been reported for Antarctic microbial mats (Vincent et al., 1993). In addition, in some of the mats high concentrations of red-scytonemin, a reduced product
of scytonemin, were detected; scytonemin and red-scytonemin are sheath pigments, which protect cyanobacterial cells against UV-A radiation. Chl.-_b_ and lutein, pigments specific to
Chlorophyta, were identified in most microbial mats. These pigments could potentially be associated with the genera _Mougeotia_, _Zygnema_ and _Cosmarium_, which were identified
morphologically in samples from WH-Lake by Villeneuve et al. (2001). Similarly the Chlorophytes _Chlorosarcinopsis_, _Chlamydomonas_, _Chlamydocapsa_ and _Chlorella_ have been reported
previously from WIS (Mueller et al., 2005). In Pond-A, the most abundant and diverse pigments were specific for Chromophyta, including chl.-_c_2, diadinoxanthin-like, diatoxanthin,
astaxanthin, monadoxanthin-like and fucoxanthin. The second highest concentrations of pigments were characteristic for Chlorophyta in Pond-A, and cyanobacteria-specific signatures were also
identified in Pond-A, however at lower concentrations. Diatoxanthin and fucoxanthin are common in diatoms in particular (Jeffrey et al., 1997). Chromophyta-specific pigments were also
identified in the other microbial mats, however at lower concentrations. Pond-A temperatures were high compared with that in the other sites, and nutrient concentrations were elevated,
potentially due to enrichment by a population of aquatic birds (red-throated loons, _Gavia stellata_) that we observed at this site. MORPHOLOGICAL DIVERSITY As in other Arctic and Antarctic
freshwater ecosystems, mat-forming cyanobacteria were the most conspicuous members of the well-developed benthic communities. Light-microscopy results were similar to that of previous
studies of microbial mat communities from the High Arctic (Bonilla et al., 2005; Mueller et al., 2005). The microbial mat communities were made up of morphospecies within orders
Oscillatoriales, Nostocales and Chroococcales, and were similar to Antarctic microbial mats (Howard-Williams et al., 1989; Taton et al., 2003, 2006a, 2006b; Jungblut et al., 2005).
Morphospecies in Oscillatoriales were the most abundant taxa at all the sites, followed by those in Chroococcales and Nostocales. In particular, morphospecies related to _Leptolyngbya_,
_Pseudanabaena_, _Phormidium, Oscillatoria_ and _Nostoc_ are characteristic of polar mats and form their overall structure (Vincent, 2000). The morphological diversity of Chroococcales was
similar to freshwater ponds in the Larsemann and Vestfold Hills region; Antarctica, however analogous communities on the McMurdo Ice Shelf and in the McMurdo Dry Valleys, lacked any
Chroococcalean morphotypes (Taton et al., 2003; Jungblut et al., 2005). Interestingly, we did not find _Nodularia_ at any of the Arctic sites, even though it has been described regularly for
Antarctic microbial mats, in particular in the McMurdo Ice Shelf, McMurdo Dry Valleys and Larsemann and Vestfold Hills (Taton et al., 2003, 2006a; Jungblut et al., 2005). In contrast,
sequences related to _Gloeobacter_, as found here in the High Arctic, have never been reported from Antarctica. All of these closest matches are to ribotypes from temperate climatic zones,
suggesting the connectivity of Arctic environments to lower latitudes. These findings contrast with data on Antarctic mats from the McMurdo Region, which are conspicuously lacking in
Chroococcales (Taton et al., 2003; Jungblut et al., 2005). BIOGEOGRAPHY OF POLAR CYANOBACTERIA The Polar Regions offer ideal sites for testing microbial endemism since they contain parallel
environments separated by vast geographical distances and potential barriers to dispersal (Staley and Gosink, 1999). Many bacteria and microbial eukaryotes have been identified as possibly
endemic to Antarctica, including several cyanobacterial species (Komárek, 1999; Taton et al., 2006b). However, our clone-library analyses indicate that three taxa previously identified as
Antarctic endemics (_Phormidum priestleyi_ Fritsch, _L. frigida_ (Fritsch) Anagn. and Kom., and _Leptolyngbya antarctica_ (West and West) Anagn. and Kom.; Komárek, 1999; Taton et al., 2006b)
were more than 99% similar to sequences from the Canadian High Arctic (Table 2); for example, ArC05 is 99.6% similar to _P. priestleyi_ (ANT.PROGRESS2.6; AY493585) and ArC13 is 99.8%
similar to _L. antarctica_ (ANT.LH18.1; AY493607). Furthermore, several of the uncultured cyanobacterial clones from East Antarctica and the McMurdo Dry Valleys identified as endemic, had
the highest percentage match, up to 99.9%, to some of our High Arctic sequences. Similarly, clone-library analysis of high-altitude saline wetland mats included a 99% match to _L. frigida_
(ANT.LH701, AY493574) and _L. antarctica_ (ANT.LH18.1, AY493607) based on partial 16S rRNA gene analyses (Dorador et al., 2008). Nadeau et al. (2001) previously reported that within another
clade of Antarctic Oscillatoriales, there was an 11-bp insertion earlier found in a Svalbard soil isolate, which implied a shared evolutionary history. In sum, these findings suggest the
presence of cold-habitat-specific cyanobacterial assemblages, with individual ribotypes that are up to 99.9% similar in the Arctic and Antarctic, and conspicuously absent from other climate
zones. Molecular-clock analysis of several bacterial taxa suggests that a 1% divergence in 16S rRNA gene sequence corresponds to an evolutionary time span of approximately 50 million years
(Moran et al., 1993; Ochman et al., 1999). This would imply that the Arctic and Antarctic ribotypes described here have been isolated or subject to reduced genetic exchange for less than 10
million years. Cyanobacteria isolated from cold environments all have temperature optima growth rates in the range 15–20 °C, suggesting that they likely had their evolutionary origins within
temperate latitudes (Tang et al., 1997; Nadeau et al., 2001) and subsequently colonized perennial cold habitats. Additional analyses using the ITS region (Comte _et al._, 2007), multi-locus
sequence analyses (Whitaker et al., 2003) and broader genomic and metagenomic analyses are needed to determine whether cold-dwelling oscillatorians belong to narrow ecotypes, analogous to
the _Synechococcus_ ecotypes from geothermal springs (Bhaya et al., 2007) and _N. commune_ in Antarctica (Novis and Smissen, 2006). An ecotype may be defined as a group of ecologically
similar cyanobacteria, with genetic diversity within the ecotype limited by a cohesive force, either periodic selection or genetic drift, or both (Cohan and Perry, 2007), where in our case
the environmental force is extreme cold. This corresponds to high-latitude and high-altitude regions where growth of higher plants is severely limited and temperatures are near-zero in
summer (Thomas et al., 2008). At least on a 16S rRNA gene level, cyanobacteria from these cold regions are more related to each other than to those in the temperate groups. Our molecular
findings suggest that microbiota of the cryosphere have been globally distributed with local habitat selection (Baas-Becking, 1934; Finlay and Fenchel, 2004). This could occur via mechanisms
of long-range transport, similar to atmospheric studies documented for microbes, such as bacteria in Saharan dust transported over the Atlantic (Griffin et al., 2002; Gorbushina et al.,
2007), and across Antarctica and the Southern Hemisphere (Hughes et al., 2004; Muñoz et al., 2004). Short-term exchange between the Arctic and Antarctica may be favored by seasonal
oscillation of the Hadley cells, which contributes to inter-hemisphere mixing in the troposphere, as revealed by model analysis of long-lived tracers (Bowman and Cohen, 1997). The present
day distribution may be accentuated over longer time scales (Cermeño and Falkowski, 2009) via global freeze-up events such as the Precambrian glaciations (Kirschvink et al., 2000) and
dispersal of microbiota throughout the cold biosphere. More recent glacial events may have also favored genetic exchange between the Polar Regions, as suggested for cold-water foraminifers
(Darling et al., 2000), although such cooling could also lead to isolation and divergence of some populations (Darling et al., 2004), and dinoflagellates (Montresor et al., 2003). The
dispersal of low-temperature ecotypes may differ from those in other extremes, for example geothermal hot-spring cyanobacteria (Papke et al., 2003; Souza et al., 2008) and hyperthermophiles
such as _Sulfolobus_ (Whitaker et al., 2003) that occupy much more localized as well as distantly separated habitats. Furthermore, cold-adapted cyanobacteria are well equipped to withstand
potential nutrient limitations, temperature fluctuation, dehydration and elevated UV radiation during long-distance aerial transport. As a result, cold-adapted cyanobacteria may show much
reduced genetic divergence in comparison with the known degree of diversification of microbial taxa at the other thermal extreme; for example, _Sulfolobus_ endemism in hot springs (Whitaker
et al., 2003). Global circulation models currently predict accelerated warming and massive contraction of glacial environments over the next few hundred years (IPCC, 2007), which may force
the cold ecotypes identified here into similarly localized habitats or extinction. ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * AF076165 * AY151728 * DQ181675 * DQ181677 * DQ181681 *
DQ181686 * DQ181742 * EF032784 * EU263766 * EU263774 * EU263787 * EU753634 * FJ977098 * FJ977164 REFERENCES * Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ . (1990). Basic local
alignment search tool. _J Mol Biol_ 215: 403–410. Article CAS Google Scholar * Anagnostidis K, Komárek J . (1988). Modern approach to the classification system of cyanophytes:
3—Oscillatoriales. _Arch Hydrobiol Suppl_ 50: 327–472. Google Scholar * Anagnostidis K, Komárek J . (1990). Modern approach to the classification of system of cyanophytes: 5—Stigonematales.
