- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Osteogenesis imperfecta (OI) comprises a group of inherited disorders characterized by bone fragility and increased susceptibility to fractures. Historically, the laboratory
confirmation of the diagnosis OI rested on cultured dermal fibroblasts to identify decreased or abnormal production of abnormal type I (pro)collagen molecules, measured by gel
electrophoresis. With the discovery of _COL1A1_ and _COL1A2_ gene variants as a cause of OI, sequence analysis of these genes was added to the diagnostic process. Nowadays, OI is known to be
genetically heterogeneous. About 90% of individuals with OI are heterozygous for causative variants in the _COL1A1_ and _COL1A2_ genes. The majority of remaining affected individuals have
recessively inherited forms of OI with the causative variants in the more recently discovered genes _CRTAP, FKBP10, LEPRE1,PLOD2, PPIB, SERPINF1, SERPINH1_ and _SP7_, or in other yet
undiscovered genes. These advances in the molecular genetic diagnosis of OI prompted us to develop new guidelines for molecular testing and reporting of results in which we take into account
that testing is also used to ‘exclude’ OI when there is suspicion of non-accidental injury. Diagnostic flow, methods and reporting scenarios were discussed during an international workshop
with 17 clinicians and scientists from 11 countries and converged in these best practice guidelines for the laboratory diagnosis of OI. SIMILAR CONTENT BEING VIEWED BY OTHERS GENETIC BASIS
OF OSTEOGENESIS IMPERFECTA FROM A SINGLE TERTIARY CENTRE IN SOUTH AFRICA Article Open access 15 December 2023 TYPE-I COLLAGEN PRODUCED BY DISTINCT FIBROBLAST LINEAGES REVEALS SPECIFIC
FUNCTION DURING EMBRYOGENESIS AND OSTEOGENESIS IMPERFECTA Article Open access 10 December 2021 GENETIC LANDSCAPE AND PHENOTYPIC SPECTRUM OF OSTEOGENESIS IMPERFECTA IN THE KAZAKHSTANI
PEDIATRIC POPULATION Article Open access 02 April 2025 INTRODUCTION CLASSIFICATION OF OSTEOGENESIS IMPERFECTA Osteogenesis imperfecta (OI) comprises a heterogeneous group of disorders
characterized by susceptibility to bone fractures with severity that ranges from death in the perinatal period to subtle increase in fracture frequency and in almost all cases presumed or
proven defects in type I (pro)collagen biosynthesis.1 In 1979, David Sillence developed a four-type classification, which is still in use for classification according to
clinical/radiological features: OI type I (mild OI with bone fragility and blue sclerae), II (perinatal lethal), III (progressive deforming) and IV (normal sclerae and mild deformity).2, 3
Dominant _COL1A1/2_ variants appeared to be causative in the majority of OI types. In 2004, OI types V and VI were added to this classification because of specific clinical/radiological
and/or histological features, absence of abnormalities of type I (pro)collagen synthesis or structure on gel electrophoresis and absence of causative variants in the _COL1A1/2_ genes.4, 5
With the discovery of rare recessive genetic causes of OI (see Table 1), it was proposed to extend the classification with OI types VII and VIII.6 However, the classification and subdivision
into different types of OI is still under discussion1 because the phenotypic spectrum that results from mutations in some of these genes is almost as broad as that with mutations in type I
collagen genes. There is also debate as to whether Bruck syndrome type I7 and II,8 characterized clinically by bone fragility and congenital contractures of the large joints, should be
classified as a type of OI. BIOSYNTHESIS OF TYPE I COLLAGEN Most individuals affected with OI are heterozygous for a causative variant in either of the two genes, _COL1A1_ or _COL1A2,_ which
encode the pro_α_1(I) and pro_α_2(I) chains of type I procollagen, respectively. Type I collagen is the major structural protein in bone, tendon and ligament. Recently, rare recessive
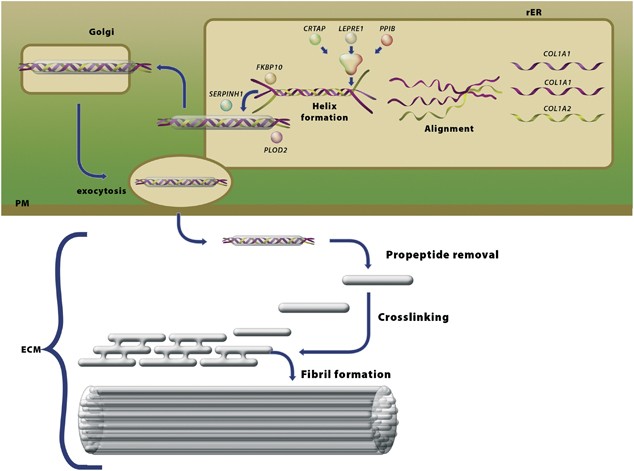
genetic causes of OI have been described and, in all but one, the identified genes encode proteins involved in the biosynthesis of type I procollagen (Figure 1, Tables 1 and 2).9, 10 GENES,
PROTEINS AND CAUSATIVE VARIANTS AUTOSOMAL DOMINANT OI The most common form of OI in most populations is OI type I. Cultured dermal fibroblasts from affected individuals produce about half
the normal amount of type I procollagen molecules, which have normal structure. OI type I usually results from variants in one _COL1A1_ allele (frameshift, nonsense and splice-site
alterations) that lead to mRNA instability and haploinsufficiency. In a small subset of individuals with OI type I, substitutions for glycine by small amino acids (cysteine, alanine and
serine) near the amino terminal ends of the triple-helical domains of either _COL1A1_ or _COL1A2_ are found. In contrast, OI types II–IV are usually caused by sequence variants in either
_COL1A1_ or _COL1A2_ that result in substitutions for glycine residues in the uninterrupted Gly-X-Y triplet repeat of the 1014-residue triple-helical domains encoded by each gene. Less
common causative variants include splice-site alterations, variants in the carboxyl-terminal propeptide coding-domains or insertion/deletion events that lead to in-frame sequence
alterations. Most of these variants result in synthesis of abnormal type I procollagen molecules characterized by post-translational over-modification of the triple-helical domain, which
