- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Gallium-modified HZSM-5 zeolites are known to increase aromatic selectivity in methanol conversion. However, there are still disputes about the exact active sites and the aromatic
formation mechanisms over Ga-modified zeolites. In this work, in situ synchrotron radiation photoionization mass spectrometry (SR-PIMS) experiments were carried out to study the behaviors of
intermediates and products during methanol conversion over Ga-modified HZSM-5. The increased formaldehyde (HCHO) yield over Ga-modified HZSM-5 was found to play a key role in the increase
in aromatic yields. More HCHO was deemed to be generated from the direct dehydrogenation of methanol, and Ga2O3 in Ga-modified HZSM-5 was found to be the active phase. The larger increase in
aromatic production over Ga-modified HZSM-5 after reduction‒oxidation treatment was found to be the result of redispersed Ga2O3 with smaller size generating a larger amount of HCHO. This
study provides some new insights into the internal driving force for promoting the production of aromatics over Ga-modified HZSM-5. SIMILAR CONTENT BEING VIEWED BY OTHERS UNVEILING THE
BRØNSTED ACID MECHANISM FOR MEERWEIN–PONNDORF–VERLEY REDUCTION IN METHANOL CONVERSION OVER ZSM-5 Article Open access 09 October 2024 STABILIZING THE FRAMEWORK OF SAPO-34 ZEOLITE TOWARD
LONG-TERM METHANOL-TO-OLEFINS CONVERSION Article Open access 02 August 2021 METHYL RADICAL CHEMISTRY IN NON-OXIDATIVE METHANE ACTIVATION OVER METAL SINGLE SITES Article Open access 15
September 2023 INTRODUCTION The catalytic conversion of methanol-to-hydrocarbons (MTH) is considered to be a promising route, which can convert coal and natural gas to more valuable fuels
and commodity chemicals through methanol (MeOH)1,2,3. Aromatic production through catalytic conversion of MeOH is a highly attractive alternative to the process of producing aromatics from
petroleum resources. It is well known that metal-modified ZSM-5 zeolites have distinct performance for increasing aromatic selectivity in MeOH conversion4,5,6,7,8,9. For Ga-modified HZSM-5
zeolites, both cationic metal species (Ga3+) and/or metal oxides (Ga2O3) have been reported to result in a significant enhancement in aromatic production7,10,11,12,13. In Ga-modified HZSM-5
prepared by impregnation or ion exchange, previous works suggested that the substitution of Brønsted acid sites (BASs) by cationic Ga species can bring about strong Lewis acid sites (LASs),
and the synergistic effect derived from the close spatial proximity between cationic Ga species and BASs leads to an enhanced Brønsted acidity7. The cationic Ga species cooperate with BASs
to facilitate the dehydrogenation-aromatization processes of cycloalkenes, which can be called the LAS-induced aromatic formation pathway12. Physical mixtures of Ga2O3 and HZSM-5 can also
improve the selectivity of aromatics5,10,11. It suggested that there may exist some certain active sites located in the interface region between Ga2O3 and zeolite to promote MeOH into
aromatics11. Formaldehyde (HCHO) is an important active intermediate in the MTH reaction, which has aroused wide interest from many investigators recently14,15,16,17. HCHO is considered to
be involved in aromatic formation in the MTH reaction, i.e., the HCHO-induced aromatic formation pathway18,19. Lercher et al. suggested that HCHO can react with olefins to form dienes via
Prins reaction (HCHO + olefins → dienes + H2O), and dienes react stepwise with HCHO to form H-poor products, aromatics and eventually cokes14. The researchers studied the behavior of HCHO in
MTH by adding HCHO into the reactants, and found that the addition of HCHO can significantly increase the selectivity to aromatics15,20,21, and accelerate catalyst deactivation22,23. In our
previous work, the critical role of HCHO in the mechanisms of aromatic formation was confirmed with the excellent time-resolved profiles obtained by in situ synchrotron radiation
photoionization mass spectrometry (SR-PIMS)24. Although the importance of HCHO in the MTH reaction is well established, the exact active centers on Ga-modified HZSM-5 zeolites and how they
affect the formation of HCHO under MTH conditions are still not clear. In this work, questions like how HCHO was generated on the Ga-modified HZSM-5 and what the active sites were, were
studied systematically. The new insights into the HCHO-induced aromatic formation pathway due to the introduction of Ga species supplemented the previous dehydrogenation-aromatization
mechanism. RESULTS AND DISCUSSION FORMALDEHYDE TREND RELATED TO AROMATICS In this work, in situ SR-PIMS (Supplementary Fig. 1) was further utilized to explore the formation and function of