_Arch Hydrobiol Suppl_ 86: 1–73. Google Scholar * Baas-Becking LGM . (1934). Geobiologie of inleiding tot de milieukunde. In: van Stockum WP, Zoon NV (eds). The Hague: Netherlands. * Bhaya
D, Grossman A, Steunou A-S, Khuri N, Cohan FM, Hamamura N _et al_. (2007). Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic
analyses. _ISME J_ 1: 703–713. Article CAS Google Scholar * Bonilla S, Villeneuve V, Vincent WF . (2005). Benthic and planktonic algal communities in a high Arctic lake: pigment structure
and contrasting responses to nutrient enrichment. _J Phycol_ 41: 1120–1130. Article CAS Google Scholar * Bowman KP, Cohen PJ . (1997). Interhemispheric exchange by seasonal modulation of
the Hadley circulation. _J Atmos Sci_ 54: 2045–2059.s. Article Google Scholar * Cermeño P, Falkowski PG . (2009). Controls on diatom biogeography in the ocean. _Science_ 325: 1539–1541.
Article Google Scholar * Cohan FM, Perry E . (2007). A systematics for discovering the fundamental units of bacterial diversity. _Curr Biol_ 17: 373–386. Article Google Scholar * Comte
K, Sabacka M, Carré-Mlouka A, Elster J, Komárek J . (2007). Relationships between the Arctic and the Antarctic cyanobacteria; three _Phormidium_-like strains evaluated by a polyphasic
approach. _FEMS Microbiol Ecol_ 59: 366–376. Article CAS Google Scholar * Darling KF, Kucera M, Pudsey CJ, Wade CM . (2004). Molecular evidence links cryptic diversification in polar
planktonic protists to quaternary climate dynamics. _Proc Natl Acad Sci USA_ 101: 7657–7662. Article CAS Google Scholar * Darling KF, Wade CW, Stewart IA, Kroon D, Dingle R, Leigh Brown
AJ . (2000). Molecular evidence for genetic mixing of Arctic and Antarctic subpolar populations of planktonic foraminifers. _Nature_ 405: 43–47. Article CAS Google Scholar * Dorador C,
Vila I, Imhoff JF, Witzel KP . (2008). Cyanobacterial diversity in Salar de Huasco, a high altitude saline wetland in northern Chile: an example of geographical dispersion? _FEMS Microbiol
Ecol_ 64: 419–432. Article CAS Google Scholar * Finlay BJ, Fenchel T . (2004). Cosmopolitan metapopulations of free-living microbial eukaryotes. _Protists_ 155: 237–244. Article Google
Scholar * Felsenstein J . (1989). PHYLIP—phylogeny inference package (version 3.2). _Cladistics_ 5: 164–166. Google Scholar * Garcia-Pichel F, Sherry ND, Castenholz RW . (1992). Evidence
for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium _Chlorogloeopsis_ sp. _Photochem Photobiol_ 56: 17–23. Article CAS Google
Scholar * Geitler L . (1932). Cyanophyceae. In: Kolkwitz R (ed). _Rabenhorst's Kryptogamenflora von Deutschland, Österreich und der Schweiz_. Akademische Verlagsgesellschaft: Leipzig,
pp 1–1196. Google Scholar * Gorbushina AA, Kort R, Schulte A, Lazarus D, Schnetger B, Brumsack HJ _et al_. (2007). Life in Darwin's dust: intercontinental transport and survival of
microbes in the nineteenth century. _Environ Microbiol_ 9: 2911–2922. Article CAS Google Scholar * Griffin DW, Kellogg CA, Garrison VH, Shinn EA . (2002). The global transport of dust.