results in alterations visible by SDS-polyacrylamide gel electrophoresis. Loss of expression of either gene because of large-scale deletions appears to be rare.11, 12, 13, 14, 15 More than
1000 distinct variants in the _COL1A1_ and _COL1A2_ genes have been identified that cause OI (R Dalgleish: Osteogenesis Imperfecta Variant Database (https://oi.gene.le.ac.uk, accessed 3 May
2011).16, 17 AUTOSOMAL RECESSIVE OI INCLUDING BRUCK SYNDROME In the last 5 years, candidate–gene approaches have identified several loci in which causative variants have been identified
which result in the long-sought causes for recessively inherited forms of OI (see Figure 1, Table 2). We classify these genes in four groups—(i) one in which the genes encode proteins that
contribute to the initial phase of chain recognition and propagation of molecular folding (_CRTAP, LEPRE1_ and _PPIB_)18, 19, 20, 21, 22, 23, 24, 25, 26 the second in which the genes encode
proteins involved in the final quality control of type I procollagen (_FKBP10_27, 28, 29 and _SERPINH1_30), the third, which involves a gene encoding proteins involved in late modification
and crosslink formation (_PLOD2_8, 31) and the fourth group, which involves recently recognized genes encoding proteins involved in bone cell differentiation (_SP7_32 and possibly
_SERPINF1_33). REASONS FOR REFERRAL PRENATAL Prenatal ultrasounds showing abnormalities suggestive of OI (diminished mineralization of skull, platyspondyly or bowing, shortening and/or
fractures of long bones) are an important reason for referral for molecular diagnostics of OI. POSTNATAL Radiographs at birth suggestive of OI, recurrent and/or unexplained fractures with or
without suspicion of non-accidental injury (NAI), primary (idiopathic) low bone mass, preferably with exclusion of secondary causes, a family history of OI and request for confirmation of
the clinical diagnosis are all reasons for referral for molecular diagnostics of OI. However, in some cases dentinogenesis imperfecta or even blue sclerae might be the only reason for
referral. As such, physicians from many specialties (gynaecologist, paediatrician, orthopaedic surgeon, clinical geneticist, general practitioner, dentist, ear nose throat specialist,
endocrinologist, internist and ophthalmologist) might refer a patient for molecular analysis. Depending on the age of presentation, OI can be difficult to distinguish from some other genetic
conditions, for example, Ehlers–Danlos syndrome arthrochalasis type (former EDS VIIA and B), isolated dentinogenesis imperfecta, blue sclerae and corneal fragility, hypophosphatasia, and
non-genetic causes of fractures including NAI and idiopathic juvenile osteoporosis.2, 34, 35, 36 NON-ACCIDENTAL INJURY Many referrals for OI diagnostics occur in the context of suspected NAI
in an attempt to exclude a genetic cause of fractures. Fractures resulting from NAI occur in 24 per 10 000 children under 3 years of age whereas the OI prevalence is 1 per 10 000–20 000.34
The incidence of OI among children evaluated for NAI is 2–5%.34 Differentiation is aided by an experienced clinician and radiologist familiar with OI36 as the nature and localization of
fractures in OI and NAI can often be distinguished.37 However, because injuries may involve infants before many of the clinical features of OI are apparent, laboratory diagnostics for OI can
certainly be helpful in this differentiation. MATERIALS AND METHODS A group of clinicians and scientists involved in OI diagnostics met on 28–29 June 2010 at a workshop in Amsterdam, the
Netherlands, to formulate Best Practice Guidelines for the molecular genetic diagnosis of OI supported by the European Molecular Genetics Quality Network (EMQN). RESULTS Discussions focussed
on diagnostic flow, methodologies, interpretation of results and reporting. Consensus guidelines were established. DISCUSSION THE DIAGNOSTIC FLOW The ‘traditional’ way to establish or
confirm the diagnosis of OI is based on studies of collagens synthesized by cultured dermal fibroblasts. Previous studies35 demonstrated that either quantitative or qualitative alterations
can be identified in about 90%38 of individuals with clinically confirmed OI. Biochemical analysis will separate individuals with quantitative defects (OI type I), from those with
qualitative defects (OI types II–IV) because of causative variants in the _COL1A1/2_, _CRTAP_, _LEPRE1_, _PPIB_ genes and those with no abnormalities detected. Electrophoretic analysis of
type I procollagen may also identify other disorders that can mimic some aspects of OI: EDS kyphoscoliotic type (type VIA), EDS arthrochalasia type (types VIIA and VIIB) and EDS
dermatosparaxis type (type VIIC).11 Also, cultured fibroblasts provides a resource for the analysis of RNA splicing and unclassified variants. However, biochemical analysis will not identify
some quantitative defects of type I procollagen, certain causative variants that alter sequences in some coding regions of the _COL1A1/COL1A2_ genes and recessive forms of OI (including
Bruck syndrome) that result from variants in _FKBP10, PLOD2, SERPINF1, SERPINH1_ or _SP7_. Furthermore, prenatal diagnosis by biochemical analysis is only possible in chorionic villus cells
and is effective with qualitative alterations9 but unsuitable for quantitative defects and delays time of diagnosis by 2–4 weeks compared with genetic analysis. In contrast, direct genomic
analysis (sequencing) of the known genes should identify causative variants in >95% of affected individuals in most populations.39, 40 Next generation sequencing has gained importance in
the laboratory diagnosis of OI – in part because of shorter time to diagnosis and in part because of the added value of the information as well as reduced costs, which is important for the
precise determination of recurrence risk (autosomal dominant versus recessive), prenatal diagnosis and pre-implantation genetic diagnosis. Given these considerations and current facilities,
the consensus of the EMQN Best Practice in OI meeting was to initiate laboratory-based diagnostic studies with direct genomic sequencing of the type I procollagen genes, _COL1A1_ and
_COL1A2_. The approach to diagnosis is detailed in Figure 2. It was agreed that the ‘traditional’ approach can still be used in the context in which genomic DNA sequencing is unavailable or
the financial barriers are high. Moreover, analysis of proteins and mRNA/cDNA from cultured fibroblasts remain valuable tools for follow-up studies. EXPLANATION OF DIAGNOSTIC WORK FLOW
SEQUENCING OF ALL TYPE I PROCOLLAGEN GENE EXONS Procollagen type I gene sequencing should identify causative variants in 90% of affected individuals,39, 40 provided that the clinical
diagnosis of OI is accurate. In some cases a follow-up study is needed to determine whether a variant is causative. This will often include genetic studies in the parents with correlation to
the phenotype observed in the parent, or analysis of mRNA splicing and protein-based biochemical studies on cultured dermal fibroblasts. If no causative variant in the _COL1A1/2_ genes is
identified by sequence analysis, the next step is to determine if a deletion or duplication of some or all of the coding regions of either gene has occurred. Strategies such as array-based
analysis, MLPA or qPCR if properly validated are considered equivalent by the working group in their detection of such alterations. From currently available data in the represented
laboratories, the added causative variants expected from this approach should be about 1–2%. In case of OI types II–IV, after identifying the causative variant in the index patient,
determination of parental mosaicism by sequence analysis of DNA from blood from each parent will provide data for genetic counselling with respect to recurrence risk and care in subsequent
pregnancies. RE-REVIEW OF CLINICAL DATA AND THE QUESTION OF NAI When no causative _COL1A1/2_ variant is found, clinical priorities should be re-considered. Failure to find a causative
variant occurs if there is technical failure, or if no causative variant is present in these genes (because of causative variants in other (un)known genes or because the patient does not
have OI). Therefore, referring physicians should be encouraged to provide a completed clinical checklist (Supplementary Table 1) and X-rays of the patient. This clinical/radiological
information should be reviewed by a clinical geneticist or a clinician with experience in OI. This can help to determine the likelihood of OI in the particular patient. With clear evidence
of OI, further analysis should proceed (Figure 2). However, if the primary reason for genetic study is to identify children with OI among the larger group suspected for NAI, analysis can
reasonably stop after _COL1A1/2_ sequencing unless there is a strong suggestion of consanguinity or recessive inheritance. Justification of this decision rests on the following
considerations. First, previous studies indicate that fewer than 5% of infants studied for suspicion of NAI are found to have OI by biochemical or DNA-based studies.34 Second, DNA-based
analysis will identify a causative variant in >90% of all individuals with OI39, 40 so that the remaining risk that an infant has OI, will be about 0.5%. Third, at present all infants
with recessive OI have obvious radiographic abnormalities fitting a diagnosis of OI and not of NAI. IDENTIFICATION AND CHARACTERIZATION OF CAUSATIVE VARIANTS IN AUTOSOMAL RECESSIVE OI
Variants in the genes causing recessive OI are estimated to account for about 5 or 6% of individuals with OI. This represents the pooled estimates from all the laboratories represented at
the workshop. Although laboratory context and clinical criteria may in some cases require otherwise, the preferred strategy is to analyse all genes at the same time to minimize turnaround
time. If no, or only one, causative variant is identified by sequence analysis, the next step is to determine by array-based analysis, MLPA or qPCR if a deletion or duplication of some or
all of the coding regions of either allele has occurred. Once recessive causative variants are identified, parental confirmation of carrier status should be completed, first to confirm that
the causative variants in the index case are inherited in _trans_ and, second, to exclude the possibility of uniparental disomy in the case of homozygosity in the infant. ANALYSIS OF
PROTEINS AND MRNA/CDNA FROM CULTURED FIBROBLASTS (FUNCTIONAL ANALYSIS) By following the guidelines to this point, virtually all causative variants in type I procollagen genes and in the
recessive OI-related genes will have been identified. However, in some cases analysis of proteins and mRNA/cDNA from cultured fibroblasts can have an additive value. First of all, mRNA/cDNA
analysis provides a tool for studying the effect of unclassified variants suspected to alter splicing. Moreover, this approach can unravel the effect of deep intronic variants that alter the
