- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Treatment with rituximab is highly effective for EBV-associated post transplant lymphoproliferative disease. However, little is known about its immunological sequelae in pediatric
allogeneic hematopoietic SCT (HSCT). Time to normal CD19+ B-lymphocyte values in blood and intravenous immunoglobulin (IVIG) substitution needed to maintain an IgG>400 mg per 100 ml in
six consecutive pediatric allogeneic HSCT patients treated with rituximab for symptomatic EBV reactivation were compared with a matched cohort of non-rituximab-treated patients. Follow-up of
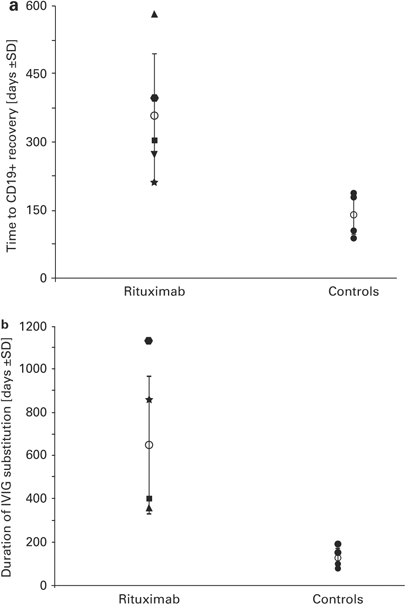
the six patients ranged from 149 to 1546 days; all but one survived. The mean (±s.d.) time to recovery of CD19+ B-lymphocytes was 353±142 days as compared with 139±42 in the controls
(_P_<0.01). Similarly, substitution of IVIG as a measure of functional B-cell recovery was extended from a mean of 122±45 to a mean of 647±320 days, and the cumulative dose of IVIG
increased from a mean of 1.86±0.51 to 4.4±0.97 g/kg, respectively (_P_<0.05). One patient had functional B-lymphocyte deficiency for >3 years and ultimately required two stem cell
boosts. Rituximab is a live-saving treatment for pediatric HSCT patients but may lead to prolonged and even persistent B-cell deficiency. Access through your institution Buy or subscribe
This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access
$259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
SAFETY AND EFFICACY OF THE LOW-DOSE MEMORY (CD45RA-DEPLETED) DONOR LYMPHOCYTE INFUSION IN RECIPIENTS OF ΑΒ T CELL-DEPLETED HAPLOIDENTICAL GRAFTS: RESULTS OF A PROSPECTIVE RANDOMIZED TRIAL IN
HIGH-RISK CHILDHOOD LEUKEMIA Article 16 February 2021 IMPACT OF ANTI-T-LYMPHOCYTE GLOBULIN DOSING ON GVHD AND IMMUNE RECONSTITUTION IN MATCHED UNRELATED MYELOABLATIVE PERIPHERAL BLOOD STEM
CELL TRANSPLANTATION Article Open access 13 July 2022 IMMUNOLOGICAL RECONSTITUTION AND INFECTIONS AFTER ALLOHCT - A COMPARISON BETWEEN POST-TRANSPLANTATION CYCLOPHOSPHAMIDE, ATLG AND
NON-ATLG BASED GVHD PROPHYLAXIS Article Open access 19 November 2024 REFERENCES * Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM _et al_. Risk of lymphoproliferative
disorders after bone marrow transplantation: a multi-institutional study. _Blood_ 1999; 94: 2208–2216. CAS PubMed Google Scholar * Faye A, Vilmer E . Post-transplant lymphoproliferative
disorder in children: incidence, prognosis, and treatment options. _Paediatr Drugs_ 2005; 7: 55–65. Article PubMed Google Scholar * Svoboda J, Kotloff R, Tsai DE . Management of patients
with post-transplant lymphoproliferative disorder: the role of rituximab. _Transpl Int_ 2006; 19: 259–269. Article CAS PubMed Google Scholar * Reff ME, Carner K, Chambers KS, Chinn PC,
Leonard JE, Raab R _et al_. Depletion of B cells _in vivo_ by a chimeric mouse human monoclonal antibody to CD20. _Blood_ 1994; 83: 435–445. CAS PubMed Google Scholar * Tedder TF, Engel P
. CD20: a regulator of cell-cycle progression of B lymphocytes. _Immunol Today_ 1994; 15: 450–454. Article CAS PubMed Google Scholar * Milpied N, Vasseur B, Parquet N, Garnier JL,
Antoine C, Quartier P _et al_. Humanized anti-CD20 monoclonal antibody (rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. _Ann Oncol_
2000; 11 (Suppl 1): 113–116. Article PubMed Google Scholar * Faye A, Quartier P, Reguerre Y, Lutz P, Carret AS, Dehée A _et al_. Chimaeric anti-CD20 monoclonal antibody (rituximab) in
post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. _Br J Haematol_ 2001; 115: 112–118. Article CAS PubMed Google Scholar * van Esser JW,
Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW _et al_. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in
high-risk patients after allogeneic stem cell transplantation. _Blood_ 2002; 99: 4364–4369. Article CAS PubMed Google Scholar * Messahel B, Taj MM, Hobson R, Hadzic N, Ramsay A, Hann I
_et al_. Single agent efficacy of rituximab in childhood immunosuppression related lymphoproliferative disease: a United Kingdom Children's Cancer Study Group (UKCCSG) retrospective
review. _Leuk Lymphoma_ 2006; 47: 2584–2589. Article CAS PubMed Google Scholar * Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A . Preemptive diagnosis and treatment of
Epstein-Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. _Bone Marrow Transplant_ 2006; 37: 539–546.