HCHO in MTH over Ga-modified HZSM-524. To alleviate the secondary reactions of HCHO, a 2 Torr pressure was applied to the catalytic reactor. The Ga-modified HZSM-5 was prepared by
impregnation and ion exchange methods, which are denoted as Ga(IM)HZSM-5 and Ga(IE)HZSM-5. Ga(IM-A/B/C)HZSM-5 represents a Ga(IM)HZSM-5 with different Ga loadings (Table 1). The
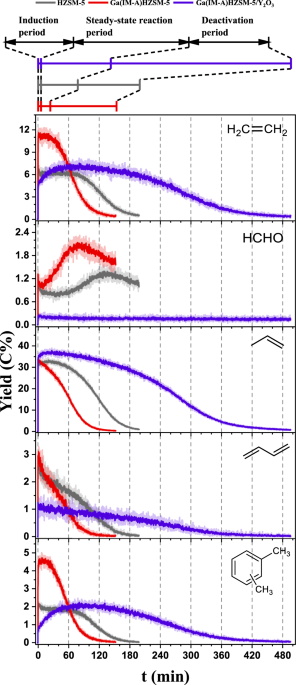
time-resolved profiles of the main product yields in MTH over the parent HZSM-5 and Ga(IM-A)HZSM-5 at 400 °C are demonstrated in Fig. 1, where the induction, steady-state reaction, and
deactivation periods could be distinguished. In terms of steady-state reaction period, compared with the parent HZSM-5, Ga(IM-A)HZSM-5 could produce more aromatics (including C7-C9
aromatics) (Fig. 1 and Supplementary Fig. 4). However, the propylene yield did not change much, while the yields of C4= and C5= olefins decreased slightly. Because the formation of ethylene
is closely associated with the aromatic-based cycle in the dual-cycle mechanism of the MTH reaction, the yield of ethylene over Ga(IM-A)HZSM-5 increased obviously with the enhanced
production of aromatics. Here, it is worth noting that the HCHO yield over Ga(IM-A)HZSM-5 was higher than that over HZSM-5, especially in the deactivation period. This promoting effect on
aromatic formation was similar to the co-feeding of formaldehyde with methanol at the expense of catalyst lifetime due to severe coke deposition (Supplementary Fig. 5). Y2O3 can decompose
HCHO into CO and H2, and has little activity for the conversion of MeOH and other stable products in the MTH reaction25. Hence, the physical mixture of Ga(IM-A)HZSM-5 with Y2O3
(Ga(IM-A)HZSM-5/Y2O3) was introduced to remove HCHO in MTH and verify whether the increase in aromatic yields promoted by Ga(IM-A)HZSM-5 was tightly connected with the increased HCHO yield.
As shown in Fig. 1, the HCHO yield over Ga(IM-A)HZSM-5/Y2O3 was significantly reduced in the whole reaction process, and the yields of aromatics over Ga(IM-A)HZSM-5/Y2O3 decreased to the
level of the parent HZSM-5 in the steady-state reaction period. The yield of 1,3-butadiene derived from the Prins reaction also decreased. In addition, the variation in stable products with
and without Y2O3 obtained by GC‒MS experiments under atmospheric pressure was similar to that of SR-PIMS experiments (Supplementary Table 2). These results indicated that the HCHO-induced
aromatic formation pathway strengthened by Ga(IM)HZSM-5 played an important role in increasing aromatic production. FORMALDEHYDE FORMATION PATHWAY OVER GA-MODIFIED HZSM-5 After that, we need
to further explore the formation mechanism of the more HCHO generated from Ga(IM)HZSM-5. HCHO in the MTH reaction was suggested to be mainly produced through three pathways, including
disproportionation of MeOH on the acid sites of HZSM-5 (Eq. (1))26,27,28, hydrogen transfer from MeOH to olefins on LAS (Eq. (2))14 and catalytic dehydrogenation of MeOH (Eq. (3))17.
$$2{{{{{{\rm{CH}}}}}}}_{3}{{{{{\rm{OH}}}}}}\to {{{{{\rm{HCHO}}}}}}+{{{{{{\rm{CH}}}}}}}_{4}+{{{{{{\rm{H}}}}}}}_{2}{{{{{\rm{O}}}}}}$$ (1)
$${{{{{{\rm{CH}}}}}}}_{3}{{{{{\rm{OH}}}}}}+{{{{{\rm{Olefin}}}}}}\to {{{{{\rm{HCHO}}}}}}+{{{{{\rm{Alkane}}}}}}$$ (2) $${{{{{{\rm{CH}}}}}}}_{3}{{{{{\rm{OH}}}}}}\to
{{{{{\rm{HCHO}}}}}}+{{{{{{\rm{H}}}}}}}_{2}$$ (3) According to the yields of main MTH products over the parent HZSM-5 and Ga(IM-A/B/C)HZSM-5 (Fig. 2a and Supplementary Table 1), Ga(IM)HZSM-5
did not produce more methane than the parent HZSM-5. In addition, the HCHO yield remained high even when the catalysts were almost completely deactivated and nearly no olefins were generated
(Fig. 1), under which hydrogen transfer from MeOH to olefins was negligible. It suggested that the increment of HCHO did not originate from the pathway of MeOH disproportionation and
hydrogen transfer. Hence, the more reasonable pathway to generate the increased HCHO yield over Ga(IM)HZSM-5 is the direct dehydrogenation of MeOH (Eq. (3)), which can be supported by the
concomitant increase in hydrogen production. The production of both H2 and HCHO was promoted with increasing Ga loading (Fig. 2b, c). The strong correlations between HCHO, H2, C2H4 and
aromatics (Supplementary Fig. 6) depicted a clear reaction network, i.e., the direct dehydrogenation of MeOH over Ga(IM)HZSM-5 enhanced the HCHO-induced aromatic cycle. A similar mechanism
was also observed over Zn/H-ZSM-5 prepared by Zn impregnation29. DETERMINATION OF ACTIVE SITES To determine the exact active sites that catalyze the dehydrogenation of MeOH, a series of
characterizations were conducted. Through the impregnation method, Ga atoms can hardly be incorporated into the zeolite framework, and the main Ga species on Ga(IM)HZSM-5 are considered to