mRNA sequences usually by conversion of cryptic exons to active exons and which would not be detected by gDNA sequencing. In these cases, biochemical analysis of type I procollagen chains
can detect quantitative defects if the new exon results in introduction of a premature stop codon and nonsense-mediated mRNA decay, or qualitative defects if the mRNA is stable and
translated into an abnormal protein. When OI type I is suspected, but genomic DNA sequencing is unavailable or too costly, _COL1A1_ null-allele14 testing on cDNA can be used to confirm the
diagnosis. However, this approach relies on the availability of informative markers and will not lead to the identification of the disease-causing variant. ANALYSIS OF REMAINING SAMPLES Some
diagnostic laboratories maintain research arms and the identification of the additional OI-related genes has profited from the repositories of cells and DNA samples from individuals with OI
because of unknown genetic causes. This practice should continue and cells and DNA samples should be banked for future analysis. If the diagnostic laboratory does not maintain such a
resource, banking with ones that do, should be considered. LABORATORY DIAGNOSIS OF OI MOLECULAR ANALYSIS FOR OI Molecular analysis for OI includes sequencing or mutation scanning and
deletion/duplication testing for _COL1A1_ and _COL1A2,_ followed by sequencing of recessive OI-related genes (see Table 1). Preferred material for testing is genomic DNA from blood, but
other sources can be used as well (see Tables 3a and b). For general issues regarding sample labelling and identification, sequence analysis and interpretation see ‘Practice guidelines for
Sanger Sequencing Analysis and Interpretation’ (http://www.cmgs.org/BPGs/pdfs%20current%20bpgs/Sequencingv2.pdf). GDNA AND CDNA SEQUENCING Current practices in sequencing of the type I
collagen genes differ among laboratories in that some use exon-by-exon amplification followed by sequence determination (Supplementary Appendix Tables 3A and B) and others use a multi-exon
substrate for analysis. If properly validated, both will provide the same mutation capture probability and their use should be determined by local preferences. To identify splice variants,
primer sequences for PCR used for direct sequencing should be designed far enough from intron–exon boundaries to allow reading of the branch sites, consensus splice donor and acceptor sites
or UTR sequences of acceptable quality. With regard to sequence analysis of cDNA, large fragments should be analysed to allow detection of multi-exon deletions, because these could be missed
when primers are located close to the exon junctions at multiple sites. The analysis of cDNA from fibroblasts is warranted when an unknown variant is found that might affect splicing. In
addition, cDNA sequence analysis may detect null-alleles without a known cause (eg, an unknown variant in the promoter region) if care is taken to analyse regions in which coding sequence
heterozygosity is known. QUANTITATIVE ANALYSIS Quantitative analysis by MLPA (kits for _COL1A1_ and _COL1A2_ have been designed by MRC-Holland, available at http://www.mlpa.com) and qPCR is
particularly important in cases without a detected molecular cause in which only genomic DNA is available and without heterozygous polymorphic variants detected by sequencing. Recently,
complete _COL1A1_ allele deletions have been reported to cause OI type I.13 For reliable quantitative testing (MLPA/qPCR) patient and control materials from the same source (eg, blood or
fibroblasts) should be used for comparison and the same DNA purification protocol should be used for all samples within a test series. PROTEIN ANALYSIS Protein analysis of type I
(pro)collagen (Supplementary Appendix Figures 3A–D) is used to detect quantitative and qualitative changes. The method used for this analysis is based on _in vitro_ labelling of collagen in
cultured fibroblasts, followed by electrophoresis on 2 M urea SDS-PAGE gels (the 2 M urea enhances chain separation) and autoradiography.41 The analysis of procollagens is achieved by
omitting the pepsin digestion step from the collagen protocol. Procollagen electrophoresis enhances detection sensitivity of quantitative type I (pro)collagen defects (OI type I), visualizes
defects in type I (pro)collagen located in the N-terminal region of the type I collagen triple helix more clearly and distinguishes between EDS VIIC and EDS VIIA and B (if samples are
analysed after labelling in the presence and absence of dextran sulphate to enhance proteolytic processing).42 PRENATAL DIAGNOSIS Prenatal diagnosis is possible in case of identification of
known disease-causing variant(s) both on genomic DNA extracted from chorionic villus sample (CVS) cells and amniocytes. ‘Testing for Maternal Cell Contamination (MCC) in Prenatal Samples for
Molecular Studies’ (http://www.cmgs.org/BPGs/pdfs%20current%20bpgs/MCC_08.pdf) should be applied. In those rare instances when post-translational over-modification of (pro)collagen type I
is visible in, for example, cells from an affected sibling but no disease-causing variant(s) have been detected in the genes involved in OI, electrophoresis of type I (pro)collagen from
cultured CVS cells can be used for prenatal diagnosis. Amniocytes are good sources of DNA but they do not make type I procollagen in sufficient abundance to permit prenatal diagnosis.
INTERPRETATION OF PERFORMED DIAGNOSTICS For the interpretation of an observed sequence variant it is essential to establish the causal role of the variant in the pathogenesis of the disease.
