- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We review two types of inorganic nanomaterials—metal chalcogenide quantum dots (QDs) and lead halide perovskites—that serve as prospective light harvesters in hybrid mesoscopic
solar cells. Metal chalcogenide QDs are introduced in three parts: chalcogenides of cadmium (CdS, CdSe and CdTe), chalcogenides of lead (PbS and PbSe) and chalcogenides of antimony (Sb2S3
and Sb2Se3). The devices made using these chalcogenide QDs in a liquid-type electrolyte showed the best cell efficiencies, ranging from 3 to 6%. For solid-state QD-sensitized solar cells
(QDSCs), the device performances were generally poor; only devices made of Sb2S3 and PbS QDs attained cell efficiencies approaching ∼7%. In contrast, nanocrystalline lead halide perovskites
have emerged since 2009 as potential photosensitizers in liquid-type sensitized TiO2 solar cells. In 2012, the efficiencies of the all-solid-state perovskite solar cells were enhanced to 9.7
and 10.9% using anodes of TiO2 and Al2O3 films, respectively, with 2,2′,7,7′-tetrakis-(_N_,_N_-di-_p_-methoxyphenylamine)9,9′-spirobifluorene (spiro-OMeTAD) as a hole-transporting material.
In 2013, the performance of a TiO2 solar cell sensitized with lead iodide perovskite (CH3NH3PbI3) was optimized further to attain an overall power conversion efficiency η=15%, which is a
new milestone for solar cells of this type having a device structure similar to that of a dye-sensitized solar cell. SIMILAR CONTENT BEING VIEWED BY OTHERS PHASE-HETEROJUNCTION ALL-INORGANIC
PEROVSKITE SOLAR CELLS SURPASSING 21.5% EFFICIENCY Article 31 July 2023 THE STUDY OF INORGANIC ABSORBER LAYERS IN PEROVSKITE SOLAR CELLS: THE INFLUENCE OF CDTE AND CIGS INCORPORATION
Article Open access 26 March 2025 HETEROJUNCTION FORMED VIA 3D-TO-2D PEROVSKITE CONVERSION FOR PHOTOSTABLE WIDE-BANDGAP PEROVSKITE SOLAR CELLS Article Open access 06 November 2023
INTRODUCTION Generating cost-effective and environmentally benign renewable energy remains a major challenge for scientific development. Solar energy, which is abundant and sustainable, has
attracted enormous interest in terms of research and development for many years. The developments in emerging photovoltaic techniques are taking place at a rapid pace. Most such devices
involve inexpensive solution processing with high device performance.1, 2, 3 Solar cells (SCs) of these new types differ from traditional SCs in their unique mesoscopic structural features,
with large surface areas that dominate their photovoltaic performance.4, 5, 6 For example, a dye-sensitized SC (DSSC) uses a monolayer of light-absorbing sensitizer anchored on
nanocrystalline TiO2 to enhance light harvesting in a mesoporous environment in which charge separation also occurs at this interface. The design of a DSSC involves an important feature: the
light harvesting and charge transport are spatially decoupled.7 Since the early success of using nanocrystalline TiO2 as a charge collector, DSSCs have been considered a low-cost
alternative to semiconductor-based photovoltaic devices.8 In 2011, Yeh, Diau and Grätzel developed an efficient DSSC based on an _ortho_-substituted porphyrin sensitizer, YD2-oC8,
co-sensitized with an organic dye (Y123) with a cobalt electrolyte to attain remarkable power conversion efficiency (PCE=12.3%),9 which opened a new research area on porphyrin-sensitized SCs
(PSSCs).10 However, the absorption spectrum of the YD2-oC8 system covers only the visible spectral region, 400–700 nm, and the lack of light-harvesting ability beyond 700 nm (near infrared)
limits the PSSC from further enhancement of the device performance. Moreover, the enduring stability of PSSCs might be an issue for future commercialization. Various inorganic light
absorbers with a light-harvesting capability that extends into the near-infrared region were hence sought to replace the delicate organic dyes. Inorganic semiconductor quantum dots (QDs)
have been extensively investigated as new photosensitizers or light absorbers to replace conventional organic-type sensitizers in DSSCs because of their excellent properties—tunable energy
band gaps via varied and controlled size and shape of the nanocrystals, large optical absorption coefficients, large dipole moments for enhanced charge separation, and multiple exciton
generation (MEG).11, 12, 13, 14, 15, 16, 17, 18 For example, the performance of QD-sensitized SCs (QDSCs) has been significantly enhanced for Sb2S3-sensitized TiO2 solar cells using a
blended layer of poly[2,6-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b;3,4-b′]-dithiophene)-_alt_-4,7-(2,1,3-benzothiadiazole)] (PCPDTBT) and [6,6]-phenyl C61-butyric acid methyl ester (PCBM)
as a hole-transporting material (HTM) to attain _η_=6.3%.15 Hybrid passivated QDSCs using PbS colloidal QDs (CQDs) as a photosensitizer attained an efficiency of more than 7.0%,13 and a
large short-circuit photocurrent density (_J_SC∼30 mA cm−2) was reported for a PbS:Hg QDSC.18 In general, using an inorganic light-harvesting material to replace a conventional organic or
organometallic sensitizer has the advantage of obtaining superior device durability. Typical semiconductor QDs have an average diameter of 1–10 nm, which indicates that surface states or
midgap trap states can be produced on the surfaces of the nanocrystals during the synthesis of the QDs via layer deposition on the photoanode of the solar cells. For mesoporous TiO2/QD solar
cells, nanocrystalline QDs can be adsorbed and deposited on the surface of the TiO2 nanoparticles, but the QD coverage on the surface of the TiO2 nanoparticles may be a problem. As a
result, these QDSC devices typically show a small open-circuit voltage (_V_OC) and a poor fill factor (FF) because of the charge loss at the interface of the TiO2/QD/HTM layers. To increase
_V_OC and FF, we must decrease the charge loss in the QD layer and suppress the charge recombination in the TiO2/QD and TiO2/HTM interfaces. For this purpose, an insulating Al2O3 scaffold,
surface passivation of QD films and interfacial engineering of buffer layers with organic and inorganic modifications have been employed to enhance the charge separation and charge transport
in solar cells.2, 13, 17 A major advance in the development of novel inorganic sensitizers occurred in 2012: Park and Grätzel reported submicrometer thin-film solid-state solar cells
attaining a PCE of 9.7% with a methyl ammonium lead-iodide perovskite sensitizer, CH3NH3PbI3, in a mesoporous TiO2 film (thickness 0.6 μm).12 This perovskite sensitizer has a band gap of 1.5
eV with the energy levels of the conduction band (CB) and the valence band (VB) matching well the CB of TiO2 and the highest occupied molecular orbital of HTM, respectively. At nearly the
same time, Snaith and co-workers11 reported a similar perovskite, CH3NH3PbI2Cl, that served as a light absorber for mesoscopic thin-film solid-state solar cells to attain a PCE of 10.9%, for
which the mesoporous Al2O3 film served as a scaffold to replace the _n_-type TiO2 electron-transporting layer. In 2013, Snaith and co-workers reported a significantly enhanced PCE of 12.3%
for perovskite CH3NH3PbI3-_x_Cl_x_ solar cells with the same device structure based on Al2O3.2 Concurrently, Seok and co-workers3 reported the same superior PCE of 12.3% for another
perovskite solar cell, CH3NH3Pb(I1-_x_Br_x_)3, with the mesoporous TiO2-based device structure. Also in 2013, Grätzel and co-workers1 reported perovskite (CH3NH3PbI3) TiO2 solar cells with a
PCE approaching 15%, which set a new record for all-solid-state hybrid mesoscopic solar cells (HMSCs). The significant discovery of perovskites as novel photovoltaic materials has hence
opened a new channel for the development of third-generation solar cells with the advantages of very high efficiency, low cost, ease of processing and considerable durability. In this
review, we summarize the recent investigations into novel inorganic materials, including both metal chalcogenide QDs and halide perovskite nanocrystals, applied as light-harvesting layers
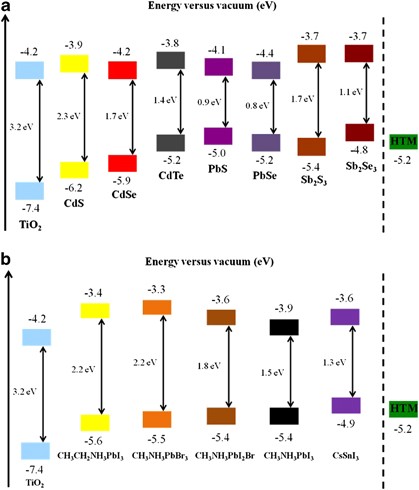
for HMSCs. Figures 1a and b are diagrams of the potential levels of various metal chalcogenide QDs and halide perovskites, respectively. Metal chalcogenide sensitizers of three types were
introduced for QDSCs: they are classified as cadmium (Cd) chalcogenides, lead (Pb) chalcogenides and antimony (Sb) chalcogenides. The band gap energies (_E_g/eV) of the metal chalcogenides
decreased systematically as the chalcogenides varied from sulfur (S) through selenium (Se) to tellurium (Te). For the halide perovskite materials, tuning the band gap of CH3NH3PbX3 was
achieved by varying the halide constituents (X=Cl, Br or I); the _E_g of the system decreased along this direction. This review provides detailed descriptions of the synthesis,
characterization, photovoltaic performance and challenges for two inorganic materials, Sb2S3 QDs and perovskite nanocrystals, because of their efficient performance as light-harvesting
materials for HMSCs. We also introduce strategies for the improvement of the performances of these QD devices and perovskite materials via surface passivation and interfacial modification
with varied device structures. CADMIUM CHALCOGENIDE QDSCS Cadmium-chalcogenide QD-based solar cells have been intensively studied over the past two decades to improve device performance and
stability in ambient conditions. The photoactive sensitizers of these solar cells are composed of cadmium sulfide (CdS),19, 20, 21, 22, 23, 24, 25 cadmium selenide (CdSe),26, 27, 28, 29, 30,
31, 32, 33 cadmium telluride (CdTe)34, 35, 36, 37, 38 and their alloy nanocrystals.39, 40, 41, 42, 43, 44, 45, 46 CdS has an optical band gap of 2.25 eV, and thus it can only absorb light
up to ∼550 nm; CdSe, with a band gap of 1.7 eV, can absorb light below ∼720 nm; CdTe has an energy gap of 1.45 eV and an optical absorption edge at ∼860 nm. The CB edges of CdSe and some
CdTe nanocrystals were located below that of the TiO2 film (Figure 2), which resulted in poor and limited electron injection from the QD to the TiO2, and substantial charge recombination
occurred at the TiO2/QD/HTM interfaces.34, 39 Moreover, the use of iodide and polysulfide electrolytes led to the degradation of these QD nanocrystals and a gradual deterioration of the
photovoltaic performances.20, 27, 28, 34 To find a solution for the above problem, co-sensitization of CdS/CdSe QDs on a TiO2 film was reported.39 The co-sensitized CdS/CdSe system showed
improved light absorption, incident photon-to-current conversion efficiency (IPCE), and PCE over individual CdS or CdSe systems because more CdSe QDs were loaded onto the TiO2 film. The rate
of electron injection from CdSe to TiO2 was significantly improved via the CdS layer and adjustment of the Fermi level, as shown in Figure 2.39 ZnS passivation served to protect the QD
sensitizers from the polysulfide electrolyte and to inhibit the charge recombination at the TiO2/electrolyte interface. For devices made of CdSe_x_Te1−_x_ alloy nanocrystals, the range of
optical absorption could be extended to ∼900 nm, which reflects the band gap tuning effect of the CdSe–CdTe alloys, especially to enhance light harvesting in the range of 750–900 nm. Figure
3 shows the procedure used to synthesize the CdSe–CdTe QD nanocrystals by means of the layer-by-layer deposition approach, for which the optical band gaps of the alloy were systematically
varied from 1.38 to 1.73 eV.40 Using core–shell nanocrystals, recombination losses at the TiO2/CdTe/CdSe interface can be effectively diminished through a cascade potential approach that
yields a superior charge separation, as shown in Figure 4. The most striking feature of this CdTe/CdSe core–shell system, with a particle size ∼10 nm, is its smaller band gap for light
absorption toward the near-infrared region (∼1000 nm).41 Instead of polysulfide electrolytes, quasi-solid-state polysulfide gel electrolytes, polymer electrolytes and solid-state HTM have
been developed and examined for this type of QDSC, but the device efficiencies were reported to be less than 5%.20, 27, 28, 42, 43 The solid-state solar cells based on CdS, CdSe and CdTe QDs
have shown PCEs less than 6% because of their large band gaps, slow electron injection rates and substantial charge recombination at the TiO2/QD/HTM interfaces. Thin-film solar cells made
of CdSe_x_Te1−_x_ alloys (_x_=0.9 or 0.8) exhibited a PCE (∼7.1%) smaller than that of a CdTe-only device (PCE=7.3% at _x_=0) because _J_SC of the former is smaller than that of the latter,
indicating that the electron transport and charge collection through the alloy layers encountered some difficulties in the devices. In summary, the ideal cadmium chalcogenide QDSC devices
should have a large recombination resistance, a small chemical capacitance and a small series resistance due to a decreased rate of recombination and fewer trap sites at the TiO2/QD
interface. Furthermore, the device performance of the CdTe/CdSe QDSCs can be further improved by increasing the QD loading on the TiO2 film to enhance the efficiency of light harvesting.
LEAD CHALCOGENIDE QDSCS Lead-chalcogenide QD-based solar cells have been intensively examined in recent years because of the small band gaps of the QDs, which allow solar energy harvesting
in the near-infrared region. For example, PbS QDs have an energy band gap in the range of 0.9–1.1 eV, and the optical absorption edge can be extended to ∼1300 nm;13, 18 the band gap energies
of PbSe QDs were tuned from 0.7 to 1.7 eV by varying the sizes of the QDs, and the optical absorption edge was further extended to ∼1500 nm.17, 47, 48 Under light illumination, the shunt
resistance of the QDSCs was smaller than in the dark; the rates of charge recombination increased at the interfaces of QD–QD and TiO2–QD because of the increased surface states and trap
states. For the PbS CQD films, a high density of midgap trap states (∼1017 cm−3 eV−1) may deteriorate the device performance (Figure 5), which could be a crucial limiting factor in improving
the device efficiency.13 PbSe QD solar cells suffered from significant current leakage and interfacial charge recombination; the film thickness of the photoactive layer was expected to be
an important parameter in optimizing the PbSe device performance.48 For PbS devices treated with CdCl2, the trap states near the middle of the PbS band gap were determined to be ∼1016 cm−3
eV−1, which is one-fifth of that in organic cross-linked and inorganic-treated PbS films.13 The PbS film treated with CdCl2 showed the greatest hole mobility, _μ_p=4.2 × 10−3 cm2 V−1 s−1,
and CdCl2 plays a major role in the passivation of the midgap trap states and charge transport in the valence band. Greater photocurrent and voltage are achieved through decreased
recombination and improved charge transport in the PbS device. Using PbS:Hg nanocrystals, the short-circuit photocurrent density of the device attained 30 mA cm−2.18 The CB of the PbS QDs
shifted upward with Hg2+ doping, and more rapid electron injection became feasible in the PbS:Hg QDSCs, as shown in Figure 6. The structural reinforcement of the Pb–S bonds and the stability
provided by the addition of Hg2+ were two major factors for the decreased charge recombination and enhanced charge transport in the PbS:Hg device. For the hydrazine-treated PbSe QDs, the
external quantum efficiency (EQE) and internal quantum efficiency (IQE) were 114% and 130%, respectively, proving the MEG effect in the PbSe device shown in Figure 7.17 PbSe QDs were treated
with ethane dithiol and hydrazine to increase the electronic coupling of the QD nanocrystals and decrease the surface states and trap states in the PbSe film. Approximately 1 mA cm−2, or
∼4% of the total photocurrent, was estimated to derive from the MEG effect in the PbSe device.17 In the core–shell structure, the PbSe core was covered with a PbS layer (thickness 0.5 nm).
PbSe/PbS core–shell QDs have an absorption edge at 960 nm (_E__g_ ∼1.29 eV).49 The PbS shell covering the PbSe core acted as surface passivation to protect the PbSe core, thus improving the
chemical stability of the QD film. The IPCE reached ∼100% at ∼420 nm, indicating that the core–shell QDs on the TiO2 surface had efficient light harvesting, enhanced electron injection from
the QD to the TiO2 CB, superior charge transport in the TiO2 layer and retarded charge recombination in the QD film. For PbS0.9Se0.1 alloy QDSCs, an EQE above 100% at a photon energy of 2.76
eV (∼440 nm) was obtained.50 PbS0.9Se0.1 alloy QDs showed a sharp absorption peak at 1076 nm due to strong quantum confinement. PbS0.9Se0.1 alloy QD films were treated with ethane dithiol
to increase electronic coupling in the PbS0.9Se0.1 QDs. Greater EQE and _J_SC values were obtained upon increasing the film thickness to 360 nm because of the increased optical absorption
and higher charge generation in the device. Other PbS(Se) QD solar cells have been reported with efforts to improve the charge transport and retard the charge recombination in the
devices.51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83 ANTIMONY CHALCOGENIDE QDSCS SYNTHESIS OF SB2S3 QDS
ON A MESOPOROUS TIO2 FILM Antimony sulfide Sb2S3 QDs have been synthesized on a mesoporous TiO2 film by means of the chemical bath deposition (CBD) method.14, 15, 84 Messina _et al._
reported a CBD procedure to form an Sb2S3 thin film coating on microscope glass slides.84 SbCl3 (1.3 g) was dissolved in acetone (5 ml); this SbCl3 solution was kept at a temperature below 4
°C for several hours. A Na2S2O3 solution (50 ml, 1 M) and deionized water (145 ml) were also kept at a temperature below 4 °C for several hours before the solutions were mixed. The cold
SbCl3 and Na2S2O3 solutions were mixed with continuous stirring, and the cold deionized water was added to the mixed solution. This final mixing initially gave a nearly clear solution. The
substrates containing the TiO2 films were placed vertically in the CBD bath for 2–3 h in a refrigerator (4–7 °C). The color of the Sb2S3-coated TiO2 film changed from yellow to orange-yellow
and finally to brown during the CBD. Thereafter, the TiO2 films coated with Sb2S3 were carefully washed with deionized water and dried at around 23 °C for several hours in air. As the
CBD-processed Sb2S3 exhibited an amorphous phase, the Sb2S3-coated TiO2 films were annealed at 300–330 °C for 30 min under Ar (or N2) inert conditions to form a crystalline stibnite
structure. Itzhaik _et al._85 reported that this annealing method, which forms an Sb2O3 surface passivation layer on the surface of the Sb2S3 sensitizer, improved the device performance,
resulting in a PCE of 3.4%. This passivation layer on the Sb2S3 surface can inhibit the electron-hole recombination through its insulating nature (_E_g ∼3.7 eV), but an Sb2O3 layer that was
thick enough to act as an energy barrier, disturbing the electron injection from the Sb2S3 sensitizer to the TiO2 film, thus degrading the device performance significantly. ELECTRONIC
PROPERTIES OF SB2S3 SENSITIZED ON TIO2 FILMS Patrick and Giustino86 reported on the structural and electronic properties of Sb2S3-sensitized QDSCs and found an almost perfect interface
between the TiO2 film and the QD sensitizer because of the lattice match of the TiO2 substrate with the Sb2S3 layer. As Figure 8 shows, no significant structural defects in the TiO2/Sb2S3
interface were found; the electronic distributions corresponding to the CB of Sb2S3 show a direct interfacial Ti–S coupling between Sb2S3 and TiO2. The CB edge (_E_CB) of the Sb2S3
sensitizer is located ∼0.5 eV above the _E_CB of the TiO2 film (Figure 1a); superior electron injection from the CB of Sb2S3 to the CB of TiO2 would hence be expected. For the TiO2/Sb2S3
solar cells, the theoretical value of _V_OC can be predicted to be as high as 1.6 V, based on the calculated energy-level diagram; this value indicates that _V_OC might be increased
experimentally by optimizing the other parameters related to hole-transporting materials. OPTICAL ABSORPTION AND IPCE OF THE SB2S3-BASED SOLAR CELLS Crystalline Sb2S3 has an optical
absorption coefficient _α_ ∼105 cm−1 in the visible region. Stibnite Sb2S3 nanocrystals deposited on a mesoporous TiO2 film have a particle size of ∼5–10 nm.14, 15 The TiO2/Sb2S3 system
shows an excellent match of energy levels for electron injection and transport after light harvesting by the Sb2S3 sensitizer. The corresponding TiO2/Sb2S3 solar cell (SC) showed IPCE values
between 70 and 90% in the visible region with various hole-transporting materials, such as CuSCN, spiro-OMeTAD and poly(3-hexylthiophene-2,5-diyl) (P3HT).14, 15, 87, 88, 89, 90, 91, 92, 93,
94 The IPCE curves feature an optical absorption onset at ∼750 nm, which is consistent with the crystalline Sb2S3 band gap of ∼1.65 eV. Seok and co-workers reported an IPCE loss15 in the
wavelength range of 450–650 nm, as P3HT can absorb light in this wavelength region, but the charges generated by P3HT were incompletely transferred to the Au counter electrode (CE) in the
TiO2/Sb2S3/P3HT device. This IPCE loss was fully recovered by adding PCBM for additional conducting channels, as shown in Figure 9. PHOTOVOLTAIC PROPERTIES OF THE SB2S3-BASED SOLAR CELLS
Etgar and co-workers fabricated Sb2S3/P3HT solar cells with a 1-decyl phosphonic acid (DPA) surface treatment to improve the device performance.94 Such a device attained a PCE of 3.9%, which
was significantly superior to that of a device without this surface treatment (_η_=3.1%). The enhanced performance was due to the smaller dark current and greater electron lifetime after
the DPA surface treatment. For Sb2S3/CuSCN solar cells, oxygen plays an important role during the fabrication of solar cells: oxygen can decrease the series resistance of the device to
enhance the overall device performance.87 It was also reported that the formation of the Sb2O3 surface passivation layer helps to promote the device performance.85 For the Sb2S3/spiro-OMeTAD
solar cells, _J_SC increased nonlinearly with increasing light intensity.92 This condition might indicate that the hole diffusion from spiro-OMeTAD to the Au electrode is a bottleneck
responsible for the nonlinear behavior of the photocurrents with increasing light intensity. The charge losses at higher light intensities can be decreased by improving the