_Am Sci_ 90: 228–235. Article Google Scholar * Hawes I, Schwarz A-M . (2001). Absorption and utilization of irradiance by cyanobacterial mats in two ice-covered Antarctic lakes with
contrasting light climates. _J Phycol_ 37: 5–15. Article CAS Google Scholar * Howard-Williams C, Pridmore R, Downes M, Vincent WF . (1989). Microbial biomass, photosynthesis and
chlorophyll _a_ related pigments in the ponds of the McMurdo Ice Shelf, Antarctica. _Antarct Sci_ 1: 125–131. Article Google Scholar * Howard-Williams C, Pridmore R, Broady P, Vincent WF .
(1990). Environmental and biological variability in the McMurdo Ice Shelf ecosystem. In: Kerry K, Hempel G (eds). _Antarctic Ecosystems. Ecological Change and Conservation_. Springer
Verlag: Berlin, pp 23–31. Chapter Google Scholar * Hughes KA, McCartney HA, Lachlan-Cope TA, Peace DA . (2004). A preliminary study of airborne microbial biodiversity over Peninsular
Antarctica. _Cell Mol Biol_ 50: 537–542. CAS PubMed Google Scholar * Intergovernmental Panel on Climate Change (IPCC), Climate Change (2007). The Physical science basis. In: Solomon _et
al._ (eds). _Working Group I Contribution to the Fourth Assessment Report of the IPCC_. Cambridge University Press: Cambridge. * Jeffrey SW, Mantoura RFC, Wright SW . (1997). _Phytoplankton
Pigments in Oceanography_. SCOR UNESCO: Paris. Google Scholar * Jungblut AD, Hawes I, Mountfort D, Hitzfeld B, Dietrich DR, Burns BP _et al_. (2005). Diversity within cyanobacterial mat
communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. _Environ Microbiol_ 7: 519–529. Article CAS Google Scholar * Kirschvink JL, Gaidos EJ, Bertani LE,
Beukes NJ, Gutzmer J, Maepa LN _et al_. (2000). Paleoproterozoic snowball earth: extreme climatic and geochemical global change and its biological consequences. _Proc Natl Acad Sci USA_ 97:
1400–1405. Article CAS Google Scholar * Komárek J . (1999). Diversity of cyanoprokaryotes (cyanobacteria) of King George Island, maritime Antarctica—a survey. _Arch Hydrobiol_ 94:
181–193. Google Scholar * Komárek J, Anagnostidis K . (1989). Modern approach to the classification system of cyanophytes: 4—Nostocales. _Arch Hydrobiol Suppl_ 82: 247–345. Google Scholar
* Komárek J, Anagnostidis K . (1998). _Cyanoprokaryota 1. Teil Chroococcales_. Gustav Fischer Verlag, Jena. Google Scholar * Lozupone C, Hamady M, Knight R . (2006). UniFrac—an online tool
for comparing microbial community diversity in a phylogenetic context. _BMC Bioinformatics_ 7: 371–385. Article Google Scholar * Maidak BL, Cole JR, Lilburn TG, Parker CTJ, Saxman PR,
Farris RJ _et al_. (2001). The RDP-II (Ribosomal Databse Project). _Nucleic Acids Res_ 29: 173–174. Article CAS Google Scholar * Montresor M, Lovejoy C, Orsini L, Procaccini G, Roy S .
(2003). Bipolar distribution of the cyst-forming dinoflagellate _Polarella glacialis_. _Polar Biol_ 26: 186–194. Google Scholar * Moran NA, Munson MA, Baumann P, Ishikawa H . (1993). A
molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. _Proc R Soc Lond Ser B_ 253: 167–171. Article Google Scholar * Mueller DR, Vincent WF . (2006). Microbial
habitat dynamics and ablation control on the Ward Hunt Ice Shelf. _Hydrol Proc_ 20: 857–876. Article CAS Google Scholar * Mueller DR, Vincent WF, Bonilla S, Laurion I . (2005).
Extremotrophs, extremophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. _FEMS Microbiol Ecol_ 53: 73–87. Article CAS Google Scholar * Mueller DR, Vincent
WF, Jeffries MO . (2006). Environmental gradients, fragmented habitats, and microbiota of a northern ice shelf cryoecosystem, Ellesmere Island, Canada. _Arct Antarct Alp Res_ 38: 593–607.