Article CAS PubMed Google Scholar * Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA _et al_. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in
patients with relapsed low-grade non-Hodgkin's lymphoma. _Blood_ 1997; 90: 2188–2195. CAS PubMed Google Scholar * McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS,
Williams ME _et al_. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. _J Clin Oncol_ 1998;
16: 2825–2833. Article CAS PubMed Google Scholar * Harris NL, Ferry JA, Swerdlow SH . Posttransplant lymphoproliferative disorders: summary of Society for Hematopathology Workshop.
_Semin Diagn Pathol._ 1997; 14: 8–14. CAS PubMed Google Scholar * Bouquillon C, Dewilde A, Andreoletti L, Lambert V, Chieux V, Gerard Y _et al_. Simultaneous detection of 6 human
herpesviruses in cerebrospinal fluid and aqueous fluid by a single PCR using stair primers. _J Med Virol_ 2000; 62: 349–353. Article CAS PubMed Google Scholar * Mackay IM . Real-time PCR
in the microbiology laboratory. _Clin Microbiol Infect_ 2004; 10: 190–212. Article CAS PubMed Google Scholar * Ruiz G, Peña P, de Ory F, Echevarría JE . Comparison of commercial
real-time PCR assays for quantification of Epstein-Barr virus DNA. _J Clin Microbiol_ 2005; 43: 2053–2057. Article CAS PubMed PubMed Central Google Scholar * Choquet S, Leblond V,
Herbrecht R, Socie G, Stoppa AM, Vandenberghe P _et al_. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter
phase 2 study. _Blood_ 2006; 107: 3053–3057. Article CAS PubMed Google Scholar * Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K _et al_.
Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. _J Pediatr_ 1997; 130: 388–393. Article CAS PubMed Google Scholar * Kliegman RN,
Behrman RF, Jenson HR (eds.) _Nelson Textbook of Pediatrics, chapter 710_, 17th edn. WB Saundern: Philadelphia, PA, 2003. Google Scholar * Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T,
Obara M _et al_. Hypogamma-globulinemia with a selective delayed recovery in memory B cells and an impaired isotype expression after rituximab administration as an adjuvant to autologous
stem cell transplantation for non-Hodgkin lymphoma. _Eur J Haematol_ 2006; 77: 226–232. Article CAS PubMed Google Scholar * Nishio M, Fujimoto K, Yamamoto S, Endo T, Sakai T, Obara M _et
al_. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired _in vitro_ immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as
an adjuvant to autologous stem cell transplantation. _Br J Haematol_ 2007; 137: 349–354. Article CAS PubMed Google Scholar * Shortt J, Spencer A . Adjuvant rituximab causes prolonged
hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin's lymphoma. _Bone Marrow Transplant_ 2006; 38: 433–436. Article CAS PubMed Google Scholar * Lim SH,
Zhang Y, Wang Z, Esler WV, Beggs D, Pruitt B _et al_. Maintenance rituximab after autologous stem cell transplant for high-risk B-cell lymphoma induces prolonged and severe
hypogammaglobulinemia. _Bone Marrow Transplant_ 2005; 35: 207–208. Article CAS PubMed Google Scholar * Sidner RA, Book BK, Agarwal A, Bearden CM, Vieira CA, Pescovitz MD . _In vivo_
human B-cell subset recovery after _in vivo_ depletion with rituximab, anti-human CD20 monoclonal antibody. _Hum Antibodies_ 2004; 13: 55–62. Article CAS PubMed Google Scholar * Anolik
JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E _et al_. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. _Clin Immunol_ 2007; 122: 139–145.
Article CAS PubMed Google Scholar * Dunleavy K, Hakim F, Kim HK, Janik JE, Grant N, Nakayama T _et al_. B-cell recovery following rituximab-based therapy is associated with
perturbations in stromal derived factor-1 and granulocyte homeostasis. _Blood_ 2005; 106: 795–802. Article CAS PubMed PubMed Central Google Scholar * Arber C, Buser A, Heim D, Weisser
M, Tyndall A, Tichelli A _et al_. Septic polyarthritis with ureaplasma urealyticum in a patient with prolonged agammaglobulinemia and B-cell aplasia after allogeneic HSCT and rituximab
pretreatment. _Bone Marrow Transplant_ 2007; 40: 597–598. Article CAS PubMed Google Scholar * Imashuku S, Teramura T, Morimoto A, Naya M, Kuroda H . Prolonged hypogammaglobulinemia
following rituximab treatment for post transplant Epstein-Barr virus-associated lymphoproliferative disease. _Bone Marrow Transplant_ 2004; 33: 129–130. Article CAS PubMed Google Scholar
* Castagnola E, Dallorso S, Faraci M, Morreale G, Di Martino D, Cristina E _et al_. Long-lasting hypogammaglobulinemia following rituximab administration for Epstein-Barr virus-related
post-transplant lymphoproliferative disease preemptive therapy. _J Hematother Stem Cell Res_ 2003; 12: 9–10. Article PubMed Google Scholar * Nishio M, Endo T, Fujimoto K, Sato N, Sakai T,
Obara M _et al_. Persistent panhypogamma-globulinemia with selected loss of memory B cells and impaired isotype expression after rituximab therapy for post-transplant EBV-associated
autoimmune hemolytic anemia. _Eur J Haematol_ 2005; 75: 527–529. Article PubMed Google Scholar * Zuccaro G, Della Bella S, Polizzi B, Vanoli M, Scorza R . Common variable immunodeficiency
following Epstein-Barr virus infection. _J Clin Lab Immunol_ 1997; 49: 41–45. CAS PubMed Google Scholar * Inoue H, Shinohara K, Nomiyama J, Oeda E . Fatal aplastic anemia caused by
Epstein-Barr virus infection after autologous bone marrow transplantation for Non-Hodgkin malignant lymphoma. _Int Medicine_ 1994; 33: 303–307. Article CAS Google Scholar * Greenfield HM,
Gharib MI, Turner AJ, Guiver M, Carr T, Will AM _et al_. The impact of monitoring Epstein-Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome
and guide intervention? _Pediatr Blood Cancer_ 2006; 47: 200–205. Article PubMed Google Scholar * Comoli P, Basso S, Zecca M, Pagliara D, Baldanti F, Bernardo ME _et al_. Preemptive
therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. _Am J Transplant_ 2007; 7: 1648–1655. Article CAS PubMed Google Scholar *
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA _et al_. Prompt versus preemptive intervention for EBV lymphoproliferative disease. _Blood_ 2004; 103: 3979–3981. Article CAS
PubMed Google Scholar * Annels NE, Kalpoe JS, Bredius RG, Claas EC, Kroes AC, Hislop AD _et al_. Management of Epstein-Barr virus (EBV) reactivation after allogeneic stem cell
transplantation by simultaneous analysis of EBV DNA load and EBV-specific T cell reconstitution. _Clin Infect Dis_ 2006; 42: 1743–1748. Article CAS PubMed Google Scholar * Meij P, van
Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N _et al_. Impaired recovery of Epstein-Barr virus (EBV)--specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell
transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. _Blood_ 2003; 101: 4290–4297. Article CAS PubMed Google Scholar
* Clave E, Agbalika F, Bajzik V, Peffault de Latour R, Trillard M, Rabian C _et al_. Epstein-Barr virus (EBV) reactivation in allogeneic stem-cell transplantation: relationship between viral
load, EBV-specific T-cell reconstitution and rituximab therapy. _Transplantation_ 2004; 77: 76–84. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank
Claudia Katerkamp, Hedwig Kolve and Maria Waeltermann for administrative and technical support. The results of this analysis were presented at the 25th annual meeting of the European Society
for Paediatric Infectious Diseases, Porto, Portugal, 2–4 May 2007. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatric Hematology and Oncology, University Children's
Hospital Muenster, Muenster, Germany K Masjosthusmann, K Ehlert, H Juergens, M Fruehwald & A H Groll * Department of General Pediatrics, University Children's Hospital Muenster,
Muenster, Germany K Masjosthusmann & J Roth * Department of Medical Microbiology, University Hospital Muenster, Muenster, Germany B R Eing * Department of Surgical Pathology, University
Hospital Muenster, Muenster, Germany G Koehler Authors * K Masjosthusmann View author publications You can also search for this author inPubMed Google Scholar * K Ehlert View author
publications You can also search for this author inPubMed Google Scholar * B R Eing View author publications You can also search for this author inPubMed Google Scholar * J Roth View author
publications You can also search for this author inPubMed Google Scholar * G Koehler View author publications You can also search for this author inPubMed Google Scholar * H Juergens View
author publications You can also search for this author inPubMed Google Scholar * M Fruehwald View author publications You can also search for this author inPubMed Google Scholar * A H Groll
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A H Groll. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Masjosthusmann, K., Ehlert, K., Eing, B. _et al._ Delay in B-lymphocyte recovery and function following rituximab for EBV-associated lymphoproliferative
disease early post-allogeneic hematopoietic SCT. _Bone Marrow Transplant_ 43, 679–684 (2009). https://doi.org/10.1038/bmt.2008.385 Download citation * Received: 14 January 2008 * Revised: 19
August 2008 * Accepted: 22 August 2008 * Published: 24 November 2008 * Issue Date: May 2009 * DOI: https://doi.org/10.1038/bmt.2008.385 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * rituximab * transplantation * lymphoproliferative disease * EBV * B lymphocytes * Igs

.jpg?crop=true&anchor=29,147&q=80&color=ffffffff&u=2xkwh0&w=1998&h=1148)