be Ga2O3 and cationic Ga species (GaO+ or hydrated GaO+ ions)7,30. The FT-IR spectra of Ga-modified HZSM-5 presented a new peak at 3666 cm−1, which could be ascribed to GaOH groups31,
implying the existence of extra-framework Ga species, such as GaO(OH) and highly dispersed Ga2O3 (Supplementary Fig. 8). Nanoscale Ga2O3 nanoparticles with uniform size distributions were
also observed over HZSM-5 matrix, as shown in TEM images (Supplementary Fig. 9). In contrast, the lack of GaOH peak in FT-IR spectra and Ga2O3 particles in TEM for Ga(IE)HZSM-5 demonstrated
that cationic GaO+ was predominant after washing with plenty of deionized water. As demonstrated in Table 1, both NH3-TPD and FT-IR of pyridine adsorption were conducted to reveal the acidic
properties of the parent HZSM-5 and Ga-modified HZSM-5 zeolites. The NH3-TPD patterns presented three desorption peaks, located at 125 °C, 223 °C and 450 °C, which could be assigned to
weak, medium and strong acid sites, respectively (Supplementary Fig. 10). The total acid amount initially decreased and then remained almost unchanged with increasing Ga loading. A similar
trend of BAS indicated that ion exchange occurred between Ga species and HZSM-5. The ion exchange capacity might level off when the Ga content reached 2.4 wt%. More LAS were generated at the
expense of BAS, suggesting the appearance of new acid sites that might be cationic GaO+ or Ga(OH)2+ species12. The systematic characterizations confirmed that Ga2O3 and cationic GaO+
coexisted in Ga(IM)HZSM-5. The dominant Ga species should be Ga2O3, after all the saturated exchange capacity of GaO+ was only 0.2 wt%. To study the catalytic performance of BAS, Ga2O3 and
cationic GaO+, MeOH was directly passed through Ga(IM-B)Silicalite-1 (with 2.5 wt.% Ga loading), single Ga2O3 and Ga(IE)HZSM-5, respectively. The formation of HCHO markedly increased, but
none of hydrocarbon products were detected over Ga(IM-B)Silicalite-1, indicating that Brønsted acid sites are indispensable to the MTH process (Supplementary Fig. 12). A considerable amount
of HCHO was produced, and dimethyl ether was also generated over pure Ga2O3 (Supplementary Fig. 13). Moreover, hydrogen was detected by GC‒MS experiments under atmospheric pressure
(Supplementary Table 2). The above results indicated that Ga2O3 can dehydrogenate MeOH to HCHO with the release of H232. In MeOH conversion over Ga2O3/HZSM-5 (prepared by physically mixing
Ga2O3 and HZSM-5), although Hutchings et al. suggested that the active sites for promoting aromatic production were located in the Ga2O3/zeolite interface region11, the comparative
experiments over Ga2O3/HZSM-5 with and without Y2O3 indicated that the increase in aromatic selectivity in MTH over Ga2O3/HZSM-5 is mainly due to the more HCHO generated from the
dehydrogenation of MeOH over Ga2O3 (Supplementary Fig. 14 and Supplementary Table 2). However, Ga(IE)HZSM-5 exhibited the opposite behavior compared with Ga(IM)HZSM-5 under low-pressure
conditions. Ga(IE)HZSM-5 produced even less HCHO than HZSM-5, leading to lower yields of aromatics. However, Ga(IM-D)HZSM-5 with 0.2 wt.% Ga loading still showed a slight promoting effect on
HCHO and aromatic formation (Supplementary Fig. 15). This result firmly excluded the role of cationic GaO+ in dehydrogenating MeOH to HCHO. The lower HCHO over Ga(IE)HZSM-5 could be
attributed to the decreased acid sites for disproportionation of MeOH or hydrogen transfer, resulting in impairing aromatic cycle33. In other words, this result strongly indicates that Ga2O3
should be assigned as the active phase in Ga(IM)HZSM-5 for the dehydrogenation of MeOH to HCHO. It should be noted that Ga(IE)HZSM-5 produced more ethene and aromatics, as well as H2, than
HZSM-5 under atmospheric pressure experiments (Supplementary Table 2). There should exist another pathway for aromatic formation that involves the role of cationic GaO+. Thus, the
LAS-induced aromatic formation pathway could be confirmed. Lewis acid sites (GaO+ species) cooperated with Brønsted acid sites to promote the dehydrogenation of alkenes and further
contributed to the formation of aromatics12,34,35. The fast desorption of alkenes from catalysts under low pressure may explain the inconsistent aromatic selectivity for Ga(IE)HZSM-5
reacting at 2 Torr and atmospheric pressure. The LAS-induced dehydrogenation pathway was diminished to a negligible level during the SR-PIMS experiments. TRANSFORMATION MECHANISM AFTER
REDUCTION‒OXIDATION TREATMENT In previous works, HZSM-5 modified by a wetness impregnation method was further subjected to reduction‒oxidation treatment, and the sample showed higher
aromatic selectivity and released more H27. In this work, Ga(IM-B)HZSM-5 was further treated under H2-O2 flow, and the resultant sample was denoted as Ga(IM-B)HZSM-5(redox). Compared with