Textboxes 1 and 2 include descriptions of variants in the genes involved in OI that are likely to be pathogenic or that should be considered as unclassified variants. REPORTING General
information on requirements for variant reporting can be found in the OECD Guidelines for Quality Assurance in Molecular Genetic Testing (http://www.OECD.org/dataoecd/43/6/38839788.pdf) and
in the guidelines issued by the Swiss Medical Genetics Society (http://sgmg.ch/user_files/images/SGMG_Reporting_Guidelines.pdf). REPORTING SCENARIOS FINDING A CAUSATIVE VARIANT IN THE
COL1A1/COL1A2 GENES IN AN AFFECTED INDEX CASE The report should state that a causative variant has been detected and that this confirms the clinical diagnosis. The report should include a
description of the reason why a particular variant is considered causative. Determination of the parental origin of the detected variant(s) should be advised. Testing of relatives at risk
should be offered in conjunction with appropriate counselling. In the case of severe/lethal OI because of a causative variant in _COL1A1_ or _COL1A2_, the measured recurrence risk is 2%
after the birth of one affected child. The recurrence risk is increased after the birth of two affected individuals, presumably because the proportion of germ cells that carry the mutation
is higher.43 This information may be mentioned in the results letter. If parental mosaicism can be demonstrated in the father, the referring clinician could be encouraged to request a sperm
sample from the father to clarify the risk. When the referring physician is not a clinical geneticist, it is recommended that the patient be referred to a clinical genetics centre for
counselling. Referral to OI centres for therapy can be proposed if available. NOT FINDING A CAUSATIVE VARIANT IN COL1A1 AND COL1A2 GENES IN AN AFFECTED INDEX CASE If no causative variant is
detected in the _COL1A1_ and _COL1A2_ genes after gDNA analysis and screening for large gene deletions (eg, MLPA), an _interim report_ can be made with the recommendation for review of the
original patient diagnosis (detailed clinical information, eg, with use of the clinical checklist, radiographs, contact with the referring physician (see Supplementary Appendix Table 1)).
This review of the clinical diagnosis should involve a clinician with expertise in diagnosis and classification of OI. The outcome of this review might indicate the desirability for testing
of additional OI genes and cDNA and protein analysis of type I (pro)collagen. If applicable, a new request for additional testing should be sent by the referring physician to the laboratory.
Laboratories that do not offer analysis of the recessive genes should suggest further analysis in another laboratory. In the case of doubtful pathogenicity of the identified variant, the
‘Practice guidelines for the Interpretation and Reporting of Unclassified Variants (UV's) in Clinical Molecular Genetics’
(http://www.cmgs.org/BPGs/pdfs%20current%20bpgs/UV%20GUIDELINES%20\ratified.pdf) should be applied. If the genetic testing procedure has identified an unclassified variant as the only
sequence change, this should be reported as such. However, the report should clearly state that the clinical significance of the variant is unknown and that its identification does not
provide an explanation for the clinical phenotype of the patient. FINDING CAUSATIVE VARIANTS IN THE CRTAP, LEPRE1, PPIB, FKBP10, SERPINF1, SERPINH1, PLOD2, SP7 GENES IN AN AFFECTED INDEX
CASE The _final report_ should state that causative variants have been detected. It is necessary to investigate the carrier status of the parents and siblings in order to determine whether a
variant is causative. Testing of relatives at risk may be offered in conjunction with appropriate counselling. Such testing might be useful for prenatal diagnosis. FINDING ONE CAUSATIVE
VARIANT IN THE CRTAP, LEPRE1, PPIB, FKBP10, SERPINF1, SERPINH1, PLOD2, SP7 GENES IN AN AFFECTED INDEX CASE When one causative recessive variant has been detected, it cannot be excluded that
a second causative variant has been missed, for example when it concerns a deep intronic variant. Dependent on the laboratory, a second _interim report_ can be made indicating the
desirability for cDNA and protein analysis of type I (pro)collagen in the case of one causative variant in the _CRTAP_, _LEPRE1_ or _PPIB_ genes. When these additional tests are not
informative or cannot be performed in the laboratory, the _final report_ should state that it is not possible to confirm the clinical diagnosis OI by biochemical and/or molecular testing.
NOT FINDING CAUSATIVE VARIANTS IN THE CRTAP, LEPRE1, PPIB, FKBP10, SERPINF1, SERPINH1, PLOD2, SP7 GENES IN AN AFFECTED INDEX CASE In the case of doubtful pathogenicity of the identified
variants, the same procedure as described above for the _COL1A1_ and _COL1A2_ genes should be followed. In the case where no causative variants in the genes involved in recessive OI have
been found, the _final report_ should state that it is not possible to confirm the clinical diagnosis OI by biochemical and/or molecular testing. NOT FINDING CAUSATIVE VARIANT(S) IN THE
GENES KNOWN FOR AUTOSOMAL DOMINANT AND AUTOSOMAL RECESSIVE OI: TYPE I (PRO)COLLAGEN ELECTROPHORESIS Reports of these studies are necessarily more descriptive than the DNA-based results. They
should list the types of genetic alterations that could not be identified. When low production or post-translational over-modification of type I (pro)collagen is observed, the diagnosis of
OI can be confirmed and in the case of over-modification of type I (pro)collagen, prenatal diagnosis on chorionic villus cells is possible. In reports concerning cells and samples with no
molecular and/or biochemical diagnosis of OI, it should be stated that it is not possible to confirm the clinical diagnosis OI. PRENATAL OR PREIMPLANTATION GENETIC DIAGNOSIS Prenatal or
preimplantation genetic diagnosis with the intention of terminating a pregnancy or not selecting embryos carrying the causative variant(s) is possible in the case of identification of known
disease-causing variant(s). This service should only be offered in a clinical genetics service and must be accompanied by appropriate genetic counselling. Requests for prenatal or
preimplantation diagnosis should always be referred and announced in advance to a clinical genetics service. In addition, the original result letter of the laboratory that identified the
causative variant should accompany the request. CONCLUSIONS Best practice guidelines have been established for the molecular genetic diagnosis of OI. The most noteworthy issue is that
molecular analysis of the _COL1A1/2_ genes is recommended as the starting point in the diagnostic flow as opposed to protein analysis. It is to be expected that molecular genetic testing in
OI will only gain in importance as new OI-genes are discovered and with the development of new technologies. However, in certain selected cases protein analysis will remain important.