Article Google Scholar * Muñoz J, Felicísimo ÁM, Cabezas F, Burgaz AR, Martínez I . (2004). Wind as a long-distance dispersal vehicle in the southern hemisphere. _Science_ 304: 1144–1147.
Article Google Scholar * Nadeau TL, Milbrandt EC, Castenholz RW . (2001). Evolutionary relationships of cultivated Antarctic Oscillatorians (cyanobacteria). _J Phycol_ 37: 650–654. Article
Google Scholar * Novis PM, Smissen RD . (2006). Two genetic and ecological groups of _Nostoc commune_ in Victoria Land, Antarctica, revealed by AFLP analysis. _Antarct Sci_ 18: 573–581.
Article Google Scholar * Ochman H, Elwyn S, Moran NA . (1999). Calibrating bacterial evolution. _Proc Natl Acad Sci USA_ 96: 12638–12643. Article CAS Google Scholar * Papke RT, Ramsing
NB, Bateson MM, Ward DM . (2003). Geographical isolation in hot spring cyanobacteria. _Environ Microbiol_ 5: 650–659. Article CAS Google Scholar * Priscu JC, Fritsen CH, Adams EE,
Giovannoni SJ, Paerl HW, McKay CP _et al_. (1998). Perennial Antarctic lake ice: an oasis for life in a polar desert. _Science_ 280: 2095–2098. Article CAS Google Scholar * Schloss PD,
Handelsman J . (2005). Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. _Appl Environ Microbiol_ 71: 1501–1506. Article CAS
Google Scholar * Souza V, Eguiarte LE, Siefert J, Elser JJ . (2008). Microbial endemism: does phosphorus limitation enhance speciation? _Nat Rev Microbiol_ 6: 559–564. Article CAS Google
Scholar * Stackebrandt E, Göbel BM . (1994). A place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. _Int J Syst Bacteriol_ 44:
846–849. Article CAS Google Scholar * Staley JT, Gosink JJ . (1999). Poles apart: biodiversity and biogeography of sea ice bacteria. _Annu Rev Microbiol_ 53: 189–215. Article CAS Google
Scholar * Strainton M, Capel MJ, Armstrong FA . (1977). _The Chemical Analysis of Fresh Water_ (Fish. Mar. Serv. Misc. Spec. Publ. 25, 2nd edn.) Canadian Department of Fisheries and
Oceans: Winnipeg, Canada. Google Scholar * Tang ET, Tremblay R, Vincent WF . (1997). Cyanobacterial dominance of polar freshwater ecosystems: are high-latitude mat-formers adapted to low
temperatures. _J Phycol_ 33: 171–181. Article Google Scholar * Taton A, Grubisic S, Brambilla E, de Wit R, Wilmotte A . (2003). Cyanobacterial diversity in natural and artificial microbial
mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. _Appl Environ Microbiol_ 69: 5157–5169. Article CAS Google Scholar * Taton A, Grubisic S,
Balthazart P, Hodgson DA, Laybourn-Parry J, Wilmotte A . (2006a). Biogeographical distribution and ecological range of benthic cyanobacteria in East Antarctic lakes. _FEMS Microbiol Ecol_
57: 272–289. Article CAS Google Scholar * Taton A, Grubisic S, Ertz D, Hodgson DA, Piccardi R, Biondi N _et al_. (2006b). Polyphasic study of Antarctic cyanobacterial strains. _J Phycol_
42: 1257–1270. Article CAS Google Scholar * Taton A, Hoffmann L, Wilmotte A . (2008). Cyanobacteria in microbial mats of Antarctic lakes (East Antarctica)—a microscopical approach.