Ga(IM-B)HZSM-5, Ga(IM-B)HZSM-5(redox) did produce more aromatics in the first few minutes of the MTH reaction (Fig. 3 and Supplementary Fig. 16). An interesting phenomenon was observed in
which the HCHO yield changed significantly in MTH over Ga(IM-B)HZSM-5(redox) (Fig. 3a). Different from the time-resolved profiles of HCHO over Ga(IM)HZSM-5 without reduction‒oxidation
treatment, the HCHO yield of Ga(IM-B)HZSM-5(redox) reached the maximum value at the moment after feeding MeOH, and then rapidly decreased to a stable yield within 30 seconds due to the
competitive catalytic reactions through the rapidly formed hydrocarbon pool (HCP), as shown in Fig. 3e. After that, the HCHO yield remained stable at a high value, and only slightly
decreased before deactivation. The acid properties of Ga(IM-B)HZSM-5(redox) presented significant decrease in strong acid sites and BAS, along with distinct increase in weak and medium acid
sites and LAS (Table 1)36. This result was consistent with the reported phenomenon that Ga2O3 particles located on the external surface of zeolite crystallites could be transformed into
highly dispersed cationic Ga species after reduction‒oxidation treatment37,38. The XPS spectra of Ga(IM-B)HZSM-5(redox) displayed much lower surface Ga intensity and higher binding energy,
similar to Ga(IE)HZSM-5, further proving the migration of gallium from the external surface of the zeolite crystallites to their intracrystalline volume (Fig. 4a)39. The H2-TPR profiles
showed huge differences in reductive properties for various Ga-based samples (Fig. 4b). There was a broad peak for pure Ga2O3. The main peak near 600 °C for Ga(IM)HZSM-5 could be assigned to
the reduction of well-dispersed Ga2O3 particles, which shifted to higher temperature with higher Ga loading, consistent with the larger Ga2O3 particles over Ga(IM-C)HZSM-5 observed from TEM
images. The peaks over 800 °C could be attributed to the reduction of large and bulk Ga2O3 particles separated from the zeolite matrix. No reduction peak was observed from Ga(IE)HZSM-5 in
the profiles, which revealed that Ga species sitting at the cationic locations could hardly be reduced by H240. Remarkably, the reduction peak of Ga(IM-B)HZSM-5(redox) shifted toward much
lower temperature, indicating the generation of smaller Ga2O3 particles through redox treatment. Combined with the fact that cationic GaO+ had no activity to generate HCHO, we could
attribute the sharp increase in HCHO over Ga(IM-B)HZSM-5(redox) to the redispersed Ga2O3 active phase entering the zeolite channels. In addition, another route to aromatic formation that
originated from the dehydrogenation of alkenes would also be enhanced due to the much higher LAS concentration for Ga(IM-B)HZSM-5(redox) (Supplementary Table 2). It demonstrated that both
the LAS-induced and HCHO-induced aromatic formation pathways coexisted over Ga(IM)HZSM-5. The former pathway involved cationic GaO+ species, and the latter pathway took Ga2O3 as the active
phase (Fig. 5). GaO+ species combined with adjacent BAS promoted the dehydrogenation process of cycloalkenes and cycloalkanes to higher unsaturated hydrocarbons, up to aromatics.
Formaldehyde reacted with alkenes at BAS to form dienes and then react stepwise to form H-poor hydrocarbons and eventually aromatics (Supplementary Fig. 17). CONCLUSIONS In this work, we
studied the MTH reaction over Ga-modified HZSM-5 with the advantage of in situ SR-PIMS. Some new insights are provided here. 1) The HCHO-induced aromatic formation pathway promoted by
Ga(IM)HZSM-5 producing more HCHO was found to play an important role in increasing aromatic yields. 2) More HCHO was attributed to the direct dehydrogenation of MeOH, and Ga2O3 in
Ga-modified HZSM-5 was confirmed to be the active center. 3) Cationic GaO+ enhanced alkenes dehydrogenation and Ga2O3 promoted methanol dehydrogenation process, which contributed together to
aromatic formation. 4) Upon reduction‒oxidation treatment, redispersed Ga2O3 with smaller sizes in Ga(IM)HZSM-5(redox) was found to further enhance MeOH dehydrogenation reaction, and
therefore produce more HCHO at a faster rate. Simultaneously, the significant increase in LAS concentration after redox treatment promoted alkene dehydrogenation-aromatization processes. The
above perspectives supplement the single LAS-induced aromatic formation mechanism with new insights into the HCHO-induced aromatic formation pathway over Ga-modified zeolites and provide a
new thought to manipulate the MTH reaction. METHODS CATALYST PREPARATIONS HZSM-5 (SiO2/Al2O3 = 36) was purchased from Nankai University Catalyst Co., Ltd. (Tianjing, China). Ga(IM)HZSM-5 was
prepared by impregnation method. In the preparation, 6.0 g of HZSM-5 was added into 10 mL of Ga(NO3)3 solutions containing 0.2, 0.5, 1.0 and 0.04 g of Ga(NO3)3·xH2O. The resulting mixed