REFERENCES * van Dijk FS, Pals G, van Rijn RR, Nikkels PGJ, Cobben JM : Classification of osteogenesis imperfecta revisited. _Eur J Med Genet_ 2010; 53: 1–5. Article CAS Google Scholar *
Byers PH, Krakow D, Nunes ME, Pepin M : Genetic evaluation of suspected osteogenesis imperfecta (OI). _Genet Med_ 2006; 8: 383–388. Article Google Scholar * Sillence DO, Senn A, Danks DM :
Genetic heterogeneity in osteogenesis imperfecta. _J Med Genet_ 1979; 16: 101–116. Article CAS Google Scholar * Glorieux FH, Rauch F, Plotkin H _et al_: Type V osteogenesis imperfecta: a
new form of brittle bone disease. _J Bone Miner Res_ 2000; 15: 1650–1658. Article CAS Google Scholar * Glorieux FH, Ward LM, Rauch F, Lalic L, Roughly PJ, Travers R : Osteogenesis
imperfecta type VI: a form of brittle bone disease with mineralization defect. _J Bone Miner Res_ 2002; 17: 30–37. Article Google Scholar * Rauch F, Glorieux FH : Osteogenesis imperfecta.
_Lancet_ 2004; 363: 1377–1385. Article CAS Google Scholar * Breslau-Siderius EJ, Engelbert RH, Pals G, van der Sluijs JA : Bruck syndrome: a rare combination of bone fragility and
multiple congenital joint contractures. _J Pediatr Orthop B_ 1998; 7: 35–38. Article CAS Google Scholar * Ha-Vinh R, Alanay Y, Bank RA _et al_: Phenotypic and molecular characterization
of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by recessive mutation in _PLOD2_. _Am J Med Genet_ 2004; 131A: 115–120. Article Google Scholar *
Engel J, Prockop DJ : The zipper-like folding of collagen triple helices and the effects of mutations that might disrupt the zipper. _Annu Rev Biophys Biophys Chem_ 1991; 20: 137–152.
Article CAS Google Scholar * Byers PH, Wallis GA, Willing MC : Osteogenesis imperfecta: translation of mutation to phenotype. _J Med Genet_ 1991; 28: 433–442. Article CAS Google Scholar
* Steiner RD, Pepin MG, Byers PH : Osteogenesis imperfecta. Available at http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=oi. Accessed 3 May 2011. * Willing MC, Pruchno CJ,
Atkinson M, Byers PH : Osteogenesis imperfecta type I is commonly due to a COL1A1 null allele of type I collagen. _Am J Hum Genet_ 1992; 51: 508–515. CAS PubMed PubMed Central Google
Scholar * Willing MC, Deschenes SP, Scott DA _et al_: Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. _Am J Hum Genet_ 1994; 55: 638–647.
CAS PubMed PubMed Central Google Scholar * Nuytinck L, Sayli BS, Karen W, De Paepe A : Prenatal diagnosis of osteogenesis imperfecta type I by COL1A1 null-allele testing. _Prenat Diagn_
1999; 19: 873–875. Article CAS Google Scholar * van Dijk FS, Huizer M, Kariminejad A _et al_: Complete COL1A1 allele deletions in osteogenesis imperfecta. _Genet Med_ 2010; 12: 736–741.
Article CAS Google Scholar * Dalgleish R : The human type I collagen mutation database. _Nucleic Acids Res_ 1997; 25: 181–187. Article CAS Google Scholar * Dalgleish R : The human
collagen mutation database 1998. _Nucleic Acids Res_ 1998; 26: 253–255. Article CAS Google Scholar * Ishikawa Y, Wirz J, Vranka JA, Nagata K, Bächinger HP : Biochemical characterization
of the prolyl 3-hydroxylase 1·cartilage-associated protein·cyclophilin B complex. _J Biol Chem_ 2009; 284: 17641–17647. Article CAS Google Scholar * Barnes AM, Chang W, Morello R _et al_:
Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. _N Engl JMed_ 2006; 355: 2757–2764. Article CAS Google Scholar * Morello R, Bertin TK, Chen Y _et
al_: CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. _Cell_ 2006; 127: 291–304. Article CAS Google Scholar * Marini JC, Cabral WA,
Barnes AM : Null mutations in _LEPRE1_ and _CRTAP_ cause severe recessive osteogenesis imperfecta. _Cell Tissue Res_ 2010; 339: 59–70. Article CAS Google Scholar * Cabral WA, Chang W,
Barnes AM _et al_: Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. _Nat Genet_ 2007; 39: 359–365. Article CAS
Google Scholar * Willaert A, Malfait F, Symoens S _et al_: Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form
responsible for prolyl 3-hydroxylation. _J Med Genet_ 2009; 46: 233–241. Article CAS Google Scholar * van Dijk FS, Nesbitt IM, Zwikstra EH _et al_: _PPIB_ mutations cause severe
osteogenesis imperfecta. _Am J Hum Gen_ 2009; 85: 521–527. Article CAS Google Scholar * Barnes AM, Carter EM, Cabral WA _et al_: Lack of cyclophilin B in osteogenesis imperfecta with
normal collagen folding. _N Engl J Med_ 2010; 362: 521–528. Article CAS Google Scholar * Pyott SM, Schwarze U, Christiansen HE _et al_: Mutations in PPIB (cyclophilin B) delay type I
procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. _Hum Mol Genet_ 2011; 20: 1595–1609. Article CAS Google Scholar * Alanay Y,
Avaygan H, Camacho N _et al_: Mutations in the gene encoding RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. _Am J Hum Genet_ 2010; 86: 551–559. Article CAS Google
Scholar * Shaheen R, Al-Owain M, Sakati N, Alzayed ZS, Alkuraya FS : FKBP10 and Bruck syndrome. Phenotypic heterogeneity or call for reclassification? _Am J Hum Genet_ 2010; 87: 306–307.