Sb2S3/Spiro-OMeTAD interface and using new HTM with a greater hole mobility. The highest efficiency of Sb2S3-based solar cells was reported to be 6.3% using PCPDTBT as an HTM layer, giving
the device a remarkable photovoltaic performance, with _J_SC=16.0 mA cm−2, _V_OC=0.595 V and FF=0.655.15 Ito and co-workers89 reported TiO2/Sb2S3/CuSCN solar cells with a TiO2 surface
treatment (_η_=4.1%). The TiO2 devices in which the surface was treated with BaTiO3/MgO showed much greater efficiency compared with untreated TiO2/Sb2S3/CuSCN devices (4.1% vs 2.8%). The
TiO2/Sb2S3 interface has been improved by a BaTiO3/MgO surface treatment; passivation of the TiO2 surface was also performed at the TiO2/CuSCN interface. CHALLENGES IN SB2S3-BASED SOLAR
CELLS For Sb2S3-based solar cells, theoretical simulations by Bisquert and co-workers88 have shown an optimized efficiency of ∼8.5% upon decreasing the series and hole-transport resistances
and increasing the charge-collection efficiency. Because the electron-hole recombination occurs mostly between the electrons of the CB in the TiO2 layer and the holes in the HTM layer, the
hole-transport properties of the HTM become important to improve the device efficiency of solar cells of this type.88, 93 The challenge is hence to find new HTMs with great hole mobility and
stability. Haque and co-workers recorded nanosecond transient absorption spectra (TAS) of thin film samples with a TiO2/Sb2S3/spiro-OMeTAD configuration.93 As Figure 10 shows, the rate of
charge recombination at the TiO2/Sb2S3 interface decreased when more successive ionic layer adsorption and reaction (SILAR) cycles were involved, making the optical onsets of the Sb2S3
nanocrystals systematically red-shifted. This effect might decrease the rate of electron injection from the Sb2S3 sensitizer to the TiO2 film and thus decrease the device efficiency,
particularly in the wavelength region beyond 750 nm. Hole transfer from the Sb2S3 sensitizer to spiro-OMeTAD was much less sensitive to the spectral shift of the Sb2S3 absorption onset,
indicating that the hole transport to spiro-OMeTAD might initiate charge separation at the TiO2/Sb2S3/spiro-OMeTAD interface and thus improve the device performance. The same group
investigated Sb2S3/P3HT films using the TAS technique.95 After excitation at 567 nm, the P3HT+ polaron band at 950 nm was monitored to investigate the kinetics of hole transport and charge
recombination in Sb2S3/P3HT films. According to the TAS results, the P3HT+ polaron band was strongly dependent on the hole transfer from Sb2S3 to P3HT, indicating that this interfacial hole
transport is a key parameter for improving the device performance. Another approach for improving the device efficiency of QDSCs is to extend the optical absorption range of the QD to ∼1000
nm to improve the light-harvesting performance of the device. The Sb2S3 nanocrystals can absorb light up to 750 nm with an optical band gap of ∼1.65 eV. To further extend the optical
absorption into the near-infrared region, we must consider semiconductors with smaller band gaps, _E_g<1.3 eV, such as Sb2Se3. SB2SE3-SENSITIZED SOLAR CELLS Antimony chalcogenide
semiconductor Sb2Se3 has an optical band gap of 1.0–1.2 eV and shows a strong optical absorption, with a coefficient _α_ of ∼105 cm−1 in the visible region.84, 96, 97, 98 Theoretical
predictions for _J_SC as a function of the band gap _E_g give the upper limits as 22 and 43 mA cm−2 for Sb2S3 (_E_g=1.7 eV) and Sb2Se3 (_E_g=1.13 eV) sensitizers, respectively.96 For the
Sb2Se3-based devices, the challenges involve finding an effective synthetic method for the Sb2Se3 nanocrystals deposited on the mesoporous film and finding a suitable HTM to match the energy
levels. Haque and co-workers reported TAS results for TiO2/Sb2Se3/spiro-OMeTAD devices.99 Sb2Se3 nanocrystals were deposited on the surface of TiO2 (or ZrO2 as a control experiment) with
the SILAR method using SbCl3 dissolved in acetone and selenide dissolved in ethanol under a N2 atmosphere. The optical absorption of the Sb2Se3 nanocrystals increased and red-shifted with
increasing SILAR cycles because of the increased surface coverage (QD loading) and size of the nanoparticles. Figure 11 shows the TA profiles and the energy level diagrams for the Sb2Se3
system with TiO2 and ZrO2 films; the yield of long-lived charge-separated states in the TiO2 films was triple that in the ZrO2 films because of the more rapid charge recombination of the
latter. With increasing annealing temperature, the yield of charge separation was decreased, and the recombination lifetime also decreased because of the increased aggregation of the Sb2Se3
nanoparticles and the oxidation of the Sb2Se3 surface. A passivation layer of In_x_(OH)_y_S_z_ between TiO2 and Sb2Se3 can diminish the interfacial charge recombination and thus increase the
decay coefficients of the transient signals by ∼10 times. For the TiO2/Sb2Se3/spiro-OMeTAD devices, the spiro-OMeTAD+ charge yield was increased from 45 to 80%, and the lifetime of the
separated charges was ∼50 ms because of an effective match of the energy levels, a greater Sb2Se3 coverage on the TiO2 film and the decreased interfacial charge recombination. For the
ZrO2/Sb2Se3/spiro-OMeTAD devices, small transient signals were observed due to an electron transfer from Sb2Se3 to the spiro-OMeTAD. In this device, the transient signals in the ZrO2 films
decayed much more rapidly than those in the TiO2 film. The rapid decays observed in the ZrO2 films are related to the recombination of the localized electrons inside the Sb2Se3 layer and the
holes in the spiro-OMeTAD. The TAS results thus indicate that Sb2Se3 might be an effective material for light harvesting in a highly efficient solid-state solar cell. PEROVSKITE-BASED
MESOSCOPIC SOLAR CELLS Perovskite originally referred to a mineral containing CaTiO3, named after Russian mineralogist Lev Perovski, and the term was later extended to encompass a class of
compounds with a crystal structure of the same type as CaTiO3. Figure 12 shows the unit cell of a three-dimensional crystal structure for perovskite compounds with the general chemical
formula ABX3. Instead of oxide perovskite species (X=O), halide perovskite compounds (X=Cl, Br or I) were found to feature excellent light-harvesting and electron-conducting properties and
are perfectly suitable for use as prospective photovoltaic materials.1, 2, 3 PEROVSKITE-SENSITIZED SOLAR CELLS WITH LIQUID ELECTROLYTES In 2009, Miyasaka and co-workers100 reported the first
perovskite-sensitized TiO2 solar cell using liquid electrolytes based on iodide and bromide. According to their approach, lead bromide perovskite (CH3NH3PbBr3) was deposited on the TiO2
film with a spin-coating procedure with the precursor solution containing CH3NH3Br and PbBr2. The coated TiO2 film showed nanocrystalline particles approximately 2–3 nm in size on the
surface of the TiO2 nanoparticles. For these TiO2/CH3NH3PbBr3 solar cells, the IPCE action spectrum showed a maximum value of 65% at ∼400 nm, but it fell to zero beyond 550 nm. The
corresponding device with _J_SC=5.57 mA cm−2, _V_OC=0.96 V and FF=0.59 gave a PCE of 3.1% under one-sun illumination.100 For these TiO2/CH3NH3PbI3 solar cells, the IPCE spectrum showed
effective optical absorption up to 800 nm with a maximum value of 45% at ∼500 nm. As a result, the PCE (3.8%) was larger for the iodide perovskite device than for the bromide perovskite
device because _J_SC (11.0 mA cm−2) was much greater for the former than for the latter, even though the _V_OC of the former device was only 0.61 V. Later in 2011, Park and co-workers101
reported on the improved efficiency of TiO2 solar cells sensitized with lead iodide perovskite (CH3NH3PbI3) and an electrolyte based on iodide. In their approach, Pb(NO3)2 was used for
modification of the surface of a mesoporous TiO2 film before coating the perovskite QD, such that Pb(NO3)2 acted as a blocking layer in the solar cells. After the TiO2 surface modification,
the device showed an improved PCE of 6.5% with _J_SC=15.8 mA cm−2, _V_OC=0.70 V and FF=0.58; the improved _V_OC was due to the retarded charge recombination effected by the surface
treatment. The authors also mentioned the stability of the perovskite nanocrystals in the iodide electrolyte, whereby the perovskite QD gradually dissolved into the iodide electrolyte after
being irradiated for 10 min. The organic part of the perovskite species is modifiable through a similar synthetic approach. For example, a CH3CH2NH3PbI3 sensitizer can be deposited on a
mesoporous TiO2 film via spin coating with an equimolar mixture of CH3CH2NH3I and PbI2 in a γ-butyrolactone solution.102 The average diameter of the CH3CH2NH3PbI3 nanocrystals was
approximately 1.8 nm; the optical band gap of this species increased to ∼2.2 eV, as estimated from the diffuse reflectance spectra. For the TiO2/CH3CH2NH3PbI3 solar cells, the IPCE spectrum
attained a maximum value of ∼60% at ∼600 nm, giving a PCE of 2.4% in the iodide-based electrolyte. The poor performance of the device was due to the large optical band gap of the perovskite
sensitizer of this type, which limited light harvesting in the visible region. SYNTHESIS OF LEAD-IODIDE PEROVSKITES ON METAL-OXIDE FILMS Perovskite CH3NH3PbI3 nanocrystals were formed on
mesoporous TiO2 films via spin coating with a solution containing CH3NH3I and PbI2 in an equimolar ratio.12, 101 The concentrations of the spin-coating solution were varied from 10 to 40% by
mass in γ-butyrolactone. The nanocrystalline (CH3NH3)PbI3 layer formed on the surface of the TiO2 film after drying at temperatures from 40 to 160 °C for 30 min, typically at 100 °C for 30
min. With an increasing concentration of the coating solution, the color of the perovskite (CH3NH3)PbI3 on the TiO2 film varied from yellow (10% by mass) to black (40% by mass). These
perovskite nanocrystalline materials have an average diameter of approximately 2.5 nm, showing QD behavior on the surface of the TiO2 nanoparticles, according to the TEM images shown in
Figure 13.101 Instead of the mesoporous TiO2 film, an insulating Al2O3 layer served as a scaffold for coating the perovskite halide (CH3NH3PbI2Cl) as a light harvester on the surface of the
Al2O3 film. The perovskite nanocrystalline film was prepared from a precursor solution containing CH3NH3I and PbCl2 in a molar ratio of 3:1 in anhydrous _N_,_N_-dimethylformamide.11 After
spin-coating and drying, the halide perovskite absorber on the Al2O3 film had an I:Cl ratio of approximately 2:1, according to the energy-dispersive X-ray analysis. ELECTRONIC PROPERTIES OF
LEAD IODIDE PEROVSKITES ON METAL-OXIDE FILMS Park and co-workers reported an alignment of the energy levels in a lead iodide perovskite (CH3NH3PbI3) QDSC. The CH3NH3PbI3 nanocrystals served
as the light absorber, and spiro-OMeTAD was used as the hole-transporting layer. This perovskite nanomaterial adsorbed on a TiO2 film had a direct optical transition with an energy gap _E_g
of ∼1.5 eV.12 From the energy-level alignment shown in Figure 1, we expect excellent properties of the device for electron injection and charge separation; that is, electrons can feasibly
inject from the perovskite species into the CB of a TiO2 film, and holes can transfer from the sensitizer perovskite to the spiro-OMeTAD HTM. Snaith and co-workers11 reported on a perovskite
(CH3NH3PbI2Cl) absorber on the surface of an insulating Al2O3 film. This insulating Al2O3 has a wide band gap _E_g of 7–9 eV and acts purely as a mesoporous scaffold for the perovskite
(CH3NH3PbI2Cl) to be deposited. In this case, the photo-induced electrons could not inject into the Al2O3 film because of the insulating nature of the metal-oxide film, but the holes
generated in the perovskite could propagate into the HTM layer, as shown in Figure 14. After charge separation between the CH3NH3PbI2Cl absorber and the spiro-OMeTAD HTM, the photo-induced
electrons were transported directly from the perovskite on the surface of the Al2O3 to the FTO electrode. The perovskite species thus acts as a bi-functional material to perform both
light-absorbing and electron-transport functions. The authors also found that the rates of electron transport (diffusion) in the Al2O3-based devices were 10 times those in TiO2-based
devices, according to the data for the transient photocurrent decay shown in Figure 14. These data demonstrate the superior photovoltaic performance of the perovskite SCs using a mesoporous
insulating metal-oxide layer rather than a conventional mesoporous TiO2 film. PHOTOVOLTAIC CHARACTERISTICS OF PEROVSKITE SOLAR CELLS Perovskite CH3NH3PbI3 nanocrystals have large
coefficients of optical absorption in the visible region, ranging from 104 to 105 cm−1.12 These nanocrystals also have excellent matching of energy levels with those of TiO2. Interfacial
bonding of perovskites with TiO2 and spiro-OMeTAD was effective in ensuring uniform penetration and complete filling of the pores. Figure 15 shows the photovoltaic performance of the
corresponding device; the IPCE values attained a maximum higher than 60% at 450 nm and were maintained greater than 50% up to 750 nm. The IPCE characteristics indicate that the CH3NH3PbI3
nanocrystals deposited on the mesoporous TiO2 film feature excellent light-harvesting properties, bestowing an excellent quantum efficiency to the device in the visible region. The
perovskite device of 0.6-μm film thickness showed a high photocurrent density (_J_SC=17.6 mA cm−2) and _V_OC (=0.88 V), a satisfactory FF (=0.62) and excellent overall device performance
(_η_=9.7%) under one-sun irradiation.12 With increased thickness of the TiO2 film, both _V_OC and FF of the device decreased because of the increased dark current and resistance to electron
transport. The results obtained from the TAS measurements indicate that the holes were completely transferred from the perovskite sensitizer to the spiro-OMeTAD HTM after charge separation
in the sensitizer; the normalized photocurrent density was linearly proportional to the light intensity (Figure 15), without charge loss.12 Mixed-halide perovskite (CH3NH3PbI2Cl)
nanocrystals have an optical absorption onset (_λ_∼800 nm) similar to that of the iodide perovskite (CH3NH3PbI3) mentioned previously. Figure 16 presents a comparison of the photovoltaic
performance of the CH3NH3PbI3 SCs using either the TiO2 or the Al2O3 film as the metal-oxide layer.11 For the Al2O3-based device, the IPCE curve attains a maximum value greater than 80% at
400 nm, and the values are maintained at greater than 60% up to 700 nm; for the TiO2-based device, the IPCE values are slightly smaller than those of the Al2O3-based device, with similar
_J_SC for both devices.11 The _V_OC of the Al2O3 device was, however, much greater than that of the TiO2 device because the loss of injection potential was much less for the former than for
the latter. As a result, _V_OC significantly increased (∼200 mV) for the devices from the TiO2 film to the insulating Al2O3 film, and the best device efficiency of 10.9% was found for the
Al2O3 device under one-sun conditions.11 In 2013, Snaith and co-workers2 reported highly efficient perovskite CH3NH3Pb(I,Cl)3 solar cells, with the efficiency of the best device approaching
12.3% based on a mesoporous Al2O3 film that was ∼0.4 μm thick. For the Al2O3 films with a thickness less than 0.4 μm, the perovskite solar cell had an efficiency of ∼9.1% with a capping
layer of thickness of 0.3–0.4 μm. For the Al2O3 films with a thickness greater than 0.4 μm, the perovskite solar cells had, in contrast, an efficiency near 12% without a perovskite capping
layer. Seok and co-workers reported on perovskite CH3NH3Pb(I,Br)3 solar cells with a maximum efficiency of 12.3%.3 In contrast with the approach of Snaith, using the Al2O3 film as a scaffold
for perovskites, the best device performance of Seok’s method was achieved using TiO2 films of thickness ∼0.6 μm. For the mesoporous TiO2-based devices, the efficiency was optimized by
using an alloy comprising both CH3NH3PbI3 and CH3NH3PbBr3 crystals, resulting in increased device efficiency and superior device stability. The mixing ratios of the solutions containing
perovskites CH3NH3Pb(I1−_x_Br_x_)3 were reported for _x_ varying in the range of 0.06–0.20. Figure 17 presents the stability data of the devices, indicating that the devices containing less
Br (_x_=0.06 or zero) had a higher efficiency than the others in the initial stage of the test, whereas those containing more Br (_x_=0.20 or 0.29) exhibited superior durability under
ambient conditions because of their compact nanocrystalline structure. Seok and co-workers also fabricated perovskite CH3NH3PbI3/poly-triarylamine (PTAA) solar cells with an efficiency of
12%.103 The device using PTAA as an HTM layer showed much better performance than those using other polymeric hole conductors such as P3HT (_η_∼6.7%), PCPDTBT (_η_∼5.3%) and PCDTBT
(_η_∼4.2%). Park and co-workers104 demonstrated perovskite CH3NH3PbI3 solar cells based on rutile TiO2 nanorods with an efficiency of 9.4%. Park’s results indicate that the photovoltaic
performance was strongly dependent on the length of the TiO2 nanorods because of the efficiency of charge generation rather than because of the kinetics of charge recombination. Hagfeldt and
co-workers105 reported perovskite CH3NH3PbI3/spiro-OMeTAD solar cells with an efficiency of 8.5%. The spiro-OMeTAD device showed a much improved performance compared with devices using P3HT
(_η_∼4.5%) and 4-(diethylamino)-benzaldehyde diphenylhydrazone (DEH) (_η_∼1.6%) because of the greater electron lifetime of the former. Other perovskite-based HMSCs have been studied, with
various TiO2 nanostructures and HTMs.106, 107, 108, 109 Hagfeldt and co-workers110 reported ZrO2/CH3NH3PbI3/spiro-OMeTAD solar cells with an efficiency of 10.8%. Perovskite
CH3NH3PbI3/fullerene heterojunction hybrid solar cells have also been fabricated.111 Chung _et al._112 reported the use of perovskite CsSnI3 as an HTM in solid-state DSSCs. Perovskite CsSnI3
is a direct band gap _p_-type semiconductor with an optical absorption onset at 1.3 eV (Figure 1b), and it has a large hole mobility (_μ_h=585 cm2V−1 s−1) near 23 °C; the latter value is
100–1000 times that of a polymer-type HTM. As a result, the device consisting of CsSnI2.95F0.05/N719 dye/TiO2 attained a PCE of 8.5% with an enhanced IPCE in the red visible region.112 Chen
_et al._113 reported Schottky solar cells based on perovskite CsSnI3 thin films; these films were prepared by coating multiple layers of SnCl2 and CsI on a glass substrate followed by
annealing at 175 °C on a hotplate for 1 min under ambient conditions. The CsSnI3 film was formed during annealing from the SnCl2/CsI stack. The _JV_ characteristic curve showed a small PCE
(0.88%) for an SC of this type. The perovskite mesoscopic solar cell without an HTM layer was reported to possess a PCE of 5.5%.114 CHALLENGES IN PEROVSKITE-BASED SOLAR CELLS The synthesis
of perovskite CH3NH3PbI3 nanocrystals is sensitive to ambient humidity. According to results reported elsewhere,101 the perovskites gradually dissolved into a liquid-type electrolyte after
irradiation for 10 min. As a result, anhydrous starting materials should be used in a dry box during synthesis. Another issue is the thickness and porosity of the HTM layer (such as
spiro-OMeTAD) coated on the surface of the perovskite. For example, the contact resistance and series resistance at the interface of the CH3NH3PbI3/spiro-OMeTAD/cathode must be decreased by
optimizing the thickness of the spiro-OMeTAD layer. A thinner spiro-OMeTAD layer is required to decrease the series resistance and hole-transport resistance through the spiro-OMeTAD to the
cathode. As the conductivity of a typical halide perovskite is on the order of 10−3 S cm−1, the spiro-OMeTAD layer should be thick enough to prevent an electrical short circuit between the
perovskite layer and the counter electrode. Greater FF and PCE of the devices can be achieved by employing new types of HTMs with greater mobility and by introducing a thin capping layer on
the perovskite sensitizer. Nevertheless, the best-performing perovskite solar cell was reported in 2013 with a structure of TiO2/perovskite/spiro-OMeTAD/Au, which exhibited a remarkable PCE
of 15.0% (_J_SC=20.0 mA cm−2, _V_OC=0.993 V, FF=0.73) under an illumination of 96.4 mW cm−2.1 It is worth noting that when the device was encapsulated under argon, it could maintain greater
than 80% of its initial PCE after 500 h.1 CONCLUSIONS Highly efficient dye-sensitized nanocrystalline TiO2 solar cells have been developed since 19918; the most promising devices are based
on ruthenium or porphyrin dyes with efficiencies of power conversion exceeding 12%,9, 10 but these traditional DSSCs have durability problems due to (i) instability of the organic
sensitizers and (ii) leakage of the devices containing typical liquid-type electrolytes. To solve these critical problems for DSSCs, various inorganic light harvesters have been intensively
developed, in combination with hole-conducting materials of organic or polymer type. Therefore, herein we have reviewed inorganic nanomaterials of two types—metal chalcogenides and halide
perovskites—as prospective photosensitizers or light absorbers for hybrid mesoscopic solar cells. A summary of the photovoltaic performances of these inorganic photosensitizers in
liquid-type electrolytes appears in Table 1. The power conversion efficiencies of these devices are randomly distributed from 2 to 6%, and their performances are much poorer than those of
the liquid-type DSSCs, according to the comparison shown in Figure 18. Although a large photocurrent was reported for a mercury-doped PbS-sensitized SC (_J_SC=30 mA cm−2),18 its poor
photovoltage is a typical problem for QD-based solar cells. All-solid-state mesoscopic solar cells have been rapidly developing and have shown astonishing progress in their device
performance during the period 2012–2013. A summary of the photovoltaic performances of all-solid-state quantum-dot-sensitized solar cells and lead halide perovskite-based solar cells appears
in Table 2; their progress trends are demonstrated in Figure 19. The first all-solid-state DSSC was reported in 1998 with a small PCE (0.74%); it had a ruthenium complex dye and an organic
HTM (spiro-OMeTAD) as a solid-state electrolyte.116 The device efficiency of an all-solid-state DSSC quickly rose to 4.0% in 2005 (Z907)117 and to 5.0% in 2010 (C106).118 In 2011, two
metal-free organic dyes, C220119 and Y123,120 showed promising performances for all-solid-state devices, attaining efficiencies of 6.1 and 7.2%, respectively. Since 2010, metal chalcogenide
QDs have played an important role as suitable sensitizers for solid-state mesoscopic TiO2 solar cells. The two most promising QD sensitizers are PbS and Sb2S3; the PCEs of these QDSCs
reached ∼7% in 2012. The most remarkable advancement in the development of all-solid-state mesoscopic solar cells was the discovery of light harvesters of the perovskite type. As Figure 19
shows, the development of perovskite-based solar cells has progressed expeditiously, with achieved PCEs of 10%11, 12 in 2012, followed by 12%2, 3, 103 and then 15%1 in 2013. Such striking
and rapid progress in the development of organic-inorganic hybrid photovoltaic devices indicates the threshold of a new era for the commercialization of all-solid-state mesoscopic solar
cells in the near future. REFERENCES * Burschka, J., Pellet, N., Moon, S. J., Humphry-Baker, R., Gao, P., Nazeeruddin, M. K. & Grätzel, M Sequential deposition as a route to
high-performance perovskite-sensitized solar cells. _Nature_ 499, 316–319 (2013). CAS Google Scholar * Ball, J. M., Lee, M. M., Hey, A. & Snaith, H. J. Low-temperature processed
meso-superstructured to thin-film perovskite solar cells. _Energy Environ. Sci._ 6, 1739–1743 (2013). CAS Google Scholar * Noh, J. H., Im, S. H., Heo, J. H., Mandal, T. N. & Seok, S.
I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. _Nano Lett._ 13, 1764–1769 (2013). CAS Google Scholar * Kamat, P. V. Quantum
dot solar cells. The next big thing in photovoltaics. _J. Phys. Chem. Lett._ 4, 908–918 (2013). CAS Google Scholar * Ruhle, S., Shalom, M. & Zaban, A Quantum-dot-sensitized solar
cells. _ChemPhysChem_ 11, 2290–2304 (2010). Google Scholar * Grätzel, M., Janssen, R. A., Mitzi, D. B. & Sargent, E. H. Materials interface engineering for solution-processed
photovoltaics. _Nature_ 488, 304–312 (2012). Google Scholar * Grätzel, M. Recent advances in sensitized mesoscopic solar cells. _Acc. Chem. Res._ 42, 1788–1798 (2009). Google Scholar *
O'Regan, B. & Grätzel, M. A low-cost, high efficiency solar cell based on dye-sensitized colloidal TiO2 films. _Nature_ 353, 737–740 (1991). CAS Google Scholar * Yella, A., Lee,
H. W., Tsao, H. N., Yi, C., Chandiran, A. K., Nazeeruddin, M. K., Diau, E. W., Yeh, C. Y., Zakeeruddin, S. M. & Gratzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based
redox electrolyte exceed 12 percent efficiency. _Science_ 334, 629–634 (2011). CAS Google Scholar * Li, L. L. & Diau, E. W. Porphyrin-sensitized solar cells. _Chem. Soc. Rev._ 42,
291–304 (2013). CAS Google Scholar * Lee, M. M., Teuscher, J., Miyasaka, T., Murakami, T. N. & Snaith, H. J. Efficient hybrid solar cells based on meso-superstructured organometal
halide perovskites. _Science_ 338, 643–647 (2012). CAS Google Scholar * Kim, H. S., Lee, C. R., Im, J. H., Lee, K. B., Moehl, T., Marchioro, A., Moon, S. J., Humphry-Baker, R., Yum, J. H.,
Moser, J. E., Gratzel, M. & Park, N. G. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. _Sci. Rep_ 2, 591
(2012). Google Scholar * Ip, A. H., Thon, S. M., Hoogland, S., Voznyy, O., Zhitomirsky, D., Debnath, R., Levina, L., Rollny, L. R., Carey, G. H., Fischer, A., Kemp, K. W., Kramer, I. J.,
Ning, Z., Labelle, A. J., Chou, K. W., Amassian, A. & Sargent, E. H. Hybrid passivated colloidal quantum dot solids. _Nature Nanotech_ 7, 577–582 (2012). CAS Google Scholar * Chang, J.
A., Rhee, J. H., Im, S. H., Lee, Y. H., Kim, H. J., Seok, S. I., Nazeeruddin, M. K. & Gratzel, M. High-performance nanostructured inorganic-organic heterojunction solar cells. _Nano
Lett._ 10, 2609–2612 (2010). CAS Google Scholar * Chang, J. A., Im, S. H., Lee, Y. H., Kim, H. J., Lim, C. S., Heo, J. H. & Seok, S. I. Panchromatic photon-harvesting by
hole-conducting materials in inorganic-organic heterojunction sensitized-solar cell through the formation of nanostructured electron channels. _Nano Lett._ 12, 1863–1867 (2012). CAS Google
Scholar * Nozik, A. J. Multiple exciton generation in semiconductor quantum dots. _Chem. Phys. Lett._ 457, 3–11 (2008). CAS Google Scholar * Semonin, O. E., Luther, J. M., Choi, S., Chen,
H. Y., Gao, J., Nozik, A. J. & Beard, M. C. Peak external photocurrent quantum efficiency exceeding 100% via MEG in a quantum dot solar cell. _Science_ 334, 1530–1533 (2011). CAS
Google Scholar * Lee, J. W., Son, D. Y., Ahn, T. K., Shin, H. W., Kim, I. Y., Hwang, S. J., Ko, M. J., Sul, S., Han, H. & Park, N. G. Quantum-dot-sensitized solar cell with
unprecedentedly high photocurrent. _Sci. Rep._ 3, 1050 (2013). Google Scholar * Sun, W. T., Yu, Y., Pan, H.-Y., Gao, X.-F., Chen, Q. & Peng, L. M. CdS quantum dots sensitized TiO2
nanotube-array photoelectrodes. _J. Am. Chem. Soc._ 130, 1124–1125 (2008). CAS Google Scholar * Ren, S., Chang, L. Y., Lim, S. K., Zhao, J., Smith, M., Zhao, N., Bulovic, V., Bawendi, M.
& Gradecak, S. Inorganic-organic hybrid solar cell: bridging quantum dots to conjugated polymer nanowires. _Nano Lett._ 11, 3998–4002 (2011). CAS Google Scholar * Lee, H., Leventis, H.
C., Moon, S.-J., Chen, P., Ito, S., Haque, S. A., Torres, T., Nüesch, F., Geiger, T., Zakeeruddin, S. M., Grätzel, M. & Nazeeruddin, M. K. PbS and CdS quantum dot-sensitized solid-state
solar cells: ‘old concepts, new results’. _Adv. Funct. Mater._ 19, 2735–2742 (2009). CAS Google Scholar * Brennan, T. P., Ardalan, P., Lee, H.-B.-R., Bakke, J. R., Ding, I. K., McGehee,
M. D. & Bent, S. F. Atomic layer deposition of cds quantum dots for solid-state quantum dot sensitized solar cells. _Adv. Energy Mater._ 1, 1169–1175 (2011). CAS Google Scholar *
Dowland, S., Lutz, T., Ward, A., King, S. P., Sudlow, A., Hill, M. S., Molloy, K. C. & Haque, S. A. Direct growth of metal sulfide nanoparticle networks in solid-state polymer films for
hybrid inorganic-organic solar cells. _Adv. Mater._ 23, 2739–2744 (2011). CAS Google Scholar * Qian, J., Liu, Q. S., Li, G., Jiang, K. J., Yang, L. M. & Song, Y. P3HT as hole transport
material and assistant light absorber in CdS quantum dots-sensitized solid-state solar cells. _Chem. Comm._ 47, 6461–6463 (2011). CAS Google Scholar * Balis, N., Dracopoulos, V.,
Stathatos, E., Boukos, N. & Lianos, P. A solid-state hybrid solar cell made of nc-TiO2, CdS quantum dots, and P3HT with 2-amino-1-methylbenzimidazole as an interface modifier. _J. Phys.
Chem. C_ 115, 10911–10916 (2011). CAS Google Scholar * Diguna, L. J., Shen, Q., Kobayashi, J. & Toyoda, T. High efficiency of CdSe quantum-dot-sensitized TiO2 inverse opal solar cells.
_Appl. Phys. Lett._ 91, 023116 (2007). Google Scholar * Celik, D., Krueger, M., Veit, C., Schleiermacher, H. F., Zimmermann, B., Allard, S., Dumsch, I., Scherf, U., Rauscher, F. &
Niyamakom, P. Performance enhancement of CdSe nanorod-polymer based hybrid solar cells utilizing a novel combination of post-synthetic nanoparticle surface treatments. _Solar Energy Mater.
Solar Cells_ 98, 433–440 (2012). CAS Google Scholar * Lee, Y. H., Im, S. H., Chang, J. A., Lee, J.-H. & Seok, S. I. CdSe-sensitized inorganic–organic heterojunction solar cells: the
effect of molecular dipole interface modification and surface passivation. _Org. Electron._ 13, 975–979 (2012). CAS Google Scholar * Shalom, M., Buhbut, S., Tirosh, S. & Zaban, A
Design rules for high-efficiency quantum-dot-sensitized solar cells: a multilayer approach. _J. Phys. Chem. Lett._ 3, 2436–2441 (2012). CAS Google Scholar * Tachan, Z., Hod, I., Shalom,
M., Grinis, L. & Zaban, A The importance of the TiO2/quantum dots interface in the recombination processes of quantum dot sensitized solar cells. _Phys. Chem. Chem. Phys._ 15, 3841–3845
(2013). CAS Google Scholar * Barcelo, I., Campina, J. M., Lana-Villarreal, T. & Gomez, R. A solid-state CdSe quantum dot sensitized solar cell based on a quaterthiophene as a hole
transporting material. _Phys. Chem. Chem. Phys._ 14, 5801–5807 (2012). CAS Google Scholar * Philias, J.-M. & Marsan, B. All-solid-state photoelectrochemical cell based on a polymer
electrolyte containing a new transparent and highly electropositive redox couple. _Electrochim. Acta_ 44, 2915–2926 (1999). CAS Google Scholar * Kniprath, R., Rabe, J. P., McLeskey, J. T.,
Wang, D. & Kirstein, S. Hybrid photovoltaic cells with II–VI quantum dot sensitizers fabricated by layer-by-layer deposition of water-soluble components. _Thin Solid Films_ 518, 295–298
(2009). CAS Google Scholar * Chen, Z., Zhang, H., Yu, W., Li, Z., Hou, J., Wei, H. & Yang, B. Inverted hybrid solar cells from aqueous materials with a PCE of 3.61%. _Adv. Energy
Mater._ 3, 433–437 (2013). CAS Google Scholar * Yue, G., Wu, J., Xiao, Y., Lin, J., Huang, M., Lan, Z. & Fan, L. CdTe quantum dots-sensitized solar cells featuring PCBM/P3HT as hole
transport material and assistant sensitizer provide 3.40% efficiency. _Electrochim. Acta_ 85, 182–186 (2012). CAS Google Scholar * Chen, H. C., Lai, C. W., Wu, I. C., Pan, H. R., Chen, I.
W., Peng, Y. K., Liu, C. L., Chen, C. H. & Chou, P. T. Enhanced performance and air stability of 3.2% hybrid solar cells: how the functional polymer and CdTe nanostructure boost the
solar cell efficiency. _Adv. Mater._ 23, 5451–5455 (2011). CAS Google Scholar * Sun, S., Liu, H., Gao, Y., Qin, D. & Chen, J. Controlled synthesis of CdTe nanocrystals for high
performanced Schottky thin film solar cells. _J. Mater. Chem._ 22, 19207 (2012). CAS Google Scholar * Olson, J. D., Rodriguez, Y. W., Yang, L. D., Alers, G. B. & Carter, S. A. CdTe
Schottky diodes from colloidal nanocrystals. _Appl. Phys. Lett._ 96, 242103 (2010). Google Scholar * Lee, Y.-L. & Lo, Y.-S. Highly efficient quantum-dot-sensitized solar cell based on
co-sensitization of CdS/CdSe. _Adv. Funct. Mater._ 19, 604–609 (2009). Google Scholar * MacDonald, B. I., Martucci, A., Rubanov, S., Watkins, S. E., Mulvaney, P. & Jasieniak, J. J.
Layer-by-layer assembly of sintered CdSexTe1−x nanocrystal solar cells. _ACS Nano_ 6, 5995–6004 (2012). CAS Google Scholar * Itzhakov, S., Shen, H., Buhbut, S., Lin, H. & Oron, D.
Type-II quantum-dot-sensitized solar cell spanning the visible and near-infrared spectrum. _J. Phys. Chem. C_ doi:10.1021/jp312190x (2013) (in press). * Yu, Z., Zhang, Q., Qin, D., Luo, Y.,
Li, D., Shen, Q., Toyoda, T. & Meng, Q. Highly efficient quasi-solid-state quantum-dot-sensitized solar cell based on hydrogel electrolytes. _Electrochem. Comm._ 12, 1776–1779 (2010).
CAS Google Scholar * Karageorgopoulos, D., Stathatos, E. & Vitoratos, E. Thin ZnO nanocrystalline films for efficient quasi-solid state electrolyte quantum dot sensitized solar cells.
_J. Power Sour._ 219, 9–15 (2012). CAS Google Scholar * Santra, P. K. & Kamat, P. V. Mn-doped quantum dot sensitized solar cells: a strategy to boost efficiency over 5%. _J. Am. Chem.
Soc._ 134, 2508–2511 (2012). CAS Google Scholar * Santra, P. K. & Kamat, P. V. Tandem-layered quantum dot solar cells: tuning the photovoltaic response with luminescent ternary cadmium
chalcogenides. _J. Am. Chem. Soc._ 135, 877–885 (2013). CAS Google Scholar * Chi, C.-F., Chen, P., Lee, Y.-L., Liu, I. P., Chou, S.-C., Zhang, X.-L. & Bach, U. Surface modifications
of CdS/CdSe co-sensitized TiO2 photoelectrodes for solid-state quantum-dot-sensitized solar cells. _J. Mater. Chem._ 21, 17534 (2011). CAS Google Scholar * Ma, W., Swisher, S. L., Ewers,
T., Engel, J., Ferry, V. E., Atwater, H. A. & Alivisatos, A. P. Photovoltaic performance of ultrasmall pbse quantum dots. _ACS Nano_ 5, 8140–8147 (2011). CAS Google Scholar * Choi, J.
J., Lim, Y. F., Santiago-Berrios, M. B., Oh, M., Hyun, B. R., Sun, L., Bartnik, A. C., Goedhart, A., Malliaras, G. G., Abruna, H. D., Wise, F. W. & Hanrath, T. PbSe nanocrystal excitonic
solar cells. _Nano Lett._ 9, 3749–3755 (2009). CAS Google Scholar * Etgar, L., Yanover, D., Čapek, R. K., Vaxenburg, R., Xue, Z., Liu, B., Nazeeruddin, M. K., Lifshitz, E. & Gratzel,
M Core/shell PbSe/PbS QDs TiO2 heterojunction solar cell. _Adv. Funct. Mater._ 23, 2736–2741 (2013). CAS Google Scholar * Zhai, G., Church, C. P., Breeze, A. J., Zhang, D., Alers, G. B.
& Carter, S. A. Quantum dot PbS0.9Se0.1/TiO2 heterojunction solar cells. _Nanotechnology_ 23, 405401 (2012). Google Scholar * Ning, Z., Ren, Y., Hoogland, S., Voznyy, O., Levina, L.,
Stadler, P., Lan, X., Zhitomirsky, D. & Sargent, E. H. All-inorganic colloidal quantum dot photovoltaics employing solution-phase halide passivation. _Adv. Mater._ 24, 6295–6299 (2012).
CAS Google Scholar * Tang, J., Kemp, K. W., Hoogland, S., Jeong, K. S., Liu, H., Levina, L., Furukawa, M., Wang, X., Debnath, R., Cha, D., Chou, K. W., Fischer, A., Amassian, A., Asbury,
J. B. & Sargent, E. H. Colloidal-quantum-dot photovoltaics using atomic-ligand passivation. _Nature Mater_ 10, 765–771 (2011). CAS Google Scholar * Jeong, K. S., Tang, J., Liu, H.,
Kim, J., Schaefer, A. W., Kemp, K., Levina, L., Wang, X., Hoogland, S., Debnath, R., Brzozowski, L., Sargent, E. H. & Asbury, J. B. Enhanced mobility-lifetime products in PbS colloidal
quantum dot photovoltaics. _ACS Nano_ 6, 89–99 (2012). CAS Google Scholar * Tang, J., Liu, H., Zhitomirsky, D., Hoogland, S., Wang, X., Furukawa, M., Levina, L. & Sargent, E. H.
Quantum junction solar cells. _Nano Lett._ 12, 4889–4894 (2012). CAS Google Scholar * Song, T., Zhang, F., Lei, X., Xu, Y., Lee, S. & Sun, B. Core-shell structured photovoltaic devices
based on PbS quantum dots and silicon nanopillar arrays. _Nanoscale_ 4, 1336–1343 (2012). CAS Google Scholar * Kramer, I. J., Zhitomirsky, D., Bass, J. D., Rice, P. M., Topuria, T.,
Krupp, L., Thon, S. M., Ip, A. H., Debnath, R., Kim, H. C. & Sargent, E. H. Ordered nanopillar structured electrodes for depleted bulk heterojunction colloidal quantum dot solar cells.
_Adv. Mater._ 24, 2315–2319 (2012). CAS Google Scholar * Kim, S., Im, S. H., Kang, M., Heo, J. H., Seok, S. I., Kim, S. W., Mora-Sero, I. & Bisquert, J. Air-stable and efficient
inorganic-organic heterojunction solar cells using PbS colloidal quantum dots co-capped by 1-dodecanethiol and oleic acid. _Phys. Chem. Chem. Phys._ 14, 14999–15002 (2012). CAS Google
Scholar * Im, S. H., Kim, H.-j., Kim, S. W., Kim, S.-W. & Seok, S. I. All solid state multiply layered PbS colloidal quantum-dot-sensitized photovoltaic cells. _Energy Environ. Sci._ 4,
4181–4186 (2011). CAS Google Scholar * Xu, F., Benavides, J., Ma, X. & Cloutier, S. G. Interconnected TiO2 nanowire networks for PbS quantum dot solar cell applications. _J.
Nanotech._ 2012, 6 (2012). Google Scholar * Klem, E. J. D., Gregory, C. W., Cunningham, G. B., Hall, S., Temple, D. S. & Lewis, J. S. Planar PbS quantum dot/C60 heterojunction
photovoltaic devices with 5.2% power conversion efficiency. _Appl. Phys. Lett._ 100, 173109 (2012). Google Scholar * Rath, A. K., Bernechea, M., Martinez, L., Arquer, F. P. G., Osmond, J.
& Konstantatos, G. Solution-processed inorganic bulk nano-heterojunctions and their application to solar cells. _Nature Photon_ 6, 529–534 (2012). CAS Google Scholar * Etgar, L.,
Zhang, W., Gabriel, S., Hickey, S. G., Nazeeruddin, M. K., Eychmuller, A., Liu, B. & Gratzel, M. High efficiency quantum dot heterojunction solar cell using anatase (001) TiO2
nanosheets. _Adv. Mater._ 24, 2202–2206 (2012). CAS Google Scholar * Gao, J., Perkins, C. L., Luther, J. M., Hanna, M. C., Chen, H. Y., Semonin, O. E., Nozik, A. J., Ellingson, R. J. &
Beard, M. C. n-Type transition metal oxide as a hole extraction layer in PbS quantum dot solar cells. _Nano Lett._ 11, 3263–3266 (2011). CAS Google Scholar * Park, H., Chang, S., Jean,
J., Cheng, J. J., Araujo, P. T., Wang, M., Bawendi, M. G., Dresselhaus, M. S., Bulovic, V., Kong, J. & Gradecak, S. Graphene cathode-based ZnO nanowire hybrid solar cells. _Nano Lett._
13, 233–239 (2013). CAS Google Scholar * Brown, P. R., Lunt, R. R., Zhao, N., Osedach, T. P., Wanger, D. D., Chang, L. Y., Bawendi, M. G. & Bulovic, V. Improved current extraction from
ZnO/PbS quantum dot heterojunction photovoltaics using a MoO3 interfacial layer. _Nano Lett._ 11, 2955–2961 (2011). CAS Google Scholar * Chen, H.-Y., Hou, J., Dayal, S., Huo, L.,
Kopidakis, N., Beard, M. C. & Luther, J. M. A p-Type quantum dot/organic donor:acceptor solar-cell structure for extended spectral response. _Adv. Energy Mater._ 1, 528–533 (2011). CAS
Google Scholar * Zhang, Y., Li, Z., Ouyang, J., Tsang, S.-W., Lu, J., Yu, K., Ding, J. & Tao, Y. Hole transfer from PbS nanocrystal quantum dots to polymers and efficient hybrid solar
cells utilizing infrared photons. _Org. Electron._ 13, 2773–2780 (2012). CAS Google Scholar * Zhou, N., Chen, G., Zhang, X., Cheng, L., Luo, Y., Li, D. & Meng, Q. Highly efficient
PbS/CdS co-sensitized solar cells based on photoanodes with hierarchical pore distribution. _Electrochem. Comm._ 20, 97–100 (2012). CAS Google Scholar * Wang, D., Baral, J. K., Zhao, H.,
Gonfa, B. A., Truong, V.-V., El Khakani, M. A., Izquierdo, R. & Ma, D. Controlled fabrication of PbS quantum-dot/carbon-nanotube nanoarchitecture and its significant contribution to
near-infrared photon-to-current conversion. _Adv. Funct. Mater._ 21, 4010–4018 (2011). CAS Google Scholar * Ehrler, B., Wilson, M. W., Rao, A., Friend, R. H. & Greenham, N. C. Singlet
exciton fission-sensitized infrared quantum dot solar cells. _Nano Lett._ 12, 1053–1057 (2012). CAS Google Scholar * Lin, C.-Y., Teng, C.-Y., Li, T.-L., Lee, Y.-L. & Teng, H.
Photoactive p-type PbS as a counter electrode for quantum dot-sensitized solar cells. _J. Mater. Chem. A_ 1, 1155–1162 (2013). CAS Google Scholar * Yang, Y., Zhu, L., Sun, H., Huang, X.,
Luo, Y., Li, D. & Meng, Q. Composite counter electrode based on nanoparticulate PbS and carbon black: towards quantum dot-sensitized solar cells with both high efficiency and stability.
_ACS Appl. Mater. Interfaces_ 4, 6162–6168 (2012). CAS Google Scholar * Sung, S. D., Lim, I., Kang, P., Lee, C. & Lee, W. I. Design and development of highly efficient PbS quantum
dot-sensitized solar cells working in an aqueous polysulfide electrolyte. _Chem. Comm._ 49, 6054–6056 (2013). CAS Google Scholar * Yu, K., Ouyang, J., Zhang, Y., Tung, H. T., Lin, S.,
Nagelkerke, R. A., Kingston, D., Wu, X., Leek, D. M., Wilkinson, D., Li, C., Chen, I. G. & Tao, Y. Low-temperature noninjection approach to homogeneously-alloyed PbSexS1-x colloidal
nanocrystals for photovoltaic applications. _ACS Appl. Mater. Interfaces_ 3, 1511–1520 (2011). CAS Google Scholar * Ma, W., Luther, J. M., Zheng, H., Wu, Y. & Alivisatos, A. P.
Photovoltaic devices employing ternary PbSxSe1-x nanocrystals. _Nano Lett._ 9, 1699–1703 (2009). CAS Google Scholar * Ouyang, J., Schuurmans, C., Zhang, Y., Nagelkerke, R., Wu, X.,
Kingston, D., Wang, Z. Y., Wilkinson, D., Li, C., Leek, D. M., Tao, Y. & Yu, K. Low-temperature approach to high-yield and reproducible syntheses of high-quality small-sized PbSe
colloidal nanocrystals for photovoltaic applications. _ACS Appl. Mater. Interfaces_ 3, 553–565 (2011). CAS Google Scholar * Kuo, C.-Y., Su, M.-S., Ku, C.-S., Wang, S.-M., Lee, H.-Y. &
Wei, K.-H. Ligands affect the crystal structure and photovoltaic performance of thin films of PbSe quantum dots. _J. Mater. Chem._ 21, 11605–11612 (2011). CAS Google Scholar * Kuo, C.-Y.,
Su, M.-S., Hsu, Y.-C., Lin, H.-N. & Wei, K.-H. An organic hole transport layer enhances the performance of colloidal PbSe quantum dot photovoltaic devices. _Adv. Funct. Mater._ 20,
3555–3560 (2010). CAS Google Scholar * Liu, Y., Gibbs, M., Perkins, C. L., Tolentino, J., Zarghami, M. H., Bustamante, J. Jr. & Law, M. Robust, functional nanocrystal solids by
infilling with atomic layer deposition. _Nano Lett._ 11, 5349–5355 (2011). CAS Google Scholar * Benehkohal, N. P., González-Pedro, V., Boix, P. P., Chavhan, S., Tena-Zaera, R., Demopoulos,
G. P. & Mora-Seró, I. Colloidal PbS and PbSeS quantum dot sensitized solar cells prepared by electrophoretic deposition. _J. Phys. Chem. C_ 116, 16391–16397 (2012). Google Scholar *
Cui, D., Xu, J., Zhu, T., Paradee, G., Ashok, S. & Gerhold, M. Harvest of near infrared light in PbSe nanocrystal-polymer hybrid photovoltaic cells. _Appl. Phys. Lett._ 88, 183111
(2006). Google Scholar * Tan, Z., Zhu, T., Thein, M., Gao, S., Cheng, A., Zhang, F., Zhang, C., Su, H., Wang, J., Henderson, R., Hahm, J.-i., Yang, Y. & Xu, J. Integration of planar and
bulk heterojunctions in polymer/nanocrystal hybrid photovoltaic cells. _Appl. Phys. Lett._ 95, 063510 (2009). Google Scholar * Nam, M., Kim, S., Kim, T., Kim, S.-W. & Lee, K.-K.
Broadband energy-harvesting hybrid solar cells employing nanocomposites of polythiophene:ternary PbSSe nanocrystals. _Appl. Phys. Lett._ 99, 233115 (2011). Google Scholar * Messina, S.,
Nair, M. T. S. & Nair, P. K. Antimony sulfide thin films in chemically deposited thin film photovoltaic cells. _Thin Solid Films_ 515, 5777–5782 (2007). CAS Google Scholar * Itzhaik,
Y., Niitsoo, O., Page, M. & Hodes, G. Sb2S3-sensitized nanoporous TiO2 solar cells. _J. Phys. Chem. C_ 113, 4254–4256 (2009). CAS Google Scholar * Patrick, C. E. & Giustino, F.
Structural and electronic properties of semiconductor-sensitized solar-cell interfaces. _Adv. Funct. Mater._ 21, 4663–4667 (2011). CAS Google Scholar * Nezu, S., Larramona, G., Choné, C.,
Jacob, A., Delatouche, B., Péré, D. & Moisan, C. Light soaking and gas effect on nanocrystalline TiO2/Sb2S3/CuSCN photovoltaic cells following extremely thin absorber concept. _J. Phys.
Chem. C_ 114, 6854–6859 (2010). CAS Google Scholar * Boix, P. P., Larramona, G., Jacob, A., Delatouche, B., Mora-Seró, I. & Bisquert, J. Hole transport and recombination in all-solid
Sb2S3-sensitized TiO2 solar cells using CuSCN as hole transporter. _J. Phys. Chem. C_ 116, 1579–1587 (2012). CAS Google Scholar * Tsujimoto, K., Nguyen, D.-C., Ito, S., Nishino, H.,
Matsuyoshi, H., Konno, A., Kumara, G. R. A. & Tennakone, K TiO2 surface treatment effects by Mg2+, Ba2+, and Al3+on Sb2S3 extremely thin absorber solar cells. _J. Phys. Chem. C_ 116,
13465–13471 (2012). CAS Google Scholar * Lim, C. S., Im, S. H., Kim, H. J., Chang, J. A., Lee, Y. H. & Seok, S. I. Enhancing the device performance of Sb2S3-sensitized heterojunction
solar cells by embedding Au nanoparticles in the hole-conducting polymer layer. _Phys. Chem. Chem. Phys._ 14, 3622–3626 (2012). CAS Google Scholar * Boix, P. P., Lee, Y. H.,
Fabregat-Santiago, F., Im, S. H., Mora-Sero, I., Bisquert, J. & Seok, S. I. From flat to nanostructured photovoltaics: balance between thickness of the absorber and charge screening in
sensitized solar cells. _ACS Nano_ 6, 873–880 (2012). CAS Google Scholar * Moon, S. J., Itzhaik, Y., Yum, J. H., Zakeeruddin, S. M., Hodes, G. & Gratzel, M. Sb2S3-based mesoscopic
solar cell using an organic hole conductor. _J. Phys. Chem. Lett._ 1, 1524–1527 (2010). CAS Google Scholar * O'Mahony, F. T. F., Lutz, T., Guijarro, N., Gómez, R. & Haque, S. A.
Electron and hole transfer at metal oxide/Sb2S3/spiro-OMeTAD heterojunctions. _Energy Environ. Sci._ 5, 9760–9764 (2012). CAS Google Scholar * Fukumoto, T., Moehl, T., Niwa, Y.,
Nazeeruddin, M. K., Grätzel, M. & Etgar, L. Effect of interfacial engineering in solid-state nanostructured Sb2S3 heterojunction solar cells. _Adv. Energy Mater._ 3, 29–33 (2013). CAS
Google Scholar * Bansal, N., O'Mahony, F. T. F., Lutz, T. & Haque, S. A. Solution processed polymer-inorganic semiconductor solar cells employing Sb2S3 as a light harvesting and
electron transporting material. _Adv. Energy Mater._ 3, 986–990 (2013). CAS Google Scholar * Messina, S., Nair, M. T. S. & Nair, P. K. Solar cells with Sb2S3 absorber films. _Thin
Solid Films_ 517, 2503–2507 (2009). CAS Google Scholar * Messina, S., Nair, M. T. S. & Nair, P. K. Antimony selenide absorber thin films in all-chemically deposited solar cells. _J.
Electrochem. Soc._ 156, H327–H332 (2009). CAS Google Scholar * Barrios-Salgado, E., Nair, M. T. S., Nair, P. K. & Zingaro, R. A. Chemically deposited thin films of PbSe as an absorber
component in solar cell structures. _Thin Solid Films_ 519, 7432–7437 (2011). CAS Google Scholar * Guijarro, N., Lutz, T., Lana-Villarreal, T., O’Mahony, F., Gómez, R. & Haque, S. A.
Toward antimony selenide sensitized solar cells: efficient charge photogeneration at spiro-OMeTAD/Sb2Se3/metal oxide heterojunctions. _J. Phys. Chem. Lett._ 3, 1351–1356 (2012). CAS Google
Scholar * Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. _J. Am. Chem. Soc._ 131, 6050–6051
(2009). CAS Google Scholar * Im, J. H., Lee, C. R., Lee, J. W., Park, S. W. & Park, N. G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. _Nanoscale_ 3, 4088–4093 (2011).
CAS Google Scholar * Im, J. H., Chung, J., Kim, S. J. & Park, N. G. Synthesis, structure, and photovoltaic property of a nanocrystalline 2H perovskite-type novel sensitizer
(CH3CH2NH3)PbI3 . _Nanoscale Res. Lett._ 7, 353 (2012). Google Scholar * Heo, J. H., Im, S. H., Noh, J. H., Mandal, T. N., Lim, C.-S., Chang, J. A., Lee, Y. H., Kim, H.-J., Sarkar, A.,
Nazeeruddin, M. K., Gratzel, M. & Seok, S. I. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. _Nature Photon_
7, 486–491 (2013). CAS Google Scholar * Kim, H. S., Lee, J. W., Yantara, N., Boix, P. P., Kulkarni, S. A., Mhaisalkar, S., Gratzel, M. & Park, N. G. High efficiency solid-state
sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. _Nano Lett._ 13, 2412–2417 (2013). CAS Google Scholar * Bi, D., Yang, L., Boschloo,
G., Hagfeldt, A. & Johansson, E. M. J. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskite-sensitized mesoscopic solar cells. _J. Phys. Chem. Lett._ 4,
1532–1536 (2013). CAS Google Scholar * Cai, B., Xing, Y., Yang, Z., Zhang, W.-H. & Qiu, J. High performance hybrid solar cells sensitized by organolead halide perovskites. _Energy
Environ. Sci._ 6, 1480–1485 (2013). CAS Google Scholar * Qiu, J., Qiu, Y., Yan, K., Zhong, M., Mu, C., Yan, H. & Yang, S. All-solid-state hybrid solar cells based on a new organometal
halide perovskite sensitizer and one-dimensional TiO2 nanowire arrays. _Nanoscale_ 5, 3245–3248 (2013). CAS Google Scholar * Edri, E., Kirmayer, S., Cahen, D. & Hodes, G. High
open-circuit voltage solar cells based on organic–inorganic lead bromide perovskite. _J. Phys. Chem. Lett._ 4, 897–902 (2013). CAS Google Scholar * Abrusci, A., Stranks, S. D., Docampo,
P., Yip, H. L., Jen, A. K. & Snaith, H. J. High performance perovskite-polymer hybrid solar cells via electronic coupling with fullerene monolayers. _Nano Lett._ 13, 3124–3128 (2013).
CAS Google Scholar * Bi, D., Häggman, L., Boschloo, G., Yang, L., Johansson, E. M. J. & Hagfeldt, A. Using two-step deposition technique to prepare perovskite (CH3NH3PbI3) for thin
film solar cells based on ZrO2 and TiO2 mesostructures. _RSC Adv._ doi:10.1039/C3RA43228A(in press) (2013). * Jeng, J. Y., Chiang, Y. F., Lee, M. H., Peng, S. R., Guo, T. F., Chen, P. &
Wen, T. C. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. _Adv. Mater._ 25, 3727–3732 (2013). CAS Google Scholar * Chung, I., Lee, B., He, J., Chang, R. P. &
Kanatzidis, M. G. All-solid-state dye-sensitized solar cells with high efficiency. _Nature_ 485, 486–489 (2012). CAS Google Scholar * Chen, Z., Wang, J. J., Ren, Y., Yu, C. & Shum, K.
Schottky solar cells based on CsSnI3 thin-films. _Appl. Phys. Lett._ 101, 093901 (2012). Google Scholar * Etgar, L., Gao, P., Xue, Z., Peng, Q., Chandiran, A. K., Liu, B., Nazeeruddin, M.
K. & Gratzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. _J. Am. Chem. Soc._ 134, 17396–17399 (2012). CAS Google Scholar * Im, S. H., Kim, H.-j., Rhee, J. H., Lim, C.-S.
& Seok, S. I. Performance improvement of Sb2S3-sensitized solar cell by introducing hole buffer layer in cobalt complex electrolyte. _Energy Environ. Sci._ 4, 2799–2802 (2011). CAS
Google Scholar * Bach, U., Lupo, D., Comte, P., Moser, J. E., Weissortel, F., Salbeck, J., Spreitzer, H. & Gratzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high
photon-to-electron conversion efficiencie. _Nature_ 395, 583 (1998). CAS Google Scholar * Schmidt-Mende, L., Zakeeruddin, S. M. & Grätzel, M. Efficiency improvement in
solid-state-dye-sensitized photovoltaics with an amphiphilic Ruthenium-dye. _Appl. Phys. Lett._ 86, 013504 (2005). Google Scholar * Wang, M., Liu, J., Cevey-Ha, N.-L., Moon, S.-J., Liska,
P., Humphry-Baker, R., Moser, J.-E., Grätzel, C., Wang, P. & Zakeeruddin, S. M. High efficiency solid-state sensitized heterojunction photovoltaic device. _Nano Today_ 5, 169–174 (2010).
CAS Google Scholar * Cai, N., Moon, S. J., Cevey-Ha, L., Moehl, T., Humphry-Baker, R., Wang, P., Zakeeruddin, S. M. & Gratzel, M. An organic D-π-A dye for record efficiency
solid-state sensitized heterojunction solar cells. _Nano Lett._ 11, 1452–1456 (2011). CAS Google Scholar * Burschka, J., Dualeh, A., Kessler, F., Baranoff, E., Cevey-Ha, N. L., Yi, C.,
Nazeeruddin, M. K. & Gratzel, M. Tris(2-(1H-pyrazol-1-yl)pyridine)cobalt(III) as p-type dopant for organic semiconductors and its application in highly efficient solid-state
dye-sensitized solar cells. _J. Am. Chem. Soc._ 133, 18042–18045 (2011). CAS Google Scholar Download references ACKNOWLEDGEMENTS The National Science Council of Taiwan and the Ministry of
Education of Taiwan, under the ATU program, provided support for this project. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Applied Chemistry and Institute of Molecular
Science, National Chiao Tung University, Hsinchu, Taiwan Jae Hui Rhee, Chih-Chun Chung & Eric Wei-Guang Diau Authors * Jae Hui Rhee View author publications You can also search for this
author inPubMed Google Scholar * Chih-Chun Chung View author publications You can also search for this author inPubMed Google Scholar * Eric Wei-Guang Diau View author publications You can
also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Eric Wei-Guang Diau. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rhee, J.,
Chung, CC. & Diau, EG. A perspective of mesoscopic solar cells based on metal chalcogenide quantum dots and organometal-halide perovskites. _NPG Asia Mater_ 5, e68 (2013).
https://doi.org/10.1038/am.2013.53 Download citation * Received: 10 July 2013 * Revised: 08 August 2013 * Accepted: 09 August 2013 * Published: 18 October 2013 * Issue Date: October 2013 *
DOI: https://doi.org/10.1038/am.2013.53 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * metal chalcogenide * perovskite * quantum dot * solar
cell