solution was continuously stirred at 50 °C until it became dry. The dry mixture was subsequently dried in flowing air (50 sccm) at 110 °C for 6 h, and then calcined in flowing air (50 sccm)
at 550 °C for 5 h. The three Ga(IM)HZSM-5 samples with different Ga loadings were denoted as Ga(IM-A)HZSM-5, Ga(IM-B)HZSM-5, Ga(IM-C)HZSM-5 and Ga(IM-D)HZSM-5, respectively. The same
procedure as Ga(IM-B)HZSM-5 was applied for preparation of Ga(IM-B)Silicalite-1. The prepared Ga(IM)HZSM-5 was further treated under 50 sccm of pure hydrogen at 450 °C for 1 h at a heating
rate of 10 °C min-1, and then under 50 sccm of dry air at 450 °C for 1 h. The obtained sample was denoted as Ga(IM)HZSM-5(redox). Ga(IE)HZSM-5 was prepared by ion exchange method. In total,
3.0 g of HZSM-5 was dispersed in 50 mL of 1 M Ga(NO3)3 solution. After stirring at 80 °C for 4 h, the zeolite was filtered and washed with large amount of deionized water, followed by drying
at 100 °C for 6 h. The above steps were repeated three times. Finally, the resulting sample was calcined at 550 °C for 5 h at a heating rate of 1 °C min-1. Ga(IM)HZSM-5/Y2O3 was prepared by
physically mixing the prepared Ga(IM)HZSM-5 with Y2O3 at a mass ratio of 1:1 using an agate mortar and pestle. Ga2O3/HZSM-5 and Ga2O3/HZSM-5/Y2O3 were also prepared by physical mixing
method. The mass ratio of Ga2O3 and HZSM-5 in Ga2O3/HZSM-5 is 0.2:3, and the mass ratio of Ga2O3, HZSM-5 and Y2O3 in Ga2O3/HZSM-5/Y2O3 is 0.2:3:3. All kinds of powder samples were
pelletized, crushed and sieved to 30-40 mesh before catalytic testing. CATALYST CHARACTERIZATIONS The Ga contents in the prepared samples were determined by an inductively coupled
plasma-atomic emission spectroscopy (ICP‒AES) method using Thermo iCAP 7600 equipment. In a typical test, 50 mg of zeolite sample was dissolved in the mixture of 2 mL of HClO4, 8 mL of HF
and 4 mL of HNO3, and then diluted to 250 mL with deionized water. Powder X-ray diffraction (XRD) patterns were obtained on a Smartlab diffractometer using Cu Kα radiation at 40 KV and 150
mA. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-2011 instrument operating at 200 KV. Nitrogen adsorption/desorption isotherms were tested at −196 °C on a
TriStar II 3020 M system. Before measurement, the samples were degassed at 300 °C for 30 min under vacuum conditions. The specific surface area was determined by the Brunauer‒Emmett‒Teller
(BET) method, and the pore volume was calculated by the t-Plot method. X-ray photoelectron spectra (XPS) were recorded with an ESCALAB 250Xi spectrometer. The FT-IR experiments of the
catalysts, pressed into tablets with KBr before scanning, were performed by Nicolet 8700. The zeolite/KBr tablet was heated at 105 °C for 12 h to remove water prior to analysis.
Temperature-programmed reduction (H2-TPR) was measured by a VDSorb-91i-VAP-HB chemical adsorption instrument. Before testing, 100 mg of catalyst was heated in Ar flow at 500 °C for 1 h at a
heating rate of 10 °C min-1. Afterward, the TPR test was performed from 200 °C to 1000 °C at a heating rate of 10 °C min-1 in a mixture of 10% H2/Ar flow. NH3 temperature-programmed
desorption (NH3-TPD) was also employed on a VDSorb-91i-VAP-HB chemical adsorption instrument. Typically, 100 mg of sample was pretreated at 500 °C for 1 h at a heating rate of 10 °C min-1
under He flow. After cooling to 40 °C, 1% NH3/He flow was introduced and adsorbed for 1 h, followed by purging in He flow for another 1 h to remove the physisorbed NH3. Subsequently, a
temperature program was performed from 40 °C to 800 °C at a heating rate of 10 °C min-1. The FT-IR spectra of pyridine adsorption were recorded by a Nicolet 5700 FTIR spectrometer. In total,
15 mg of catalyst sample was pressed into self-supporting wafers (R. 6.5 mm) and pretreated in vacuum at 400 °C for 2 h at a heating rate of 10 °C min-1. Then, they were exposed to
excessive amount of pyridine and further evacuated at 200 °C for 1 h. After that, the system was heated to 200 °C, 300 °C and 400 °C under vacuum, and the IR spectra were recorded. The
concentrations of Brønsted acid sites (BASs) and Lewis acid sites (LASs) were quantified from the IR spectra at 200 °C and based on the band areas at 1510–1564 cm−1 and 1421–1466 cm−1,
respectively. CATALYTIC TESTING Catalytic reactions were carried out under both low-pressure and atmospheric pressure conditions. To capture the intermediately formed formaldehyde, an in
situ low-pressure catalytic reactor combined with in situ synchrotron radiation photoionization mass spectrometry (SR-PIMS) was employed to perform the catalytic conversion of methanol. The
co-feeding of formaldehyde was conducted by the mixture of 5 wt.% trioxane in methanol41. The low-pressure catalytic experiment apparatus mainly included a bubbler feeding system, a
low-pressure catalytic reactor and a homemade orthogonal time-of-flight mass spectrometer (_oa_ TOF-MS) (Supplementary Fig. 1). The ionization source used in the mass spectrometer was the
synchrotron VUV light. The catalyst was placed in a quartz reactor (O.D. 8 mm, I.D. 6 mm, L. 150 mm) of the low-pressure catalytic reactor. A K-type thermocouple wrapped in a quartz tube was
inserted adjacent to the catalyst. In this way, the temperature of the catalyst can be detected in real time. During the low-pressure experiment, the pressure of the catalytic reactor was
maintained at 2 Torr, which was achieved by a closed-loop control system consisting of a pressure sensor, a butterfly valve (Model 61232-KEGG-0002, VAT, Switzerland) and a vacuum pump. For
the atmospheric pressure experiment, the catalysts were placed in front of the quartz sand plate in a quartz reactor (O.D. 10 mm, I.D. 7 mm, L. 340 mm) (Supplementary Fig. 2), and quartz
wool was placed before and after the catalysts for fixation. The effluent products flowed through the heated transfer line into GC‒MS (Agilent 5977B GC/MSD & 8890 GC System) for online
analysis. The transfer line was heated to 200 °C to avoid condensation of volatile products. In this GC‒MS, the thermal conductivity detector (TCD) equipped with a HayeSep Q column was used
for inorganic product analysis, and the flame ionization detector (FID) equipped with a HP-PLOT/Q capillary column was used for organic compound analysis. In the catalytic testing, 150 mg of
samples for HZSM-5, Ga(IM)HZSM-5, single Ga2O3, Ga(IM)HZSM-5(redox), Silicalite-1 and Ga(IM)Silicalite-1 were used. To ensure that the amounts of the zeolite contained in the physically
mixed samples were the same, 300 mg of Ga(IM)HZSM-5/Y2O3, 160 mg of Ga2O3/HZSM-5 and 310 mg of Ga2O3/HZSM-5/Y2O3 were used, respectively. Before the catalytic reaction, the catalysts were
pretreated in Ar flow (250 sccm) at 500 °C for two hours at a heating rate of 10 °C min-1. DATA AVAILABILITY All results are reported in the main paper and Supplementary information. All
other data are available from the authors upon request. REFERENCES * Olsbye, U. et al. Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity.
_Angew. Chem. Int. Ed. Engl._ 51, 5810–5831 (2012). Article PubMed CAS Google Scholar * Ilias, S. & Bhan, A. Mechanism of the catalytic conversion of methanol to hydrocarbons. _ACS
Catal._ 3, 18–31 (2013). Article CAS Google Scholar * Yarulina, I., Chowdhury, A. D., Meirer, F., Weckhuysen, B. M. & Gascon, J. Recent trends and fundamental insights in the
methanol-to-hydrocarbons process. _Nat. Catal._ 1, 398–411 (2018). Article CAS Google Scholar * Ono, Y., Adachi, H. & Senoda, Y. Selective conversion of methanol into
aromatic-hydrocarbons over zinc-exchanged ZSM-5 zeolites. _J. Chem. Soc. Faraday Trans._ 84, 1091–1099 (1988). Article CAS Google Scholar * Freeman, D., Wells, R. P. K. & Hutchings,
G. J. Methanol to hydrocarbons: enhanced aromatic formation using a composite Ga2O3-H-ZSM-5 catalyst. _Chem. Commun_. 1754–1755 (2001). * Zhang, J. G., Qian, W. Z., Kong, C. Y. & Wei, F.
Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst. _ACS Catal._ 5, 2982–2988 (2015). Article CAS Google Scholar * Gao, P.
et al. Bronsted/Lewis acid synergy in methanol-to-aromatics conversion on Ga-modified ZSM-5 zeolites, as studied by solid-state NMR spectroscopy. _ACS Catal._ 8, 69–74 (2018). Article CAS
Google Scholar * Pinilla-Herrero, I. et al. High Zn/Al ratios enhance dehydrogenation vs hydrogen transfer reactions of Zn-ZSM-5 catalytic systems in methanol conversion to aromatics. _J.
Catal._ 362, 146–163 (2018). Article CAS Google Scholar * Zhang, Y. K., Qu, Y. X., Wang, D. L., Zeng, X. C. & Wang, J. D. Cadmium modified HZSM-5: a highly efficient catalyst for
selective transformation of methanol to aromatics. _Ind. Eng. Chem. Res._ 56, 12508–12519 (2017). Article CAS Google Scholar * Freeman, D., Wells, R. P. K. & Hutchings, G. J.
Conversion of methanol to hydrocarbons over Ga2O3/H-ZSM-5 and Ga2O3/WO3 catalysts. _J. Catal._ 205, 358–365 (2002). Article CAS Google Scholar * Lopez-Sanchez, J. A. et al. Reactivity of
Ga2O3 clusters on zeolite ZSM-5 for the conversion of methanol to aromatics. _Catal. Lett._ 142, 1049–1056 (2012). Article CAS Google Scholar * Gao, P. et al. A mechanistic study of
methanol-to-aromatics reaction over Ga-modified ZSM-5 zeolites: understanding the dehydrogenation process. _ACS Catal._ 8, 9809–9820 (2018). Article CAS Google Scholar * Lai, P.-C., Chen,
C.-H., Lee, C.-H. & Lin, Y.-C. Methanol conversion to aromatics over Ga-supported HZSM-5 with evolved meso- and microporosities by desilication. _Chemistryselect_ 1, 6335–6344 (2016).