Article CAS Google Scholar * Kelley BP, Malfait F, Bonafe L _et al_: Mutations in _FKBP10_ cause recessive osteogenesis imperfecta and Bruck syndrome. _J Bone Miner Res_ 2011; 26:
666–672. Article CAS Google Scholar * Christiansen HE, Schwarze U, Pyott SM _et al_: Homozygosity for a missense mutation in _SERPINH1_, which encodes the collagen chaperone protein
HSP47, results in severe recessive osteogenesis imperfecta. _Am J Hum Genet_ 2010; 86: 389–398. Article CAS Google Scholar * van der Slot AJ, Zuurmond AM, Bardoel AFJ _et al_:
Identification of _PLOD2_ as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. _J Biol Chem_ 2003; 278: 40967–40972. Article CAS Google Scholar * Lapunzina P, Aglan M,
Temtamy S _et al_: Identification of a frameshift mutation in _Osterix_ in a patient with recessive osteogenesis imperfecta. _Am J Hum Genet_ 2010; 87: 110–114. Article CAS Google Scholar
* Becker J, Semler O, Gilissen C _et al_: Exome sequencing identifies truncating mutations in human _SERPINF1_ in autosomal-recessive osteogenesis imperfecta. _Am J Hum Genet_ 2011; 88:
362–371. Article CAS Google Scholar * Marlowe A, Pepin MG, Byers PH : Testing for osteogenesis imperfecta in cases of suspected non-accidental injury. _J Med Gen_ 2002; 39: 382–386.
Article CAS Google Scholar * Ablin DS, Greenspan A, Reinhart M, Grix A : Differentiation of child abuse from osteogenesis imperfecta. _Am J Roentgenol_ 1990; 154: 1035–1046. Article CAS
Google Scholar * Steiner RD, Pepin M, Byers PH : Studies of collagen synthesis and structure in the differentiation of child abuse from osteogenesis imperfecta. _J Pediatr_ 1996; 128:
542–547. Article CAS Google Scholar * Bilo RAC, Robben SGF, van Rijn RR : _Forensic Aspects of Paediatric Fractures; Differentiating Accidental Trauma from Child Abuse_. 1st edn.
Springer: Heidelberg, Dordrecht, London, New York, 2010. Book Google Scholar * Wenstrup RJ, Willing MC, Starman BJ, Byers PH : Distinct biochemical phenotypes predict clinical severity in
nonlethal variants of osteogenesis imperfecta. _Am J Hum Genet_ 1990; 46: 975–982. CAS PubMed PubMed Central Google Scholar * Körkkö J, Ala-Kokko L, De Paepe A, Nuytinck L, Earley J,
Prockop DJ : Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with
osteogenesis imperfecta type I:identification of common sequences of null-allele mutations. _Am J Hum Genet_ 1998; 62: 98–110. Article Google Scholar * Sykes B, Ogilvie D, Wordsworth P _et
al_: Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. _Am J Hum Genet_ 1990; 46: 293–307. CAS PubMed PubMed Central
Google Scholar * Steinmann B, Rao VH, Vogel A, Bruckner P, Gitzelmann R, Byers PH : Cysteine in the triple-helical domain of one allelic product of the α1(I) gene of type I collagen
produces a lethal form of osteogenesis imperfecta. _J Biol Chem_ 1984; 259: 11129–11138. CAS Google Scholar * Bateman JF, Golub SB : Assessment of procollagen processing defects by
fibroblasts cultured in the presence of dextran sulfate. _Biochem J_ 1990; 267: 573–577. Article CAS Google Scholar * Pyott SM, Schwarze U, Christiansen HE _et al_: Recurrence of
perinatal lethal osteogenesis imperfecta in sibships: parsing the risk between parental mosaicism for dominant mutations and autosomal recessive inheritance. _Genet Med_ 2011; 13: 125–130.
Article Google Scholar * Dalgleish R, Flicek P, Cunningham F _et al_: Locus Reference Genomic sequences: an improved basis for describing human DNA variants. _Genome Med_ 2010; 2: 24.
Article Google Scholar Download references ACKNOWLEDGEMENTS Funding for the Best Practice Meeting was provided by the European Molecular Genetics Quality Network (http://www.emqn.org).