_Algological Studies_ 126: 173–208. Article Google Scholar * Thomas DN, Fogg GE, Convey P, Fritsen CH, Gili J-M, Gradinger R _et al_. (2008). _The Biology of the Polar Regions_. Oxford
University Press: Oxford, pp 394. Book Google Scholar * Thompson JD, Higgins DG, Gibson TJ . (1994). CLUSTALw; improving the sensitivity of progressive sequence alignment through sequence
weighting, position specific gap penalties and weight martix choice. _Nucleic Acids Res_ 22: 4673–4680. Article CAS Google Scholar * Tillett D, Neilan BA . (2000). Xanthogenate nucleic
acid isolation from cultured and environmental cyanobacteria. _J Phycol_ 36: 251–258. Article CAS Google Scholar * Villeneuve V, Vincent WF, Komárek J . (2001). Community structure and
microhabitat characteristics of cyanobacterial mats in an extreme high Arctic environment, Ward Hunt Lake. _Nova Hedw_ 123: 199–224. Google Scholar * Vincent WF . (2000). Cyanobacterial
dominance in the Polar Regions. In: Whitton BA, Potts M (eds). _The Ecology of Cyanobacteria_. Kluwer Academic Publisher: the Netherlands, pp 321–340. Google Scholar * Vincent WF, Downes
MT, Castenholz RW, Howard-Wiliams C . (1993). Community structure and pigment organisation of cyanobacteria-dominated microbial mats in Antarctica. _Eur J Phycol_ 28: 213–221. Article
Google Scholar * Vincent WF, Mueller DR, Bonilla S . (2004). Ecosystems on ice: the microbial ecology of Markham Ice Shelf in the high Arctic. _Cryobiol_ 48: 103–122. Article Google
Scholar * Ward DM, Cohan FM, Bhaya D, Heidelberg JF, Kühl M, Grossman A . (2008). Genomics, environmental genomics and the issue of microbial species. _Heredity_ 100: 207–219. Article CAS
Google Scholar * Whitaker RJ, Grogan DW, Taylor JW . (2003). Geographic barriers isolate endemic populations of hyperthermophilic archaea. _Science_ 301: 976–978. Article CAS Google
Scholar * Zakhia F, Jungblut AD, Taton A, Vincent WF, Wilmotte A . (2007). Cyanobacteria in cold environments. In: Margesin R, Schinner F, Marx JC, Gerday C (eds). _Psychrophiles: from
Biodiversity to Biotechnology_. Springer-Verlag: Berlin, pp 121–135. Google Scholar * Zapata M, Rodriguez F, Garrido JL . (2000). Separation of chlorophylls and carotenoids from marine
phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine containing mobile phases. _Mar Ecol Prog Ser_ 195: 29–45. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We acknowledge financial support from the Natural Sciences and Engineering Research Council, the Canada Research Chair in Aquatic Ecosystem Studies, the Network of Centres
of Excellence program ArcticNet and the International Polar Year Programme MERGE. Logistical support was supplied by the Polar Continental Shelf Project (this is PCSP publication number
04109). Field assistance was provided by Denis Sarrazin, Julie Veillette, Caroline Chénard, Dermot Antoniades, Jérémie Pouliot and Alexandra Pontefract. We also thank the staff of
Quttinirpaaq National Park, Parks Canada, for support and facilities, Marie-Josée Martineau for laboratory assistance and four anonymous reviewers for insightful comments and suggestions.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Département de Biologie and Centre d'Etudes Nordiques, Université Laval, Quebec City, Quebec, Canada Anne D Jungblut & Warwick F
Vincent * Département de Biologie, Québec-Océan, and Institut de biologie intégrative et des systèmes (IBIS), Université Laval, Quebec City, Quebec, Canada Connie Lovejoy Authors * Anne D
Jungblut View author publications You can also search for this author inPubMed Google Scholar * Connie Lovejoy View author publications You can also search for this author inPubMed Google
Scholar * Warwick F Vincent View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Anne D Jungblut. ADDITIONAL
INFORMATION Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej) SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 (PPT 746 KB)
SUPPLEMENTARY FIGURE S2 (PPT 71 KB) SUPPLEMENTARY TABLE S1 (DOC 20 KB) SUPPLEMENTARY TABLE S2 (DOC 32 KB) SUPPLEMENTARY TABLE S3 (DOC 78 KB) SUPPLEMENTARY TABLE S4 (DOC 61 KB) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jungblut, A., Lovejoy, C. & Vincent, W. Global distribution of cyanobacterial ecotypes in the cold biosphere.
_ISME J_ 4, 191–202 (2010). https://doi.org/10.1038/ismej.2009.113 Download citation * Received: 09 July 2009 * Revised: 28 September 2009 * Accepted: 28 September 2009 * Published: 05
November 2009 * Issue Date: February 2010 * DOI: https://doi.org/10.1038/ismej.2009.113 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * 16S rRNA
gene * biogeography * cyanobacteria * dispersal * microbial mats * polar