Article CAS Google Scholar * Muller, S. et al. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. _J. Am. Chem. Soc._ 138, 15994–16003 (2016).
Article PubMed Google Scholar * Martinez-Espin, J. S. et al. New insights into catalyst deactivation and product distribution of zeolites in the methanol-to-hydrocarbons (MTH) reaction
with methanol and dimethyl ether feeds. _Catal. Sci. Technol._ 7, 2700–2716 (2017). Article CAS Google Scholar * Hwang, A. & Bhan, A. Deactivation of zeolites and zeotypes in
methanol-to-hydrocarbons catalysis: mechanisms and circumvention. _Acc. Chem. Res._ 52, 2647–2656 (2019). Article PubMed CAS Google Scholar * Liu, Y. et al. Formation mechanism of the
first carbon‒carbon bond and the first olefin in the methanol conversion into hydrocarbons. _Angew. Chem. Int. Ed. Engl._ 55, 5723–5726 (2016). Article PubMed CAS Google Scholar *
Martinez-Espin, J. S. et al. Hydrogen transfer versus methylation: on the genesis of aromatics formation in the methanol-to-hydrocarbons reaction over H-ZSM-5. _ACS Catal._ 7, 5773–5780
(2017). Article CAS Google Scholar * Liu, Y. et al. Critical role of formaldehyde during methanol conversion to hydrocarbons. _Nat. Commun._ 10, 9 (2019). CAS Google Scholar * Arora, S.
S. & Bhan, A. The critical role of methanol pressure in controlling its transfer dehydrogenation and the. corresponding effect on propylene-to-ethylene ratio during
methanol-to-hydrocarbons catalysis on H-ZSM-5. _J. Catal._ 356, 300–306 (2017). Article CAS Google Scholar * Ni, Y. M., Zhu, W. L. & Liu, Z. M. H-ZSM-5-catalyzed hydroacylation
involved in the coupling of methanol and formaldehyde to aromatics. _ACS Catal._ 9, 11398–11403 (2019). Article CAS Google Scholar * Muller, S. et al. Coke formation and deactivation
pathways on H-ZSM-5 in the conversion of methanol to olefins. _J. Catal._ 325, 48–59 (2015). Article Google Scholar * Hwang, A., Kumar, M., Rimer, J. D. & Bhan, A. Implications of
methanol disproportionation on catalyst lifetime for methanol-to-olefins conversion by HSSZ-13. _J. Catal._ 346, 154–160 (2017). Article CAS Google Scholar * Wen, W. et al. Formation and
fate of formaldehyde in methanol-to-hydrocarbon reaction: in situ synchrotron radiation photoionization mass spectrometry study. _Angew. Chem. Int. Ed. Engl._ 59, 4873–4878 (2020). Article
PubMed CAS Google Scholar * Hwang, A. & Bhan, A. Bifunctional strategy coupling Y2O3-catalyzed alkanal decomposition with methanol-to-olefins catalysis for enhanced lifetime. _ACS
Catal._ 7, 4417–4422 (2017). Article CAS Google Scholar * Kubelková, L., Nováková, J. & Jírů, P. Reaction of small amounts of methanol on Hzsm-5, Hy and modified Y zeolites. _Stud.
Surf. Sci. Catal._ 18, 217–224 (1984). Article Google Scholar * Sun, X. Y. et al. On reaction pathways in the conversion of methanol to hydrocarbons on HZSM-5. _J. Catal._ 317, 185–197
(2014). Article CAS Google Scholar * Comas-Vives, A., Valla, M., Coperet, C. & Sautet, P. Cooperativity between Al Sites promotes hydrogen transfer and carbon‒carbon bond formation
upon dimethyl ether activation on alumina. _ACS Cent. Sci._ 1, 313–319 (2015). Article PubMed PubMed Central CAS Google Scholar * Ni, Y., Zhu, W. & Liu, Z. Formaldehyde intermediate
participating in the conversion of methanol to aromatics over zinc modified H-ZSM-5. _J. Energy Chem._ 54, 174–178 (2021). Article CAS Google Scholar * Chen, Y. Y., Chang, C. J., Lee, H.
V., Juan, J. C. & Lin, Y. C. Gallium-immobilized carbon nanotubes as solid templates for the synthesis of hierarchical Ga/ZSM-5 in methanol aromatization. _Ind. Eng. Chem. Res._ 58,
7948–7956 (2019). Article CAS Google Scholar * Kazansky, V. B., Subbotina, I. R., van Santen, R. A. & Hensen, E. J. M. DRIFTS study of the chemical state of modifying gallium ions in
reduced Ga/ZSM-5 prepared by impregnation - I. Observation of gallium hydrides and application of CO adsorption as a molecular probe for reduced gallium ions. _J. Catal._ 227, 263–269
(2004). Article CAS Google Scholar * Merko, M., Busser, G. W. & Muhler, M. Non-oxidative dehydrogenation of methanol to formaldehyde over bulk beta-Ga2O3. _ChemCatChem_, 14 (2022). *
Westgard Erichsen, M., Svelle, S. & Olsbye, U. The influence of catalyst acid strength on the methanol to hydrocarbons (MTH) reaction. _Catal. Today_ 215, 216–223 (2013). Article Google
Scholar * Lai, P. C., Chen, C. H., Hsu, H. Y., Lee, C. H. & Lin, Y. C. Methanol aromatization over Ga-doped desilicated HZSM-5. _Rsc Adv._ 6, 67361–67371 (2016). Article CAS Google
Scholar * Hsieh, C. Y., Chen, Y. Y. & Lin, Y. C. Ga-Substituted Nanoscale HZSM-5 in Methanol Aromatization: The Cooperative Action of the Bronsted Acid and the Extra-Framework Ga
Species. _Ind. Eng. Chem. Res._ 57, 7742–7751 (2018). Article CAS Google Scholar * Xiao, H. et al. Mechanistic insight to acidity effects of Ga/HZSM-5 on its activity for propane
aromatization. _Rsc Adv._ 5, 92222–92233 (2015). Article CAS Google Scholar * Nowak, I., Quartararo, J., Derouane, E. G. & Vedrine, J. C. Effect of H-2-O-2 pre-treatments on the state
of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. _Appl. Catal. A-Gen._ 251, 107–120 (2003). Article CAS Google Scholar * Hensen, E. J. M. et al. In situ GaK edge XANES study of
the activation of Ga/ZSM-5 prepared by chemical vapor deposition of trimethylgallium. _Catal. Lett._ 101, 79–85 (2005). Article CAS Google Scholar * Nowak, I., Quartararo, J., Derouane,
E. G. & Vedrine, J. C. Effects of reducing and oxidising pre-treatments on the state of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. _Stud. Surf. Sci. Catal._ 145, 201–204
(2003). Article CAS Google Scholar * Xin, M. D. et al. Ga substitution during modification of ZSM-5 and its influences on catalytic aromatization performance. _Ind. Eng. Chem. Res._ 58,
6970–6981 (2019). Article CAS Google Scholar * Li, N. et al. Bifunctional zeolites-silver catalyst enabled tandem oxidation of formaldehyde at low temperatures. _Nat. Commun_. 13 (2022).
Download references ACKNOWLEDGEMENTS This work was supported by grants from the National Key Research and Development Program of China (2017YFA0402800), the Natural Science Foundation of
China (No. 91845203, 92045301 and 91945302), and the CAS Key Technology Talent Program. AUTHOR INFORMATION Author notes * These authors contributed equally: Wu Wen, Tianci Xiao. AUTHORS AND
AFFILIATIONS * National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029, Anhui, P. R. China Wu Wen, Tianci Xiao, Beibei Feng, Jiuzhong Yang,
Zhandong Wang, Jun Bao, Chengyuan Liu & Yang Pan * Key Laboratory for Power Machinery and Engineering of Ministry of Education, Shanghai Jiao Tong University, Shanghai, 200240, P. R.
China Chaoqun Zhou, Hao Ma, Zhongyue Zhou & Fei Qi * Shanghai Research Institute of Petrochemical Technology SINOPEC, Shanghai, 201208, P. R. China Jian Li * Department of Chemistry,
University of Science and Technology of China, Hefei, 230029, Anhui, P. R. China Ying Zhang Authors * Wu Wen View author publications You can also search for this author inPubMed Google
Scholar * Tianci Xiao View author publications You can also search for this author inPubMed Google Scholar * Beibei Feng View author publications You can also search for this author inPubMed
Google Scholar * Chaoqun Zhou View author publications You can also search for this author inPubMed Google Scholar * Jian Li View author publications You can also search for this author
inPubMed Google Scholar * Hao Ma View author publications You can also search for this author inPubMed Google Scholar * Zhongyue Zhou View author publications You can also search for this
author inPubMed Google Scholar * Ying Zhang View author publications You can also search for this author inPubMed Google Scholar * Jiuzhong Yang View author publications You can also search
for this author inPubMed Google Scholar * Zhandong Wang View author publications You can also search for this author inPubMed Google Scholar * Fei Qi View author publications You can also
search for this author inPubMed Google Scholar * Jun Bao View author publications You can also search for this author inPubMed Google Scholar * Chengyuan Liu View author publications You can
also search for this author inPubMed Google Scholar * Yang Pan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W. Wen and T. Xiao designed
the experiments, prepared the catalysts, performed most of the catalytic tests, analyzed the experimental data and wrote the paper. B. Feng performed the GC‒MS experiments of the MTH
reaction under atmospheric pressure. C. Zhou, J. Li and Y. Zhang performed catalysts characterization. H. Ma and J. Bao provided technical support for catalyst preparation. Z. Zhou, J. Yang
and F. Qi provided the support of synchrotron radiation photoionization mass spectrometry technology, as well as the corresponding qualitative and quantitative methods. Z. Wang provided the
technology and method of catalytic evaluation under atmospheric pressure. C. Liu and Y. Pan conceived and designed the research, analyzed the experimental results and cowrote the paper. All
authors participated in the discussion of the results and the preparation of the paper. CORRESPONDING AUTHORS Correspondence to Chengyuan Liu or Yang Pan. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Chemistry_ thanks Zhangfeng Qin and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PAN_PR FILE SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wen, W., Xiao, T., Feng, B. _et al._ Role of formaldehyde in
promoting aromatic selectivity during methanol conversion over gallium-modified zeolites. _Commun Chem_ 5, 153 (2022). https://doi.org/10.1038/s42004-022-00771-8 Download citation *
Received: 30 June 2022 * Accepted: 04 November 2022 * Published: 19 November 2022 * DOI: https://doi.org/10.1038/s42004-022-00771-8 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative