- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Inflammation is one of the early phases in the development of gastric cancer. Therefore, several studies have examined the association of polymorphisms in tumour-necrosis factor-A
gene (_TNF-A_) with gastric cancer risk. This meta-analysis reviews and summarises published evidence for these associations. Searching several databases yielded 24 independent studies that
reported on the associations between _TNF-A_ polymorphisms and gastric cancer risk. We analysed available data for the most commonly investigated polymorphisms: _TNF-A_ –308G>A (23
studies), _TNF-A_ –238G>A (9 studies), and _TNF-A_ –857C>T (5 studies). Summary odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated in the random-effects model
using the DerSimonian–Laird method. _Q_-statistic and _I__2_-statistic were calculated to examine heterogeneity, and funnel plots were plotted to examine small study effects. The overall ORs
(95% CIs) for AG and AA genotypes _vs_ GG genotype for _TNF-A_ –308 were 1.09 (0.94–1.27) and 1.49 (1.11–1.99), respectively. For _TNF-A_ –238, the corresponding ORs (95% CIs) were 1.05
(0.84–1.33) and 1.25 (0.30–5.26), respectively. The overall ORs (95% CIs) for CT and TT genotypes (_vs_ CC) for _TNF-A_ –857 were 1.06 (0.89–1.27) and 1.57 (0.91–2.70), respectively. The
statistically significant association between _TNF-A_ –308GG and gastric cancer was limited to western populations. This association showed little heterogeneity (_I__2_=0) and remained
consistently strong when analyses were limited to anatomic and histologic subtypes of gastric cancer, or limited to studies in which genotype frequencies were in Hardy–Weinberg equilibrium,
or limited to larger studies. These same subgroup analyses did not change results associated with other polymorphisms. In conclusion, _TNF-A_ –308AA genotype was associated with a
statistically significant increased risk of gastric cancer, whereas other studied polymorphisms were not. The association between _TNF-A_ –857TT genotype and gastric cancer was near
significant, and may become significant if more studies are published. SIMILAR CONTENT BEING VIEWED BY OTHERS _TNF_ GENETIC POLYMORPHISM (RS1799964) MAY MODIFY THE EFFECT OF THE DIETARY
INFLAMMATORY INDEX ON GASTRIC CANCER IN A CASE–CONTROL STUDY Article Open access 03 September 2020 NO EVIDENT CAUSAL ASSOCIATION BETWEEN _HELICOBACTER PYLORI_ INFECTION AND COLORECTAL
CANCER: A BIDIRECTIONAL MENDELIAN RANDOMIZATION STUDY Article Open access 29 October 2023 THE RELATIONSHIP BETWEEN DIVERTICULAR DISEASE OF INTESTINE AND CIRRHOSIS: A TWO-SAMPLE MENDELIAN
RANDOMIZATION STUDY Article Open access 28 September 2024 MAIN Inflammation in the form of chronic superficial gastritis is one of the early phases in the development of gastric cancer
(Correa, 1992). Therefore, a stronger inflammatory response by the host may modify gastric cancer risk. The first published epidemiologic evidence for this hypothesis came from a study
published in _Nature_, which showed an increased risk of gastric cancer and its precursor lesions associated with proinflammatory polymorphisms in interleukin-1B (_IL-1B_) and _IL-1RN_ genes
(El Omar et al, 2000). Subsequent studies presented more data on the associations between several proinflammatory polymorphisms and gastric cancer. Some studies suggested that these
polymorphisms increase gastric cancer risk, and, in some studies, this increase in risk was observed only in certain subgroups such as certain anatomical subsites (ie, noncardia) (El Omar et
al, 2003) or histological subtypes (ie, intestinal type) (Machado et al, 2001) of gastric cancer. Other studies, however, showed no association or even inverse associations. In addition to
polymorphisms in interleukin genes, the polymorphisms in the promoter region of tumour-necrosis factor-A (_TNF-A_) gene have been extensively studied in relation to gastric cancer. Three
polymorphisms in _TNF-A_ genes have been studied more than the other polymorphisms. _TNF-A_ –308G>A is associated with an increased production of TNF-_α_ (Jang et al, 2001), which is a
central mediator of the immune response and shares many biologic properties with IL-1. The function and significance of _TNF-A_ −238G>A is less clear, but because a putative repressor
site is located in a 25-base stretch that includes position −238, this polymorphism may be functional (Jang et al, 2001). _TNF-A_ −857C>T is also associated with higher transcriptional
activity of _TNF-A_ (Hohjoh and Tokunaga, 2001). Since the previous results have been inconclusive regarding the associations between _TNF-A_ genotypes and gastric cancer risk, the purpose
of this meta-analysis is to review studies that have examined those polymorphisms. Where possible, we examine these associations by anatomical or histological subtypes of gastric cancer, and
by _Helicobacter pylori_ positivity. METHODS SELECTION OF STUDIES We conducted a comprehensive search by examining several databases for all papers that had been published on the
association between _TNF-A_ polymorphisms and gastric cancer risk. All results were updated on 15 October 2007. The following terms were used in PubMed Databases search: (‘Interleukins’
[MeSH] OR ‘Tumor Necrosis Factor-alpha’ [MeSH] OR (Tumor Necrosis) OR TNF) AND (‘Stomach Neoplasms’ [MeSH] OR (gastric cancer) OR (stomach cancer)) AND (‘Polymorphism, Genetic’ [MeSH] OR
polymorphism OR polymorphisms). The following terms were used in ISI Database search: (TS=(Interleukins) OR TS=(Tumor Necrosis Factor-alpha) OR TS=(Tumor Necrosis) OR TS=(TNF)) AND
(TS=(Stomach Neoplasms) OR TS=(gastric cancer) OR TS=(stomach cancer)) AND (TS=(Polymorphism, Genetic) OR TS=(polymorphism) OR TS=(polymorphisms)). Other databases and search terms were
MedCarib, LILACS, IMEMR, IndMed, and PAHO databases, searched for (gastric OR stomach) AND (cancer OR carcinoma OR neoplasms); IMSEAR database, searched for combinations of gastric or
stomach with cancer or carcinoma or neoplasms; and J-EAST database, searched for combinations of gastric or stomach with cancer or carcinoma or neoplasms plus polymorphism. In addition,
references of cited articles were reviewed. Two of the authors reviewed results of each of the database searches to make sure that published papers are not missed. In addition, where overall
data were missing, we contacted the authors for further information. Using these approaches, reports on _TNF-A_ polymorphisms in relation to gastric cancer was found in a total of 29
articles (Jang et al, 2001; Wu et al, 2002, 2003, 2004; El Omar et al, 2003; Garza-Gonzalez et al, 2003, 2005; Machado et al, 2003; Fei et al, 2004; Glas et al, 2004; Lee et al, 2004, 2005;
Ohyama et al, 2004; Torres et al, 2004; Guo et al, 2005; Li et al, 2005; Lu et al, 2005; Perri et al, 2005; Rocha et al, 2005; Zambon et al, 2005; Kamangar et al, 2006a; Kim et al, 2006;
Morgan et al, 2006; Shirai et al, 2006; Deans et al, 2007; Garcia-Gonzalez et al, 2007; Hou et al, 2007; Seno et al, 2007; Sugimoto et al, 2007). Three studies (Garza-Gonzalez et al, 2003;
Wu et al, 2003; Shirai et al, 2006) were excluded from the analyses because their results were reported in other studies (Ohyama et al, 2004; Wu et al, 2004; Garza-Gonzalez et al, 2005). One
more study was excluded because the results had been reported for a combination of oesophageal and gastric cancers (Deans et al, 2007). Another study (Seno et al, 2007) was excluded because
genotype frequencies were not reported. Therefore, a total of 24 studies were used for calculating summary statistics. DATA EXTRACTION AND STATISTICAL ANALYSIS For _TNF-A_ −308 (rs1800629)
and _TNF-A_ −238 (rs361525), numbers and percentages of GG, GA, and AA genotypes, and for _TNF-A_ −857 (rs1799724), numbers and percentages of CC, CT, and TT genotypes were extracted by case
status. For GG and GA _vs_ GG genotypes (_TNF-A_ −308 and −238) and for TT and TC _vs_ CC genotypes (_TNF-A_ −857), odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated.
We performed similar calculations for AA _vs_ a combination of GA and GG genotypes (_TNF-A_ −308 and −238), and for TT _vs_ a combination of TC and CC genotypes (_TNF-A_ −857) to examine
the applicability of recessive models. Likewise, we did similar analyses for a combination of AA and GA _vs_ GG genotypes (_TNF-A_ −308 and −238) and for combination of TT and TC _vs_ CC
genotypes (_TNF-A_ −857) to examine whether dominant models apply. We used both random-effects models (DerSimonian–Laird method) and fixed-effects models (Mantel–Haenszel method) to
calculate overall summary ORs and 95% CIs. Because these two methods yielded similar results, we chose only random-effects models (Moayyedi, 2004) to present forest plots, and all other
analyses described from here onwards. Some of the published studies found associations only with certain anatomical subsites (ie, noncardia) or histological subtypes (ie, intestinal type) of
gastric cancer. Therefore, we calculated summary ORs and 95% CIs for noncardia cancer, where genotype data were presented by anatomical location, and for intestinal-type cancer, where data
on histology were available. We examined the association between _TNF-A_ −308 and gastric cancer in studies that reported this association among _H. pylori_-positive subjects. It has been
suggested that proinflammatory polymorphisms in interleukins may be associated with higher risk of gastric cancer in western countries, but not in East Asian countries. Therefore, study
populations were classified as western (Europe and Americas) _vs_ East Asian (China, Korea, Taiwan, and Japan), and subgroup analyses were performed for each group. In all, 12 studies were
from western and 12 studies were from East Asian countries. We examined the effect of Hardy–Weinberg equilibrium (HWE) on the results of our meta-analysis by calculating summary ORs and 95%
CIs for studies in which these alleles were in HWE among controls. The strategy was to exclude studies in which genotypes violated HWE at _α_=0.05. We plotted Begg's funnel plot to
examine small study effects (Sterne et al, 2001). We also used the method of Begg and Mazumdar (1994) to calculate _P_ for rank correlation and Egger's weighted regression method (Egger
et al, 1997) to calculate _P_ for bias. For sensitivity analysis, we excluded smaller studies and recalculated the summary ORs (95% CIs) using only larger studies. To examine result
heterogeneity among studies, the _Q_-statistic for homogeneity (using Mantel–Haenszel weights) and the _I__2_-statistic (Higgins et al, 2003) were calculated. All analyses were done using
STATA software, version 9.2 (STATA Corporation, College Station, TX, USA). Throughout the paper, two-sided _P_-values <0.05 were considered as statistically significant. RESULTS
Twenty-four studies with a total number of 4399 cases and 6855 controls were included in this analysis (Table 1). The most commonly investigated genotypes were _TNF-A_ −308, −238, and −857,
which were reported in 23, 9, and 5 studies, respectively. Since other genotypes, such as _TNF-A_ −1031, were investigated in a very small number of studies, only data on the above mentioned
three genotypes were analysed. Most studies used healthy volunteers or blood donors as control subjects. The frequency of _TNF-A_ −308A and _TNF-A_ −238A alleles ranged from 0.9 to 16.2%
and from 1.2 to 10.3%, respectively. The frequency of _TNF-A_ −857T allele ranged from 14.3 to 19.6%. Median frequencies of _TNF-A_ −308A allele were 12.5 and 6.8% in western populations and
East Asian populations, respectively. Corresponding frequencies for _TNF-A_ −238A allele were 4.9 and 3.8%, and for _TNF-A_ −857T allele were 19.6 and 15.0%, respectively. _TNF-A_ −308
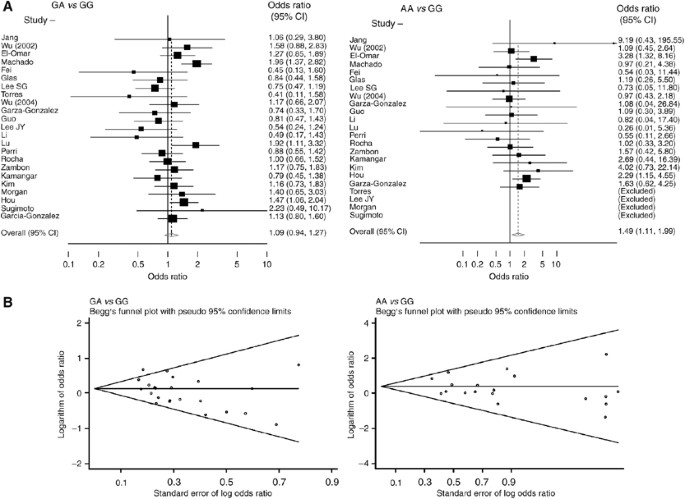
Study-specific and summary ORs (95% CIs) are shown in Figure 1A. For all gastric cancers, the random-effect overall ORs (95% CIs) associated with heterozygous (GA _vs_ GG) and homozygous (AA
_vs_ GG) proinflammatory genotypes were 1.09 (0.94–1.27) and 1.49 (1.11–1.99), respectively, and the corresponding fixed-effect ORs (95% CIs) were 1.14 (1.02–1.27) and 1.51 (1.14–1.99),
respectively. Recessive model was the best-fitting inheritance model for _TNF-A_ −308. However, because GG genotype was much more frequent than GA and AA genotypes, comparing GA+GG _vs_ AA
genotype did not have a material effect on the test power. Therefore, the results are reported separately for GA _vs_ GG and AA _vs_ GG genotypes. Table 2 summarises overall and
subgroup-specific summary ORs and 95% CIs. Only a fraction of studies presented data on subgroups. For example, only 6 out of the 23 studies reported their results by intestinal _vs_ diffuse
histology types. Among these, summary ORs (95% CIs) for GA _vs_ GG and AA _vs_ GG genotypes were 1.17 (0.85–1.59) and 2.88 (1.74–4.77) for noncardia cancers and 0.98 (0.68–1.40) and 2.17
(1.17–4.03) for intestinal-type gastric cancers, respectively. When we limited our analysis to _H. pylori_-positive cases and controls, these ORs (95% CIs) were 0.66 (0.33–1.30) and 3.23
(0.10–99.4), respectively. The summary ORs (95% CIs) for GA _vs_ GG and AA _vs_ GG genotypes were 1.14 (0.95–1.37) and 1.74 (1.21–2.51) for western countries, and 1.02 (0.78–1.34) and 1.14
(0.70–1.84) for eastern countries, respectively. In three studies, the distributions of _TNF-A_ −308 genotypes among controls were not in HWE (Table 1). Limiting the analysis to the studies
with HWE, the summary ORs (95% CIs) for GA _vs_ GG and AA _vs_ GG genotypes were 1.08 (0.91–1.28) and 1.73 (1.22–2.44), respectively. Figure 1B shows Begg's funnel plot for the
association between _TNF-A_ −308 and gastric cancer. This figure shows logarithm of OR (_Y_ axis) _vs_ its standard error (SE) (_X_ axis). Smaller studies have larger SEs, therefore points
showing these studies are in the right-hand side of the graph. For GA _vs_ GG genotypes, there was evidence for bias using Egger's weighted regression method (_P_ for bias=0.03) and
using the method of Begg and Mazumdar (_P_=0.07). There was no evidence of bias for AA _vs_ GG genotypes using either Egger's method (_P_ for bias=0.44) or Begg's method
(_P_=0.87). We excluded small studies, defined as those having an SE>0.5. After these exclusion, the summary OR (95% CI) changed to 1.13 (0.97–1.32) for GA _vs_ GG, and 1.68 (1.08–2.61)
for AA _vs_ GG. Table 2 shows the _Q_- and _I__2_-statistics for the overall and subgroup analyses. For the overall analysis, the _Q_-statistic was significant (_P_=0.02) and _I__2_ (34%)
showed a moderate variation for GA _vs_ GG genotypes. For AA _vs_ GG genotypes, the _Q_-statistic was not significant (_P_=0.74) and _I__2_ was equal to zero. Subgroup analyses showed
similar patterns as overall analyses. While for most subgroup analyses, the _P_-value for GA _vs_ GG genotypes was significant or remained close to significance level and _I__2_ showed
moderate heterogeneity among studies, _P_ for heterogeneity was not significant for AA _vs_ GG genotypes (Table 2). _TNF-A_ −238 Figure 2A summarises the ORs and 95% CIs for the associations
between _TNF-A_ −238A carrying genotypes and gastric cancer risk. For all gastric cancers, the random-effect overall ORs (95% CIs) were 1.05 (0.84–1.33) for GA _vs_ GG genotypes and 1.25
(0.30–5.26) for AA _vs_ GG genotypes. The fixed-effect ORs (95% CIs) were 1.04 (0.83–1.31) and 0.87 (0.41–1.87), respectively. When we investigated the inheritance models, _TNF-A_ −238
showed neither recessive nor dominant models (data not shown). Therefore, the results are reported separately as GA _vs_ GG and AA _vs_ GG genotypes. For noncardia cancers, the ORs (95% CIs)
were 1.04 (0.71–1.52) for GA genotype and 1.38 (0.01–157.0) for AA genotype. Limiting the results to intestinal-type cancers, the ORs (95% CIs) were 1.40 (0.93–2.10) for GA genotype and
0.13 (0.01–2.20) for AA genotype. For this last comparison, one of the three studies was excluded because of the null values for AA genotype frequency among both cases and controls. For
studies from western countries, summary ORs (95% CIs) for GA and AA genotypes were 1.05 (0.78–1.41) and 1.25 (0.01–171.9), respectively. For the studies from East Asian countries, these
corresponding ORs (95% CIs) were 1.04 (0.69–1.58) and 1.28 (0.39–4.21), respectively. In three studies, _TNF-A_ −238 genotype in control subjects were not in HWE (Table 1). After excluding
these three studies, the summary ORs (95% CIs) for GA and AA genotypes were 1.08 (0.75–1.55) and 4.04 (0.57–28.8), respectively. Begg's funnel plot for the association between _TNF-A_
−238 and gastric cancer is shown in Figure 2B. For both GA _vs_ GG and AA _vs_ GG genotypes, there was no evidence for bias using either the method of Begg and Mazumdar (_P_ for rank
correlation=1.00 and 0.85, respectively) or Egger's weighted regression method (_P_ for bias=0.84 and 0.75, respectively). For GA genotype, after excluding studies with an SE>0.5,
the summary OR (95% CI) was 1.04 (0.82–1.33). For AA genotype, all of the studies had an SE>0.5. For GA genotype, the _Q_-statistic was nonsignificant for the overall association
(_P_=0.55) and subgroup analyses, and _I__2_-statistic showed little heterogeneity among studies (Table 2). For AA genotype, the _Q_-statistic was significant for the overall association
(_P_=0.05) and also for noncardia, intestinal-type, and western populations subgroups, where _I__2_-statistic showed moderate-to-high heterogeneity. _TNF-A_ −857 Figure 3A summarises the ORs
and 95% CIs for the associations between _TNF-A_ −857T carrying genotypes (TC and TT _vs_ CC) and gastric cancer risk. For all gastric cancers, the random-effect overall ORs (95% CIs) for
TC _vs_ CC and TT _vs_ CC genotypes were 1.06 (0.89–1.27) and 1.57 (0.91–2.70), respectively. The fixed-effect ORs (95% CIs) were 1.06 (0.89–1.27) and 1.54 (0.98–2.43), respectively. Like
_TNF-A_ −238, _TNF-A_ −857 showed neither recessive nor dominant model. Therefore, the results are reported separately for TC _vs_ CC and TT _vs_ CC genotypes. Only one study reported the
associations between _TNF-A_ −857 and noncardia cancers. This study was also the only study from western countries; the ORs (95% CIs) for TC and TT genotypes in this study were 0.94
(0.63–1.41) and 0.75 (0.17–3.38), respectively. For studies from eastern countries, summary ORs (95% CIs) for TC and TT genotypes were 1.09 (0.90–1.33) and 1.73 (0.95–3.14), respectively. In
one study, _TNF-A_ −857 genotypes among controls were not in HWE (Table 1). After excluding that study, the summary ORs (95% CIs) for TC and TT genotypes were 1.09 (0.90–1.33) and 1.73
(0.95–3.14), respectively. Begg's funnel plot for the association between _TNF-A_ −857 and gastric cancer is shown in Figure 3B. For both TC and CC genotypes, there was no evidence for
bias using either the method of Begg and Mazumdar (_P_ for rank correlation=0.81 and 0.46, respectively) or Egger's weighted regression method (_P_ for bias=0.87 and 0.89,
respectively). For TC _vs_ CC genotypes, none of the studies had an SE>0.5. For TT _vs_ CC genotypes, after excluding two studies with SEs>0.5, the summary OR (95% CI) changed to 1.66
(0.81–3.42). The _Q_-statistic was nonsignificant for the overall associations and also for the subgroup analyses. Furthermore, _I__2_-statistic showed little to moderate heterogeneity among
studies (Table 2). DISCUSSION Gastric cancer is the second most common cause of cancer death in the world (Parkin et al, 2005; Kamangar et al, 2006c). Because inflammation is one of the
initial phases of gastric carcinogenesis, especially for intestinal-type gastric cancer (Correa, 1992), inflammation-related polymorphisms, including single-nucleotide polymorphisms (SNPs)
in _TNF-A_ gene, have been extensively studied in relation to gastric cancer (Camargo et al, 2006). The most extensively studied of these proinflammatory polymorphisms are two linked SNPs in
_IL-1B_ (−511C>T and −31T>C) and a penta-allelic variable number tandem-repeat polymorphism and allele 2 (_IL-1RN_*2). So far, at least three meta-analyses of the associations between
these polymorphisms and gastric cancer risk have been published (Camargo et al, 2006; Kamangar et al, 2006b; Wang et al, 2007). However, to our knowledge, no systematic review has been
previously published on the association between _TNF-A_ SNPs and gastric cancer. Of the three polymorphisms reviewed in this report, –308G>A is studied more extensively and a biologic
role for it has been identified. An analysis of the 23 studies that presented results on _TNF-A_ −308G>A showed no association between GA genotype (_vs_ GG) and gastric cancer risk.
However, there was a statistically significant increased risk associated with AA genotype using both fixed-effects and random-effects models. This association was limited to studies from
western countries, and no association was found in studies from East Asian countries. Previous studies have suggested that frequencies of genetic markers often shows high variations among
various ethnic and racial groups (Garte et al, 2001; Ioannidis et al, 2004), whereas differences in genetic effects (in terms of ORs) are much less common (Ioannidis et al, 2004). This
meta-analysis found that the median prevalence of _TNF-A_ −308A carrier genotypes was almost twice as high in western as in East Asian populations (23.5 _vs_ 13.4%). However, unlike what has
been shown for most previous associations (Ioannidis et al, 2004), the OR associated with AA genotype showed a difference between western and East Asian studies. We cannot explain the
reasons for this latter observed difference. No SNPs have been consistently associated with gastric cancer risk (Gonzalez et al, 2002). Indeed, because several initially promising
gene–disease associations gravitated towards null over time (Ioannidis et al, 2001; Kamangar et al, 2006b), it has been suggested that journals should take a cautious approach in publishing
such associations (Ioannidis, 2006). However, as shown in the results and discussed below, within the studies from western countries, the association between _TNF-A_ −308AA genotype and
gastric cancer risk seems robust to many tests, including testing for publication bias, heterogeneity, and HWE. Interestingly, a study within the InterLymph Consortium found that this same
polymorphism was consistently associated with an increased risk of diffuse large B-cell lymphoma with a comparable magnitude of association (Rothman et al, 2006); the ORs associated with GA
and AA genotypes (_vs_ GG genotype) were 1.29 and 1.65, respectively. The studies that participated in this Consortium were all from western countries. The forest plots for _TNF-A_ −308AA
genotype did not suggest a dominant effect for any single study. We used funnel plots and two formal statistical methods (Egger's weighted regression method and the rank correlation
method of Begg and Mazumdar) to detect bias. In general, smaller studies, that is, those with higher SEs, had lower ORs, and there was some evidence for publication bias using both formal
methods. However, excluding smaller studies did not materially change the results. In 20 out of 23 studies, distribution of _TNF-A_ −308 among controls was in HWE. Limiting the analyses to
these 20 studies, the results remained essentially unchanged. The overall heterogeneity between the studies was very low, as indicated by the _I__2_ value of zero. Since subgroup analyses
are often limited by selective reporting of significant subgroup results, such analyses generally need to be interpreted with caution. However, in this report, limiting the results of
_TNF-A_ −308 polymorphism to noncardia gastric cancers or intestinal-type cancers made little difference. The summary ORs for the AG genotype remained close to null and were nonsignificant,
whereas those for the GG genotype remained statistically significantly above one. We found nine studies that examined the association between _TNF-A_ −238G>A polymorphism and gastric
cancer risk. Unlike that for _TNF-A_ −308G>A polymorphism, a clear biologic role for this polymorphism has not been found (Jang et al, 2001). There was no association between gastric
cancer risk and either the GA or the AA genotypes using either random-effects or fixed-effects models. Examining the data by anatomic and histologic subtypes did not make a difference. No
statistically significant association was found when data were limited to East Asian populations, western populations, studies with larger sample sizes, or studies in which genotype
frequencies were in HWE. We found only five studies that had examined the association between _TNF-A_ −857C>T polymorphisms and gastric cancer. No evidence was found for the association
between CT (_vs_ CC) genotype and gastric cancer. Almost all studies showed null associations, and there was little evidence for heterogeneity. The overall association with the TT genotype,
however, was near significant. The overall pattern and magnitude of association is similar to that found for _TNF-A_ −308G>A polymorphism, but further studies are needed to examine
whether gastric cancer is significantly associated with this polymorphism. The studies showed some heterogeneity, with three showing strong positive associations, one showing no association,
and one showing an inverse association. We believe that the currently available data do not provide conclusive evidence for the presence of an association, or lack thereof, between _TNF-A_
−857TT genotype and gastric cancer. Inappropriate selection of controls is a major source of bias in case–control studies. However, control groups for most of the studies used in this
meta-analysis were selected from among healthy volunteers or blood donors, and _TNF-A_ polymorphisms are unlikely to be associated with these conditions. Because _TNF_ locus is on chromosome
6 (and not sex chromosomes), the distribution of this polymorphism is not associated with sex. Therefore, in theory, matching for sex should not affect the results. Strengths of this
meta-analysis are including 24 published studies with a large number of cases and controls, presenting data on several relevant methodologic aspects of these studies, subgroup analyses
according to predefined criteria, and using other methods to examine the robustness of the summary statistics. We acknowledge that this meta-analysis also has limitations. Combining
observational studies conducted in different populations with various qualities of design to obtain summary ORs and 95% CIs can sometimes be misleading (Shapiro, 1994), and summary
statistics need to be interpreted with caution (Egger et al, 1998). However, as mentioned above, there is little evidence for improper selection of control groups or for associations within
specific subgroups. In summary, this systematic review found that _TNF-A_ −308AA genotype was moderately associated with an increased risk of gastric cancer. _TNF-A_ –308AG, _TNF-A_ −238AA
or AG, and _TNF-A_ −857CT or TT were not statistically significantly associated with gastric cancer risk. It is possible that, with increasing the number of studies, _TNF-A_ −857TT may also
be associated with an increased risk of gastric cancer. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence
terms, as noted at publication _ REFERENCES * Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. _Biometrics_ 50: 1088–1101 Article CAS
PubMed Google Scholar * Camargo MC, Mera R, Correa P, Peek Jr RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG (2006) Interleukin-1beta and interleukin-1
receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. _Cancer Epidemiol Biomarkers Prev_ 15: 1674–1687 Article CAS PubMed Google Scholar * Correa P (1992) Human
gastric carcinogenesis: a multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. _Cancer Res_ 52: 6735–6740 CAS PubMed
Google Scholar * Deans C, Rose-Zerilli M, Wigmore S, Ross J, Howell M, Jackson A, Grimble R, Fearon K (2007) Host cytokine genotype is related to adverse prognosis and systemic inflammation
in gastro-oesophageal cancer. _Ann Surg Oncol_ 14: 329–339 Article PubMed Google Scholar * Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple,
graphical test. _BMJ_ 315: 629–634 Article CAS PubMed PubMed Central Google Scholar * Egger M, Schneider M, Davey SG (1998) Spurious precision? Meta-analysis of observational studies.
_BMJ_ 316: 140–144 Article CAS PubMed PubMed Central Google Scholar * El Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N,
Lanyon G, Martin M, Fraumeni Jr JF, Rabkin CS (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. _Nature_ 404: 398–402 Article CAS PubMed Google Scholar
* El Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni Jr JF, Chow WH (2003) Increased risk of noncardia gastric
cancer associated with proinflammatory cytokine gene polymorphisms. _Gastroenterology_ 124: 1193–1201 Article CAS PubMed Google Scholar * Fei BY, Xia B, Deng CS, Xia XQ, Xie M, Crusius
JB, Pena AS (2004) Association of tumor necrosis factor genetic polymorphism with chronic atrophic gastritis and gastric adenocarcinoma in Chinese Han population. _World J Gastroenterol_ 10:
1256–1261 Article CAS PubMed PubMed Central Google Scholar * Garcia-Gonzalez MA, Lanas A, Quintero E, Nicolas D, Parra-Blanco A, Strunk M, Benito R, Angel SM, Santolaria S, Sopena F,
Piazuelo E, Jimenez P, Pascual C, Mas E, Irun P, Espinel J, Campo R, Manzano M, Geijo F, Pellise M, Gonzalez-Huix F, Nieto M, Espinos J, Tito L, Bujanda L, Zaballa M (2007) Gastric cancer
susceptibility is not linked to pro- and anti-inflammatory cytokine gene polymorphisms in whites: a nationwide multicenter study in Spain. _Am J Gastroenterol_ 102: 1878–1892 Article CAS
PubMed Google Scholar * Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, Brockmoller J, Cascorbi
I, Clapper ML, Coutelle C, Daly A, Dell'Omo M, Dolzan V, Dresler CM, Fryer A, Haugen A, Hein DW, Hildesheim A, Hirvonen A, Hsieh LL, Ingelman-Sundberg M, Kalina I, Kang D, Kihara M,
Kiyohara C, Kremers P, Lazarus P, Le Marchand L, Lechner MC, van Lieshout EM, London S, Manni JJ, Maugard CM, Morita S, Nazar-Stewart V, Noda K, Oda Y, Parl FF, Pastorelli R, Persson I,
Peters WH, Rannug A, Rebbeck T, Risch A, Roelandt L, Romkes M, Ryberg D, Salagovic J, Schoket B, Seidegard J, Shields PG, Sim E, Sinnet D, Strange RC, Stucker I, Sugimura H, To-Figueras J,
Vineis P, Yu MC, Taioli E (2001) Metabolic gene polymorphism frequencies in control populations. _Cancer Epidemiol Biomarkers Prev_ 10: 1239–1248 CAS PubMed Google Scholar *
Garza-Gonzalez E, Bosques-Padilla FJ, El Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Perez-Perez GI (2005) Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal
gastric cancer in Mexico. _Int J Cancer_ 114: 237–241 Article CAS PubMed Google Scholar * Garza-Gonzalez E, Hold G, Perez-Perez GI, Bosques-Padilla FJ, Tijerina-Menchaca R,
Maldonado-Garza HJ, El Omar E (2003) Role of polymorphism of certain cytokines in gastric cancer in Mexico. Preliminary results. _Rev Gastroenterol Mex_ 68: 107–112 PubMed Google Scholar *
Glas J, Torok HP, Schneider A, Brunnler G, Kopp R, Albert ED, Stolte M, Folwaczny C (2004) Allele 2 of the interleukin-1 receptor antagonist gene is associated with early gastric cancer. _J
Clin Oncol_ 22: 4746–4752 Article CAS PubMed Google Scholar * Gonzalez CA, Sala N, Capella G (2002) Genetic susceptibility and gastric cancer risk. _Int J Cancer_ 100: 249–260 Article
CAS PubMed Google Scholar * Guo W, Wang N, Li Y, Zhang JH (2005) Polymorphisms in tumor necrosis factor genes and susceptibility to esophageal squamous cell carcinoma and gastric cardiac
adenocarcinoma in a population of high incidence region of North China. _Chin Med J (Engl)_ 118: 1870–1878 CAS Google Scholar * Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003)
Measuring inconsistency in meta-analyses. _BMJ_ 327: 557–560 Article PubMed PubMed Central Google Scholar * Hohjoh H, Tokunaga K (2001) Allele-specific binding of the ubiquitous
transcription factor OCT-1 to the functional single nucleotide polymorphism (SNP) sites in the tumor necrosis factor-alpha gene (TNFA) promoter. _Genes Immun_ 2: 105–109 Article CAS PubMed
Google Scholar * Hou L, El Omar EM, Chen J, Grillo P, Rabkin CS, Baccarelli A, Yeager M, Chanock SJ, Zatonski W, Sobin LH, Lissowska J, Fraumeni Jr JF, Chow WH (2007) Polymorphisms in
Th1-type cell-mediated response genes and risk of gastric cancer. _Carcinogenesis_ 28: 118–123 Article CAS PubMed Google Scholar * Ioannidis JP (2006) Journals should publish all ‘null’
results and should sparingly publish ‘positive’ results. _Cancer Epidemiol Biomarkers Prev_ 15: 186 Article PubMed Google Scholar * Ioannidis JP, Ntzani EE, Trikalinos TA (2004) ‘Racial’
differences in genetic effects for complex diseases. _Nat Genet_ 36: 1312–1318 Article CAS PubMed Google Scholar * Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001)
Replication validity of genetic association studies. _Nat Genet_ 29: 306–309 Article CAS PubMed Google Scholar * Jang WH, Yang YI, Yea SS, Lee YJ, Chun JH, Kim HI, Kim MS, Paik KH
(2001) The −238 tumor necrosis factor-alpha promoter polymorphism is associated with decreased susceptibility to cancers. _Cancer Lett_ 166: 41–46 Article CAS PubMed Google Scholar *
Kamangar F, Abnet CC, Hutchinson AA, Newschaffer CJ, Helzlsouer K, Shugart YY, Pietinen P, Dawsey SM, Albanes D, Virtamo J, Taylor PR (2006a) Polymorphisms in inflammation-related genes and
risk of gastric cancer (Finland). _Cancer Causes Control_ 17: 117–125 Article PubMed Google Scholar * Kamangar F, Cheng C, Abnet CC, Rabkin CS (2006b) Interleukin-1B polymorphisms and
gastric cancer risk – a meta-analysis. _Cancer Epidemiol Biomarkers Prev_ 15: 1920–1928 Article CAS PubMed Google Scholar * Kamangar F, Dores GM, Anderson WF (2006c) Patterns of cancer
incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. _J Clin Oncol_ 24: 2137–2150
Article PubMed Google Scholar * Kim N, Cho SI, Yim JY, Kim JM, Lee DH, Park JH, Kim JS, Jung HC, Song IS (2006) The effects of genetic polymorphisms of IL-1 and TNF-A on _Helicobacter
pylori_-induced gastroduodenal diseases in Korea. _Helicobacter_ 11: 105–112 Article CAS PubMed Google Scholar * Lee JY, Kim HY, Kim KH, Kim SM, Jang MK, Park JY, Lee JH, Kim JH, Yoo JY
(2005) Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. _Cancer Lett_ 225: 207–214 Article CAS PubMed Google Scholar * Lee SG, Kim B, Yook JH, Oh ST,
Lee I, Song K (2004) TNF/LTA polymorphisms and risk for gastric cancer/duodenal ulcer in the Korean population. _Cytokine_ 28: 75–82 Article CAS PubMed Google Scholar * Li C, Xia B, Yang
Y, Li J, Xia HH (2005) TNF gene polymorphisms and _Helicobacter pylori_ infection in gastric carcinogenesis in Chinese population. _Am J Gastroenterol_ 100: 290–294 Article CAS PubMed
Google Scholar * Lu W, Pan K, Zhang L, Lin D, Miao X, You W (2005) Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric
cancer in a Chinese population. _Carcinogenesis_ 26: 631–636 Article CAS PubMed Google Scholar * Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro AC, Campos
ML, Van Doorn LJ, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoes M (2003) A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma.
_Gastroenterology_ 125: 364–371 Article CAS PubMed Google Scholar * Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F,
Sobrinho-Simoes M (2001) Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. _Gastroenterology_ 121: 823–829 Article CAS PubMed
Google Scholar * Moayyedi P (2004) Meta-analysis: can we mix apples and oranges? _Am J Gastroenterol_ 99: 2297–2301 Article PubMed Google Scholar * Morgan DR, Dominguez RL, Keku TO,
Heidt PE, Martin CF, Galanko JA, Omofoye OA, Sandler RS (2006) Gastric cancer and the high combination prevalence of host cytokine genotypes and _Helicobacter pylori_ in Honduras. _Clin
Gastroenterol Hepatol_ 4: 1103–1111 Article CAS PubMed Google Scholar * Ohyama I, Ohmiya N, Niwa Y, Shirai K, Taguchi A, Itoh A, Hirooka Y, Wakai K, Hamajima N, Mori N, Goto H (2004) The
association between tumour necrosis factor-alpha gene polymorphism and the susceptibility to rugal hyperplastic gastritis and gastric carcinoma. _Eur J Gastroenterol Hepatol_ 16: 693–700
Article CAS PubMed Google Scholar * Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. _CA Cancer J Clin_ 55: 74–108 Article PubMed Google Scholar * Perri F,
Piepoli A, Bonvicini C, Gentile A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR, Ricciardiello L, Gennarelli M, Bazzoli F, Ranzani GN, Andriulli A (2005) Cytokine gene
polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. _Cytokine_ 30: 293–302 Article CAS PubMed Google Scholar * Rocha GA, Guerra JB, Rocha
AM, Saraiva IE, da Silva DA, de Oliveira CA, Queiroz DM (2005) IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. _Int J Cancer_
115: 678–683 Article CAS PubMed Google Scholar * Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, De Sanjose S, Cocco P, Berndt SI, Brennan P,
Brooks-Wilson A, Wacholder S, Becker N, Hartge P, Zheng T, Roman E, Holly EA, Boffetta P, Armstrong B, Cozen W, Linet M, Bosch FX, Ennas MG, Holford TR, Gallagher RP, Rollinson S, Bracci PM,
Cerhan JR, Whitby D, Moore PS, Leaderer B, Lai A, Spink C, Davis S, Bosch R, Scarpa A, Zhang Y, Severson RK, Yeager M, Chanock S, Nieters A (2006) Genetic variation in TNF and IL10 and risk
of non-Hodgkin lymphoma: a report from the InterLymph Consortium. _Lancet Oncol_ 7: 27–38 Article CAS PubMed Google Scholar * Seno H, Satoh K, Tsuji S, Shiratsuchi T, Harada Y, Hamajima
N, Sugano K, Kawano S, Chiba T (2007) Novel interleukin-4 and interleukin-1 receptor antagonist gene variations associated with non-cardia gastric cancer in Japan: comprehensive analysis of
207 polymorphisms of 11 cytokine genes. _J Gastroenterol Hepatol_ 22: 729–737 Article CAS PubMed Google Scholar * Shapiro S (1994) Meta-analysis/Shmeta-analysis. _Am J Epidemiol_ 140:
771–778 Article CAS PubMed Google Scholar * Shirai K, Ohmiya N, Taguchi A, Mabuchi N, Yatsuya H, Itoh A, Hirooka Y, Niwa Y, Mori N, Goto H (2006) Interleukin-8 gene polymorphism
associated with susceptibility to non-cardia gastric carcinoma with microsatellite instability. _J Gastroenterol Hepatol_ 21: 1129–1135 Article CAS PubMed Google Scholar * Sterne JA,
Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. _BMJ_ 323: 101–105 Article CAS PubMed PubMed
Central Google Scholar * Sugimoto M, Furuta T, Shirai N, Nakamura A, Xiao F, Kajimura M, Sugimura H, Hishida A (2007) Different effects of polymorphisms of tumor necrosis factor-alpha and
interleukin-1 beta on development of peptic ulcer and gastric cancer. _J Gastroenterol Hepatol_ 22: 51–59 Article CAS PubMed Google Scholar * Torres MM, Acosta CP, Sicard DM, Groot de
Restrepo H (2004) [Genetic susceptibility and risk of gastric cancer in a human population of Cauca, Colombia]. _Biomedica_ 24: 153–162 Article PubMed Google Scholar * Wang P, Xia HH,
Zhang JY, Dai LP, Xu XQ, Wang KJ (2007) Association of interleukin-1 gene polymorphisms with gastric cancer: a meta-analysis. _Int J Cancer_ 120: 552–562 Article CAS PubMed Google Scholar
* Wu MS, Chen LT, Shun CT, Huang SP, Chiu HM, Wang HP, Lin MT, Cheng AL, Lin JT (2004) Promoter polymorphisms of tumor necrosis factor-alpha are associated with risk of gastric
mucosa-associated lymphoid tissue lymphoma. _Int J Cancer_ 110: 695–700 Article CAS PubMed Google Scholar * Wu MS, Huang SP, Chang YT, Shun CT, Chang MC, Lin MT, Wang HP, Lin JT (2002)
Tumor necrosis factor-alpha and interleukin-10 promoter polymorphisms in Epstein–Barr virus-associated gastric carcinoma. _J Infect Dis_ 185: 106–109 Article CAS PubMed Google Scholar *
Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT, Lin JT (2003) Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. _Int J Cancer_ 104: 617–623 Article CAS
PubMed Google Scholar * Zambon CF, Basso D, Navaglia F, Belluco C, Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F, Plebani M (2005) Pro- and anti-inflammatory cytokines gene
polymorphisms and _Helicobacter pylori_ infection: interactions influence outcome. _Cytokine_ 29: 141–152 Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This
research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Medicine, The Reading Hospital and Medical Center, West Reading, PA, USA F Gorouhi * Digestive Disease Research Center, Medical Sciences/University of Tehran, Tehran, Iran F Islami *
International Agency for Research on Cancer, Lyon, France F Islami * Departments of Medicine and Epidemiology, Johns Hopkins Schools of Medicine and Public Health, The Johns Hopkin's
University, Baltimore, MD, USA H Bahrami * Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA F Kamangar Authors * F
Gorouhi View author publications You can also search for this author inPubMed Google Scholar * F Islami View author publications You can also search for this author inPubMed Google Scholar *
H Bahrami View author publications You can also search for this author inPubMed Google Scholar * F Kamangar View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to F Kamangar. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons
Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Gorouhi, F., Islami, F., Bahrami, H. _et al._ Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. _Br J Cancer_ 98, 1443–1451 (2008).
https://doi.org/10.1038/sj.bjc.6604277 Download citation * Received: 28 November 2007 * Accepted: 24 January 2008 * Published: 04 March 2008 * Issue Date: 22 April 2008 * DOI:
https://doi.org/10.1038/sj.bjc.6604277 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * TNF * gastric cancer * polymorphism * inflammation *
genetic