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Clinical Genetics, VU University Medical Centre, Amsterdam, The Netherlands Fleur S van Dijk, Alessandra Maugeri, Erik A
Sistermans & Gerard Pals * Department of Pathology and Medicine, University of Washington, Seattle, WA, USA Peter H Byers * Department of Genetics, University of Leicester, Leicester, UK
Raymond Dalgleish * Center for Medical Genetics, Ghent University Hospital, Ghent, Belgium Fransiska Malfait & Sofie Symoens * Division of Metabolism, University Children's
Hospital, Zurich, Switzerland Marianne Rohrbach Authors * Fleur S van Dijk View author publications You can also search for this author inPubMed Google Scholar * Peter H Byers View author
publications You can also search for this author inPubMed Google Scholar * Raymond Dalgleish View author publications You can also search for this author inPubMed Google Scholar * Fransiska
Malfait View author publications You can also search for this author inPubMed Google Scholar * Alessandra Maugeri View author publications You can also search for this author inPubMed Google
Scholar * Marianne Rohrbach View author publications You can also search for this author inPubMed Google Scholar * Sofie Symoens View author publications You can also search for this author
inPubMed Google Scholar * Erik A Sistermans View author publications You can also search for this author inPubMed Google Scholar * Gerard Pals View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Gerard Pals. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION This paper was presented at an EMQN Best Practice Meeting, 28–29 June 2010, Amsterdam, The Netherlands. Other participants at the EMQN Best Practice Meeting were Javier
Garcia-Planells, Filomena Valentina Gentile, Anne-Sophie Lebre, Shirley McQuaid, Rebecca Pollitt, Agnieszka Rusinska, Thomas Schwarzbraun and Janneke Weiss. Affiliations can be found in
Supplementary Appendix Table 2. Supplementary Information accompanies the paper on European Journal of Human Genetics website SUPPLEMENTARY INFORMATION SUPPLEMENTARY APPENDIX (DOC 1947 KB)
APPENDIX APPENDIX boxed-text boxed-text BOX 1: TEXTBOX1 INTERPRETATION OF VARIANTS IN THE _COL1A1_ AND _COL1A2_ GENES. _COL1A1_ and _COL1A2_ genes * 1 Causative variants – considerations
specific for the fibrillar collagen genes: _The following variants are likely to have pathological consequences for the protein function:_ * a Substitution of the glycine residue of every
Gly-X-Y triplet repeat within the triple-helical region of the _α_1(I)- and _α_2(I)-collagen chains. * b Sequence variants in consensus acceptor and donor splice sites (positions +1, +2, −1,
−2) will lead to splicing errors of the mRNA, but the outcome cannot be predicted without cDNA analysis, with the exception of −1G>A acceptor-site splicing variants in the triple-helical
region of the _α_1(I) collagen chains which result in a frameshift, nonsense-mediated mRNA decay and a null-allele. −1G>A splicing variants of exon 6 of the _COL1A1_ gene, results in
skipping of exon 6, whereas for the _COL1A2_ gene this results in use of a cryptic splice site in the exon and an in-frame deletion of 15 bases. Both are associated with EDS arthrochalasis
type. * c Nonsense mutations or deletions and insertions that result in interruption of the reading frame, nonsense-mediated mRNA decay and a null-allele. * d In-frame deletions and
insertions that lengthen or shorten the _α_-chains. * e Other variants* with experimental evidence (published or own data) of their impairment of the protein's function. *Variants also
should be checked against existing entries in the Osteogenesis Imperfecta Variant Database16, 17 (https://oi.gene.le.ac.uk), which can be used for corroboration. However, it should be
recognized that the data in the database are not warranted to be accurate or fit for any particular purpose. * 2 Unclassified variants (UV) * a All other sequence variants must be considered
as ‘unclassified’ until segregation within the family has been investigated and/or functional evidence becomes available. * b It is strongly recommended that intronic nucleotide
substitutions other than splice-site mutations affecting the −1, −2 or +1, +2 intronic nucleotide positions be examined by cDNA-analysis to determine their splicing outcome. * c It is
strongly recommnded that UVs be further investigated by _in silico_ analysis (eg, PolyPhen-2, SIFT, Human Splicing Finder software, and so on). Of note: the new version of Polyphen
classifies almost all glycine substitutions as benign or tolerated. BOX 2: TEXTBOX 2 INTERPRETATION OF VARIANTS IN THE _CRTAP_, _FKBP10_, _LEPRE1_, _PLOD2_, _PPIB_, _SERPINF1_, _SERPINH1 AND
SP7_ GENES. _CRTAP, FKBP10, LEPRE1, PLOD2, PPIB, SERPINF1, SERPINH1_ and _SP7_ * 1 Causative variants _The following variants are likely to have pathological consequences for the protein
function:_ * a Sequence variants in consensus acceptor and donor splice sites that will lead to splicing errors of the mRNA. * b Nonsense mutations or deletions and insertions that result in
interruption of the reading frame, nonsense-mediated mRNA decay and a null-allele. * c Most in frame deletions and insertions that lengthen or shorten the protein. * d See 1e in Textbox 1 *
2 Unclassified variants See Textbox 1. RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a
copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE van Dijk, F., Byers, P., Dalgleish, R. _et al._
EMQN best practice guidelines for the laboratory diagnosis of osteogenesis imperfecta. _Eur J Hum Genet_ 20, 11–19 (2012). https://doi.org/10.1038/ejhg.2011.141 Download citation * Received:
04 February 2011 * Revised: 09 May 2011 * Accepted: 03 June 2011 * Published: 10 August 2011 * Issue Date: January 2012 * DOI: https://doi.org/10.1038/ejhg.2011.141 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative KEYWORDS * EMQN * best practice * osteogenesis imperfecta * type I (pro)collagen * diagnosis * reporting