- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Regulation of immune responses is a complex process that involves many signaling molecules in their specific interactions and interplays. For instance, the leukocytic integrin CD11b/CD18
plays a crucial role in leukocyte infiltration, which is commonly found in most inflammatory diseases. The highly abundant integrin CD11b/CD18 is a heterodimer of the αm (CD11b) and β2
(CD18) subunits. CD11b/CD18 is located in the cell surface of circulating leukocytes and can undergo active conformational change to mediate leukocyte adhesion, migration and accumulation at
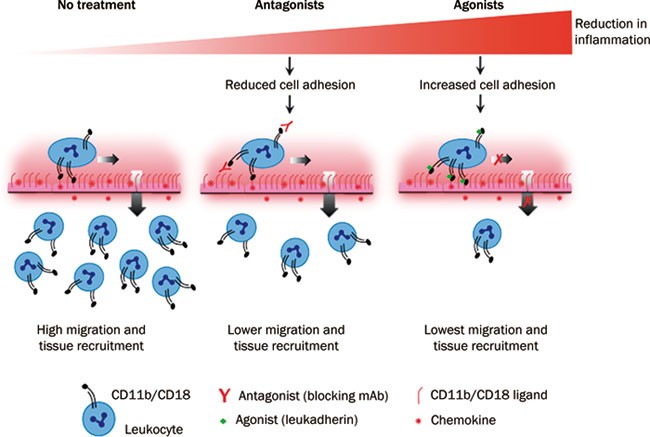
the sites of inflammation1. Current approaches for prevention of leukocyte infiltration focus on inhibiting the adhesion of leukocytic integrins to their respective ligands. For example,
the specific integrin antibody M1/70 mAb blocks the binding of integrins to ligands found on the vascular wall, thereby reducing the infiltration of leukocytes into the inflamed tissues
(Figure 1)2, 3. Despite the intensive research on identifying potent inhibitors for CD11b/CD18, such inhibitors had limited success in treating inflammatory diseases in humans. This may be
because of the large intracellular pool of CD11b/CD18 that can be mobilized to leukocyte surface and hinder the complete blockade of CD11b/CD18 with specific antibodies4, or because it is
crucial to have over 90% occupancy of active integrin receptors by inhibitors for suppression of leukocyte recruitment5. Moreover, unexpected adverse effects of antibodies against β2
integrins also prohibited the use of such blocking agent in clinical trials6. Recently, Maiguel and colleagues2 reported a new approach that involves the pharmacological activation, rather
than blockade of CD11b/CD18 by small molecules, which has enlightened as promising treatment strategy for inflammatory diseases. Maiguel and colleagues2 successfully identified several
potent small-molecule agonists (termed as leukadherins), which specifically target and enhance CD11b/CD18-dependent cell adhesion to fibrinogen or inflamed endothelium, leading to reduction
of leukocyte migration and recruitment in both _in vitro_ and _in vivo_ models (Figure 1). Their data provide scientific insight that instead of identification of blocking agents for
integrin, discovery of potent integrin CD11b/CD18 agonists could also be another effective strategy in reducing leukocyte infiltration and subsequent inflammation in humans. Through the
high-throughput screening (HTS) of a chemical library (>100 000 small molecules), three leukadherin compounds were identified to target the ligand-binding αA domain and allosterically
stabilize the active conformation of CD11b/CD18. In addition, leukadherins enhanced CD11b/CD18-dependent cell adhesion and reduced leukocyte motility, which led to a substantial reduction in
leukocyte transendothelial migration and recruitment into inflamed tissues. Several clinically relevant diseases models such as thioglycolate-induced peritonitis mice model, arterial
balloon injury rat model and zebra-fish tailfin injury model were adopted to demonstrate the physiologically relevant settings for anti-inflammatory effects of leukadherins, The results
suggest that leukadherins are therapeutic lead compounds for future optimization. Most importantly, a comparison between M1/70 mAb, a well-characterized CD11b/CD18 antibody7, and one of
these compounds revealed that leukadherin shows better efficacy and well preserves organ function upon inflammatory injury. Collectively, Maiguel's data suggested that
integrin-specific, small-molecule agonists represent an effective pharmacological approach for the treatment of inflammatory and autoimmune diseases. The phenotype of reduced migration and
recruitment of inflammatory cells and cellular adhesion due to the increase of constitutively active integrins was firstly demonstrated in knock-in mice expressing constitutively active
mutants of the integrins8. However, it is not known whether the activation of wild-type integrin receptors in normal animals could have a similar phenotype _in vivo_ and could reduce
inflammation in physiologically relevant disease models. In this respect, Maiguel and colleagues highlighted the use of chemical-biological approach in demonstrating the endogenous,
wild-type integrin protein perturbed by a specific small-molecule agonist, and provided an appropriate channel to analyze the effects of integrin activation on cellular functions _in vivo_.
On the other hand, treatment of integrin agonists _in vivo_ would increase adhesion of inflammatory cells to the vascular endothelium that may lead to vascular damage and leakage. Although
Maiguel and colleagues found no systemic signs of vascular injury or leakage from the animals administrated with compounds for more than 3 months, the bio-markers for atherosclerosis can
also be monitored in animals treated with integrin agonists for a longer treatment duration9. Now, the identification of potent anti-inflammatory compounds has become a hot topic in Chinese
herbal drug research10, therefore, Chinese medicinal herbs may serve as a potential source for the identification of novel integrin agonists in the future. REFERENCES * Hynes RO . Integrins:
bidirectional, allosteric signaling machines. _Cell_ 2002; 110: 673–87. Article CAS PubMed Google Scholar * Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, _et al_. Small
molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. _Sci Signal_ 2011; 4: ra57. Article PubMed PubMed Central Google Scholar * Cox D, Brennan M, Moran N
. Integrins as therapeutic targets: lessons and opportunities. _Nat Rev Drug Discov_ 2010; 9: 804–20. Article CAS PubMed Google Scholar * Hughes BJ, Hollers JC, Crockett-Torabi E, Smith
CW . Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. _J Clin Invest_ 1992; 90: 1687–96. Article CAS PubMed PubMed Central Google Scholar *
Lum AF, Green CE, Lee GR, Staunton DE, Simon SI . Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. _J Biol Chem_
2002; 277: 20660–70. Article CAS PubMed Google Scholar * Molloy ES, Calabrese LH . Therapy: Targeted but not trouble-free: efalizumab and PML. _Nat Rev Rheumatol_ 2009; 5: 418–9. Article
CAS PubMed Google Scholar * Springer T, Galfre G, Secher DS, Milstein C . Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. _Eur J Immunol_ 1979; 9: 301–6.
Article CAS PubMed Google Scholar * Park EJ, Mora JR, Carman CV, Chen J, Sasaki Y, Cheng G, _et al_. Aberrant activation of integrin alpha4beta7 suppresses lymphocyte migration to the
gut. _J Clin Invest_ 2007; 117: 2526–38. Article CAS PubMed PubMed Central Google Scholar * Charo IF, Taub R . Anti-inflammatory therapeutics for the treatment of atherosclerosis. _Nat
Rev Drug Discov_ 2011; 10: 365–76. Article CAS PubMed PubMed Central Google Scholar * Li HY, Cui L, Cui M . Hot topics in Chinese herbal drugs research documented in PubMed/MEDLINE by
authors inside China and outside of China in the past 10 years: based on co-word cluster analysis. _J Altern Complement Med_ 2009; 15: 779–85. Article CAS PubMed Google Scholar Download
references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory for Quality Research in Chinese Medicines, Macau University of Science and Technology, Avenida Wai Long, Taipa,
Macau, China Vincent Kam Wai Wong & Liang Liu Authors * Vincent Kam Wai Wong View author publications You can also search for this author inPubMed Google Scholar * Liang Liu View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Liang Liu. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Wong, V., Liu, L. Integrin activation as an alternative treatment approach for inflammatory diseases. _Acta Pharmacol Sin_ 32, 1309–1310 (2011).
https://doi.org/10.1038/aps.2011.150 Download citation * Published: 31 October 2011 * Issue Date: November 2011 * DOI: https://doi.org/10.1038/aps.2011.150 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative